2020 Volume 43 Issue 4 Pages 629-638
2020 Volume 43 Issue 4 Pages 629-638
Sparassis crispa (SC; Japanese name: Hanabiratake) is a mushroom with high β(1-3)-glucan content. We here studied the effects of SC and lactic acid bacteria-fermented SC (SCL) on innate immunity. In in vivo studies using mice, oral administration of SC or SCL enhanced the accumulation of macrophages, neutrophils, natural killer (NK) cells, and C–C chemokine receptor type 2- or phospho-Syk-expressing cells in the jejunum epithelial villi and spleen, with significantly higher cell numbers in the SCL group than in the SC group. In addition, mRNA levels of genes encoding tissue factor (TF) and tumor necrosis factor (TNF)-α were increased in monocytes/macrophages from the peritoneal cavity of mice orally administered SCL. In in vitro studies using cultured human monocytes, SC and SCL enhanced the expression of gees involved in blood coagulation and inflammation, as well as those encoding various innate immune-related factors, such as TF, TNF-α, plasminogen activator inhibitor (PAI)-1, monocyte chemotactic protein (MCP)-1, interleukin (IL)-1β, IL-8, IL-12β, and IL-17, in a dose-dependent manner. In particular, the expression levels of all these factors in monocytes were significantly higher with SCL treatment than with SC treatment. SCL significantly enhanced the phagocytosis of pH-sensitive fluorescent dye-labeled Escherichia coli by human monocytes compared to SC. The effect of SCL on phagocytosis was significantly reduced to approximately 30% by pre-digestion of SCL with β-glucanase, suggesting that β(1-3)-glucan in SCL is a major contributor to the effect. These data suggest that oral administration of SCL significantly enhances innate immunity in mice and possibly humans.
Sparassis crispa (SC; Hanabiratake in Japanese; Cauliflower mushroom in English) is used as an ingredient in side dishes served alongside fish, vegetables, and pickles in Japanese cuisine owing to its excellent texture and taste as well as the lack of toxicity. Commercialized artificial cultivation has recently facilitated the use of SC as a food ingredient in pot-based dishes and salads. Importantly, SC has been shown to act as a functional food, possessing natural killer (NK) cell-activating activity,1) anti-allergy effects,1) antitumor effects,1,2) tumor angiogenesis inhibitory activity,3) cytokine-inducing activity in murine splenocytes,4-7) wound healing effects,8) and skin-improving properties.9) The various biological activities exhibited by SC appear to depend on the level of β(1-3)-glucan, which constitutes at least 43% of the total SC content, higher than that of other mushroom varieties, e.g., Agaricus subrufescens (12%) or Grifola frondosa (Maitake in Japanese: 18%).10,11)
Lactic acid bacteria have been used in many foods to preserve, improve glutamate-based umami, and regulate intestinal function. They are also used as probiotics (living microorganisms with health benefits when consumed) to reduce the risk of developing various diseases.12) The main activities of lactic acid bacteria in humans include the improvement of symptoms of virally and bacterially induced diarrhea13,14) and reduction of food allergies.15) Lactic acid bacteria have also been reported to function in cancer prevention,16,17) immune system regulation,18) influenza infection prevention,19) and blood pressure reduction.20)
Based on these prior observations, a functional food (product name: Royal β), which is SC fermented with plant-derived lactic acid bacteria (L), called here SCL, has recently been developed. In this study, we aimed to clarify the effects of SCL on innate immunity. To this end, we first examined the effect of oral administration of SC extract or SCL suspension on the accumulation of innate immune-related cells in the jejunum epithelial villi and spleens of mice, and the expression of C–C chemokine receptor and a hematopoietic signaling molecule in these tissues. We also investigated the expression of various coagulation- and inflammation-related factors in monocytes/macrophages collected from peritoneal lavage fluid of SC or SCL-treated mice. Next, we investigated the direct in vitro effects of SC and SCL on the expression of genes encoding various innate immune-related factors in cultured human peripheral blood monocytes and on phagocytosis of Escherichia coli by human monocytes.
Following the pulverization of dried SC, one-liter aliquots of natural mineral water (containing 110 mg/L Ca2+ and 9.8 mg/L Mg2+) were added to 20 g of SC powder samples. The preparation was boiled for 1 h and then allowed to cool to room temperature. The obtained extract was filtered with filter paper to remove residues, and the filtrate was boiled and concentrated to 1/5 of the original volume, and then cooled. Finally, a 30% volume of 1,3-butylene glycol was blended with the mixture as a stabilizer. The resulting solution was defined as a hot water extract of SC.
Preparation of SCLTo prepare SCL, we used soybean extract prepared as follows. Soybeans (700 g) were soaked with 1 L of natural mineral water for 15 h and boiled for 3 h, and then the cooled mixture was filtrated to remove the debris. To this soybean extract, dried SC powder (250 g) and glucose (100 g) were added, and the mixture was sterilized at high pressure (120°C, 30 min), then cooled to 35°C. Lactic acid bacteria (2 × 109 bacteria/10 mL) were then added to the mixture, and allowed to ferment for 7 d. This preparation was designated as SCL (product name: Royal β; Asuka Co., Ltd., Matsuzaka, Mie, Japan). Undiluted SCL or mineral water-diluted SCL was used for oral administration in mice (in vivo experiments), and mineral water-diluted SCL was used for in vitro experiments in cultured blood monocytes.
Determination of β(1-3)-Glucan ConcentrationThe concentration of β(1-3)-glucan in hot water extracts of SC and SCL before and after digestion with β-glucanase was determined based on the bioactivity of β(1-3)-glucan in the factor-G-mediated Limulus coagulation pathway using the Limulus Amebocyte Lysate chromogenic assay kit (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) as described previously.21) In this assay, pachyman (Megazyme, Dublin, Ireland) isolated from Fungus Poria Coco was used as the β(1,3)-glucan standard.
β-Glucanase-digested SC (abbreviated as dSC) and SCL (dSCL) were prepared as follows: The hot water extract of SC or SCL (70 mg/mL) was treated with 0.56 mg/mL β-glucanase (Westase; TaKaRa Bio, Shiga, Japan) overnight at room temperature according to the manufacturer’s instructions.
Analysis of Accumulation of Innate Immune-Related Cells in the Epithelial Villi of the Jejunum and Spleen of Mice Orally Administered SC or SCLMale ICR mice (10 weeks old; seven mice per group) were orally administered 100 µL of mineral water, L suspension (containing 2 × 107 bacteria), SC solution (containing 2.5 mg SC), or SCL solution (1/100 dilution with mineral water, 1/10 dilution with mineral water, or undiluted solution containing 25 µg, 250 µg, or 2.5 mg of SC, respectively). Forty-eight hours later, the small intestine (5 cm from the upper jejunum) and the spleen were dissected out. After weighing the jejunum and spleen, the tissues were fixed with 4% phosphate-buffered paraformaldehyde for 7 d, preserved by glucose exchange, and immersed in Tissue-Tek (Sakura Finetek, Alphen aan den Rijn, Netherlands) for frozen storage. Sections (5-µm thick) were prepared from frozen samples using a cryostat, subjected to three washes with phosphate-buffered saline (PBS) (5 min each), and reacted with cell-specific primary antibodies at 4°C for 15 h. The samples were washed three times (5 min each) with PBS and then incubated with fluorescent dye-conjugated secondary antibodies for 2 h at room temperature in the dark. Samples were washed three times (5 min each) with PBS and stored in an enclosed container. Stained tissue samples were photographed using a fluorescence microscope, and images were captured using a computer. The data were analyzed using ImageJ analysis software (NIH, Bethesda, MD, U.S.A.). The number of cells in the tissue was randomly calculated across five visual fields within a fixed area, and the average number of positive cells per field was determined. The primary antibodies used in the study were anti-mouse NCAM-rat immunoglobulin G (IgG) for detection of NK cells (R&D Systems, Minneapolis, MN, U.S.A.), anti-mouse F4/80-rat IgG for detection of macrophages (R&D Systems), and anti-mouse Gr-1/Ly-6G-rat IgG for detection of neutrophils (R&D Systems) diluted at 1 : 100 with antibody diluent (Can Get Signal; Toyobo Global, Osaka, Japan). Fluorescein isothiocyanate-conjugated goat anti-rat IgG (green fluorescently labeled antibody) diluted 35-fold with antibody diluent was used as the secondary antibody.
Analysis of mRNA Expression of Coagulation- and Inflammation-Related Factors in Monocytes/Macrophages from the Peritoneal Cavity of Mice Administered SC or SCLMale ICR mice (five mice per group) were orally administered a single dose of 100 µL of SC (containing 2.5 mg SC) or SCL (containing 25, 250, or 2.5 mg SC). Forty-eight hours later, total RNA from monocytes/macrophages collected from mouse peritoneal lavage fluid was extracted using the mini spin column method (RNeasy Mini Kit; Qiagen, Hilden, Germany), and cDNA was prepared using an Omniscript Reverse Transcription Kit (Qiagen). Changes in the mRNA expression levels of genes encoding mouse tissue factor (TF), tumor necrosis factor (TNF)-α, plasminogen activator inhibitor (PAI)-1, interleukin (IL)-1β, and C-X-C motif chemokine ligand 15 (CXCL15: mouse homolog of human IL-8) were determined by PCR using the primers shown in Table 1. PCR was carried out using 35 cycles that comprised the following steps: 94°C for 30 s for denaturation, 59°C for 30 s for annealing, and 72°C for 60 s for extension. The amplified products were separated by agarose gel electrophoresis in the presence of ethidium bromide, and the electrophoretic images were semi-quantifiably analyzed using image analysis software (Bio-Rad Laboratories, Hercules, CA, U.S.A.).
| Forward primer | Reverse primer | |
|---|---|---|
| F3 | 5′-CAAGTGCTTCTCGACCACAGA-3′ | 5′-GTGCACACTGTACTGCTTCCTG-3′ |
| Tnfa | 5′-GCCTCTTCTCATTCCTGCTTG-3′ | 5′-CAGATTGACCTCAGCGCTG-3′ |
| Pai1 | 5′-CGTCTCTGTGCCCATGATG-3′ | 5′-TCGTTTACCTCGATCCTGACC-3′ |
| Il1b | 5′-TTCAAATCTCGCAGCAGCA-3′ | 5′-TCCACACTCTCCAGCTGCA-3′ |
| Cxcl15 | 5′-CGTCCCTGTGACACTCAAGAG-3′ | 5′-TCACTGCCTGTCAAGCTGACT-3′ |
F3, tissue factor; Tnfα, tumor necrosis factor-α; Pai1, plasminogen activator inhibitor-1; Il1b, interleukin-1β; Cxcl15, C-X-C motif chemokine ligand 15.
By using mononuclear cell-separating agent (human lymphocyte separating agent; FUJIFILM Wako Pure Chemical Corporation) and the centrifugation specific gravity method, mononuclear cells were obtained from heparinized whole blood collected from a healthy male’s brachial vein. The obtained mononuclear cells were washed with PBS, suspended in monocyte/macrophage exclusive serum-free medium (Gibco Macrophage-SFM; Thermo Fisher Scientific, Waltham, MA, U.S.A.), and cultured in a 6-well microplate (Becton Dickinson, Franklin Lakes, NJ, U.S.A.). After a 1-h exposure to 95% air and 5% CO2 at 37°C, floating cells were removed, washed with culture medium, and used for culture experiments (5 × 105 /well). Nonspecific esterase staining was used to confirm that monocytes made up at least 95% of the culture well-adherent cells.
Effects of SC, SCL, dSC, or dSCL on Expression of Genes Encoding Coagulation- and Inflammation-Related Factors in Human Monocytes Cultured in VitroSC or SCL (0, 0.2, 2.0, or 20 µg/mL) was added to peripheral blood monocyte culture and incubated for 7 d. Natural mineral water and the supernatant from L suspension (containing 2 × 107 bacteria) were used as controls. Cultured cells were lysed in a cell lysis preservation solution (buffer RLT; Qiagen) and stored frozen until use. Total RNA extracted from these cultured monocytes was used to prepare cDNA as described above. Expression levels of mRNAs of human coagulation- and inflammation-related factor genes encoding TF, TNF-α, PAI-1, monocyte chemotactic protein (MCP)-1, IL-1β, IL-8, IL-l2β, and IL-17 were determined by PCR using the primers shown in Table 2, and the amplified products were analyzed semi-quantitatively as described above.
| Forward primer | Reverse primer | |
|---|---|---|
| F3 | 5′-AGGCACTACAAATACTGTGGCAG-3′ | 5′-TGCAGTAGCTCCAACAGTGCTTCC-3′ |
| TNFA | 5′-AGCACTGAAAGCATGATCCG-3′ | 5′-GAAGACCCCTCCCAGATAGATG-3′ |
| SERPINE1 | 5′-CTTCATGCCCCACTTCTTCAG-3′ | 5′-TTCACTTTCTGCAGCGCCT-3′ |
| MCP1 | 5′-AAGTCTCTGCCGCCCTTCT-3′ | 5′-GAATCCTGAACCCACTTCTGC-3′ |
| IL1B | 5′-AGCTCGCCAGTGAAATGATG-3′ | 5′-TCACCAAGCTTTTTTGCTGTG-3′ |
| IL8 | 5′-GATTGAGAGTGGACCACACTGC-3′ | 5′-GTATTGCATCTGGCAACCCTAC-3′ |
| IL12B | 5′-ATTCGCTCCTGCTGCTTCA-3′ | 5′-CCCATTCGCT CCAAGATGA-3′ |
| IL17 | 5′-TGTCACTGCTACTGCTGCTGAG-3′ | 5′-CACCAGTATCTTCTCCAGCCG-3′ |
F3, tissue factor; TNFA, tumor necrosis factor-α; SERPINE1, plasminogen activator inhibitor-1; MCP1; monocyte chemotactic protein-1; IL1B, interleukin-1β; IL8, interleukin-8; IL12B, interleukin-12β; IL17, interleukin-17.
The effect of dSC or dSCL on the expression of mRNA encoding TF in monocytes was investigated as follows: Monocytes were cultured in the presence of 70 µg/mL SC, SCL, dSC, or dSCL for 48 h, and total RNA was extracted from the monocytes to prepare cDNA. The expression levels of F3 mRNA were then determined as described above.
Effect of SC, SCL, or dSCL on Phagocytosis of Human MonocytesHuman peripheral blood monocytes (1 × 106/well) were cultured at 37°C for 48 h in a 6-well microplate in the absence or presence of 70 µg/mL SC, SCL, or dSCL. Then, pH-sensitive fluorescent dye-labeled E. coli (pHrodo™ Red E. coli Bioparticles™ Conjugate for Phagocytosis; Thermo Fisher Scientific) (1 mg/mL) was added to each well and incubated for 1 h according to the manufacturer’s instruction. After washing the cells with culture medium, 4′,6-diamidino-2-phenylindole (DAPI) staining solution (Thermo Fisher Scientific) (0.5 µg/mL) was added to the wells and incubated for 3 min. Then, the cells were washed and fixed according to the manufacturer’s instructions. The monocytes stained with DAPI and the cells that had taken up fluorescent dye-labeled E. coli in the same field of view were observed under fluorescence microscopy and photographed. The E. coli-phagocytic cells were quantified regardless of the difference in fluorescence intensity, and the average value from the analysis of three different microscopic fields was considered to represent phagocytic ability.
Ethical StatementExperiments with mice were conducted in strict accordance with the recommendations of the Suzuka University of Medical Science Animal Experiment Ethics Committee (approval no. 63), which were prepared based on the guidelines from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Surgery was performed under pentobarbital anesthesia and every effort was made to minimize pain. Research using human peripheral blood monocytes and human genes was performed in accordance with the ethical principles for medical research outlined in the Declaration of Helsinki 1964 and subsequent revisions (https://www.wma.net/), and the recommendations of the Suzuka University of Medical Science Clinical Research Ethics Review Committee (approval no. 154) and Human Genome Clinical Research Ethics Review Committee (approval no. 11) .
Statistical AnalysisData were collected from five independent experiments and expressed as mean ± standard deviation. Statistical analyses were performed using ANOVA followed by the Dunnett’s or Tukey’s multiple comparison test (experimental versus control), as appropriate. All statistical analyses were performed at the significance level of α = 0.05 (p < 0.05) or α = 0.01 (p < 0.01).
The concentrations of β(1-3)-glucan in hot water extracts of SC and SCL determined using the Limulus Amebocyte Lysate assay were 0.27 µg/mL and 19.3 µg/mL, respectively. The β(1-3)-glucan concentrations in SC and SCL after β-glucanase treatment were reduced to 0.01 µg/mL and 0.03 µg/mL, respectively, indicating that more than 95% β(1-3)-glucan in SC and SCL was digested by β-glucanase.
Accumulation of Immune-Related Cells in the Jejunum and Spleen of Mice Administered SC or SCLMorphological changes and the accumulation of immune-related cells in the tissues of the jejunum epithelial villi and spleen 48 h following a single oral administration of 100 µL of mineral water, L suspension, SC (containing 2.5 mg SC), or SCL (containing 25 µg, 250 µg, or 2.5 mg SC) were assessed. Histomorphological observations using hematoxylin and eosin staining showed that there were no changes in the tissues of the jejunum epithelial villi and spleen of mice administered SC or SCL when compared to the characteristics of these tissues in mice administered mineral water (Suppl. Figs. 1a, 1b). This result indicates that oral administration of SC or SCL did not impair the spleen and epithelial villi of the jejunum. L suspension treatment also showed no effects on the jejunum and spleen tissue morphology of mice compared to that of mice receiving mineral water (data not shown). Accumulation of immune-related cells (macrophages, neutrophils, and NK cells) in the epithelial villi of the jejunum and spleen was analyzed using cell-specific antibodies for the respective cell types in mice administered mineral water, L suspension, SC, or SCL. Significantly higher numbers (p < 0.01) of macrophages (Fig. 1a, Suppl. Fig. 2a), neutrophils (Fig. 1b, Suppl. Fig. 2b), NK cells (Fig. 1c, Suppl. Fig. 2c), CCR2-expressing cells (Fig. 1d, Suppl. Fig. 2d), and phospho-Syk-expressing cells (Fig. 1e, Suppl. Fig. 2e) were observed in the epithelial villi of the jejunum of mice administered SC or SCL compared to the numbers of these cells in mice administered mineral water. In addition, SCL treatment increased the number of immune-related cells in a dose-dependent manner. Accumulation of immune-related cells, CCR2-expressing cells, and phospho-Syk-expressing cells in the jejunum epithelium was significantly increased (p < 0.01) in the SCL group administered 2.5 mg compared to the respective cell numbers in the SC group administered the same dose.

Significant differences in values between the MW group and SC group, or SC (2.5 mg) group and SCL (2.5 mg) group were determined by the Dunnett’s test and are shown as * p < 0.05 and ** p < 0.01. Significant differences in values between different doses in the SCL treatment group are shown as §p < 0.05 and §§p < 0.01, respectively.
Significantly higher numbers of macrophages (Fig. 2a, Suppl. Fig. 3a), neutrophils (Fig. 2b, Suppl. Fig. 3b), NK cells (Fig. 2c, Suppl. Fig. 3c), CCR2-expressing cells (Fig. 2d, Suppl. Fig. 3d), and phospho-Syk-expressing cells (Fig. 2e, Suppl. Fig. 3e; p < 0.01) were also observed in the spleen of mice administered SC or SCL compared to the numbers of respective cells in mice administered mineral water. These increases were also dose-dependent in the SCL group. There was no significant difference between the SC and SCL groups administered 2.5 mg in the accumulation of immune-related cells in the spleen, although the numbers of CCR2-expressing cells and phospho-Syk expressing cells were observed to be significantly higher (p < 0.05) in the SCL group than in the SC group. Treatment with L suspension did not affect the numbers of immune-related cells, CCR2-expressing cells, and phospho-Syk-expressing cells in the epithelial villi jejunum and spleen of mice compared to those of the respective cells in mice administered mineral water (data not shown).
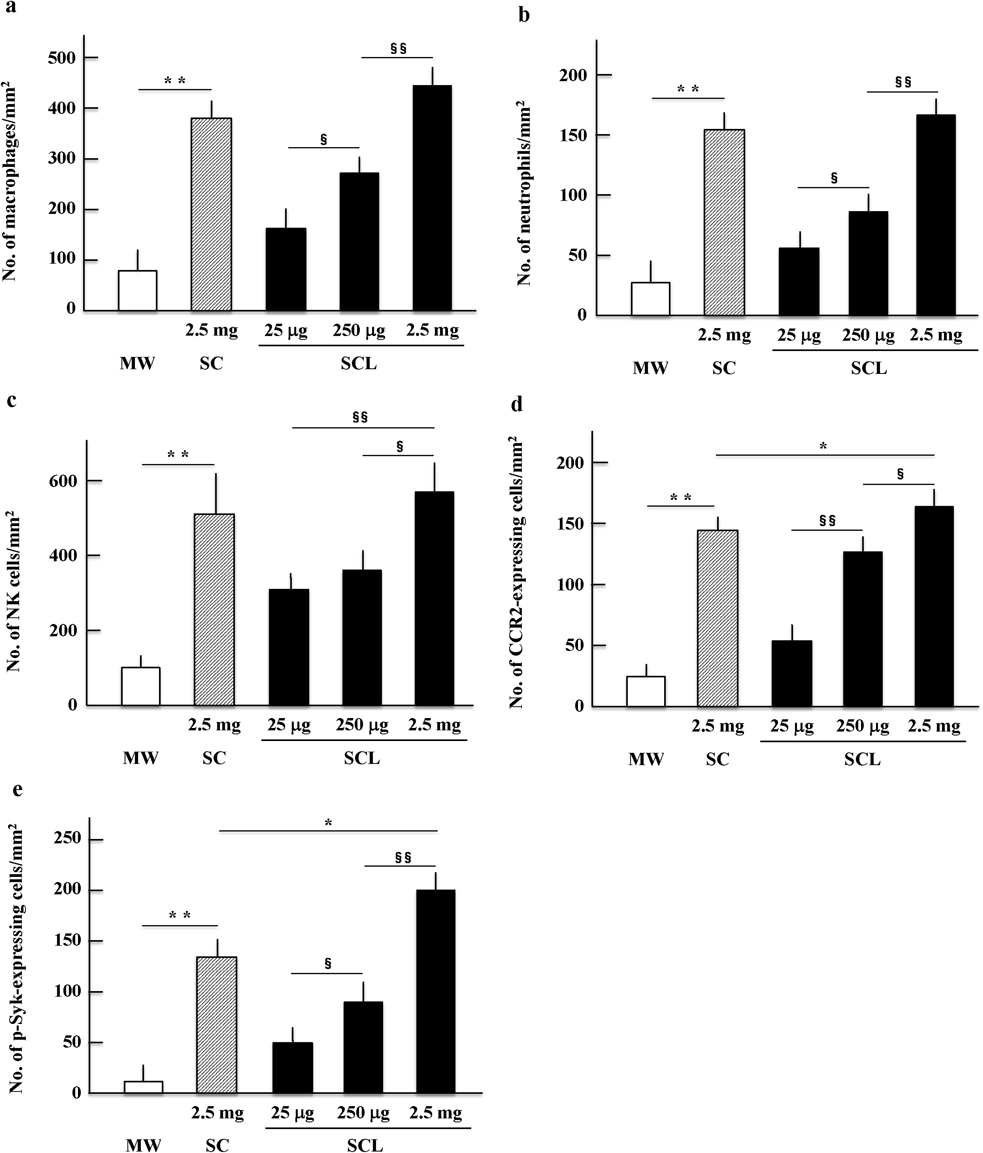
Significant differences in values between the MW group and SC group, or SC (2.5 mg) group and SCL (2.5 mg) group are shown as * p < 0.05 and ** p < 0.01. Significant differences in values between different doses in the SCL group are shown as §p < 0.05 and §§p < 0.01, respectively.
mRNA expression levels of various coagulation- and inflammation-related factors in monocytes/macrophages from the peritoneal cavity of mice at 48 h following a single oral administration of either 100 µL of SC or SCL were analyzed. The highest F3 mRNA expression levels in monocytes/macrophages were observed at doses of 250 µg SC or SCL, whereas at the higher dose (2.5 mg), the effect was lower than at 250 µg (Fig. 3a). In contrast, Tnfα mRNA expression levels were directly proportional to the dose of SCL (Fig. 3b). F3 mRNA expression levels in monocytes/macrophages of mice administered SCL (25 or 250 µg dose) were significantly higher (p < 0.01) than those of mice administered SC. Tnfα mRNA expression levels in monocytes/macrophages of mice administered SCL (250 µg or 2.5 mg dose) were significantly higher (p < 0.05 or p < 0.01) than those of mice administered SC. There was no change in expression levels of Pai1, Il1β, and Cxcl15 mRNAs after treatment with SC or SCL (data not shown).
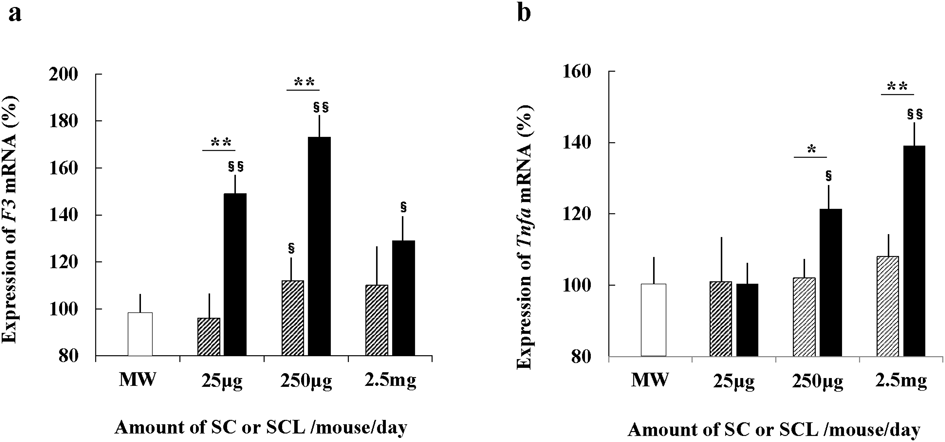
Open, gray, and closed columns indicate the effects of MW, SC, and SCL, respectively The expression level of each factor in macrophages obtained from mice administered MW was considered 100%. Significant differences in values between the MW group and SC or SCL group are shown as §p < 0.05 and §§p < 0.01, respectively. Significant differences in values between the SC group and SCL group are shown as * p < 0.05 and ** p < 0.01.
SC or SCL was added to human peripheral blood monocyte cultures, and morphological changes of monocytes cultured for 48 h were observed. In particular, monocytes with the addition of SCL had cytoplasmic chromatin aggregation, cytoplasmic expansion, and many vacuoles, and morphologically differentiated into macrophage-like cells (data not shown).
Analysis of the effects of SC or SCL on human monocyte mRNA expression levels of coagulation- and inflammation-related factor genes encoding TF, TNF-α, PAI-1, MCP-1, IL-1β, IL-8, IL-12β, and IL-17 revealed that all these factors were significantly upregulated (p < 0.01) in a dose-dependent manner after 7 d of treatment with SCL compared to those in the absence of SCL (Figs. 4a–h). mRNA levels of genes encoding TF, TNF-α, PAI-1, MCP-1, IL-1β, IL-12β, and IL-17 were significantly upregulated (p < 0.05 or p < 0.01) in a dose-dependent manner after treatment with SC compared to those in the absence of SC. Additionally, the expression levels of all these factors were significantly higher in the SCL group than in the SC group (p < 0.05 or p < 0.01). These data indicate that the stimulatory effect of SCL on the expression of various coagulation- and inflammation-related factors by human monocytes was significantly higher than that of SC treatment. The addition of the supernatant of L suspension alone did not affect expression levels of coagulation- and inflammation-related factor encoding genes in cultured monocytes compared to the effect of control mineral water (data not shown).
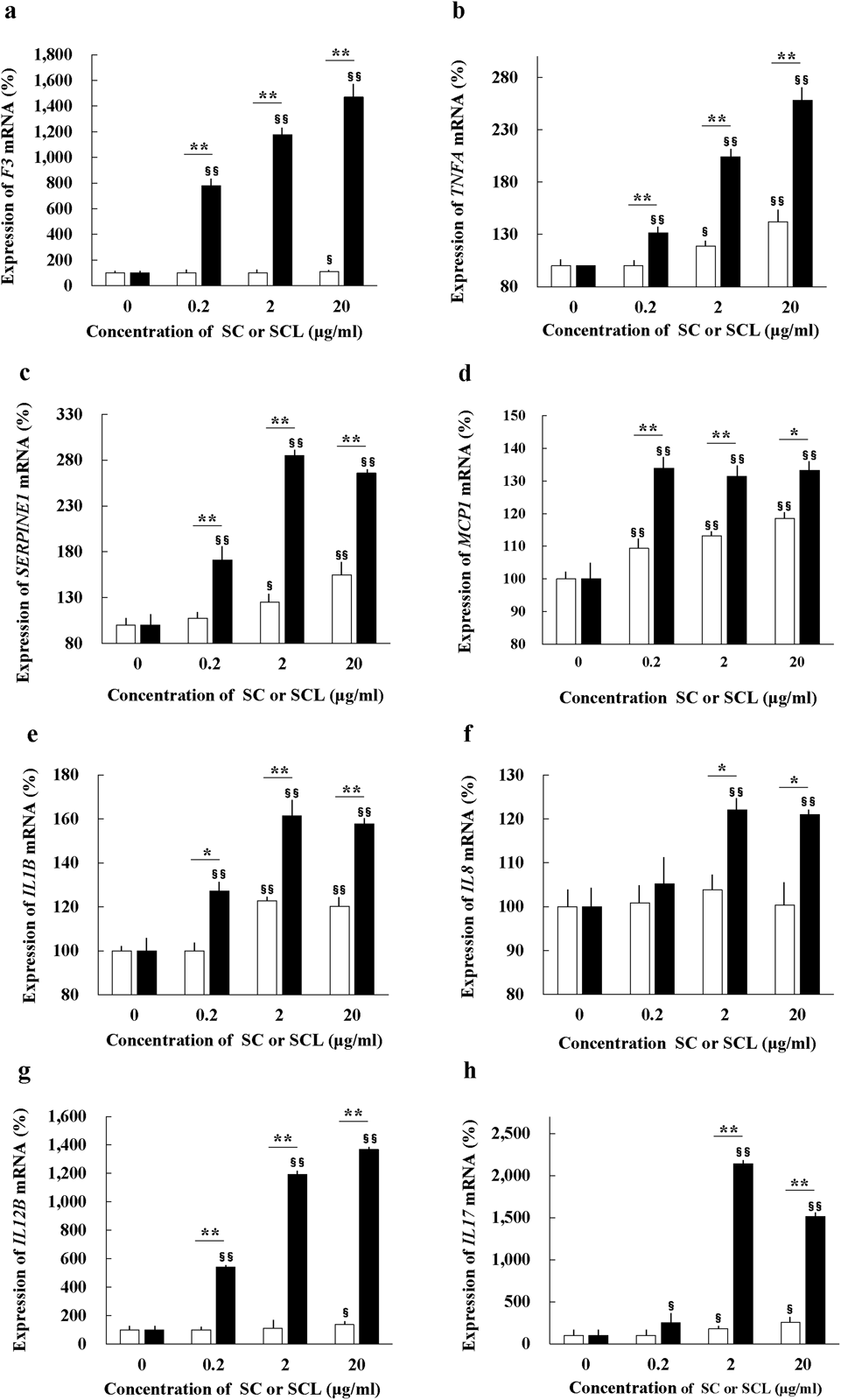
(a) TF, (b) TNF-α, (c) PAI-1, (d) MCP-1, (e) IL-1β, (f) IL-8, (g) IL-12β, (h) IL-17. Open columns and closed columns indicate the effects of SC and SCL, respectively. The expression level of each factor when SC or SCL was not present was considered 100%. Significant differences in values between different doses in the SC or SCL group are shown as §p < 0.05 and §§p < 0.01, respectively. Significant differences in values between the SC group and SCL group are shown as * p < 0.05 and ** p < 0.01.
To confirm the innate immunity-enhancing effects of SC and SCL, the phagocytic activity of monocytes stimulated with SC or SCL was examined using pH-sensitive fluorescent dye-labeled E. coli. As shown in Figs. 5a and 5b, in the control group (without SC and SCL), an average of 5.5% of DAPI-stained cells ingested E. coli, and an average of 23% of DAPI-stained cells showed E. coli uptake in the SC group. In the SCL group, almost all DAPI-stained cells showed E. coli uptake. The data show that both SC and SCL significantly (p < 0.01) enhanced monocyte phagocytosis compared to the control, and that the enhancing effect of SCL on monocyte phagocytosis was significantly greater than that of SC (p < 0.01).
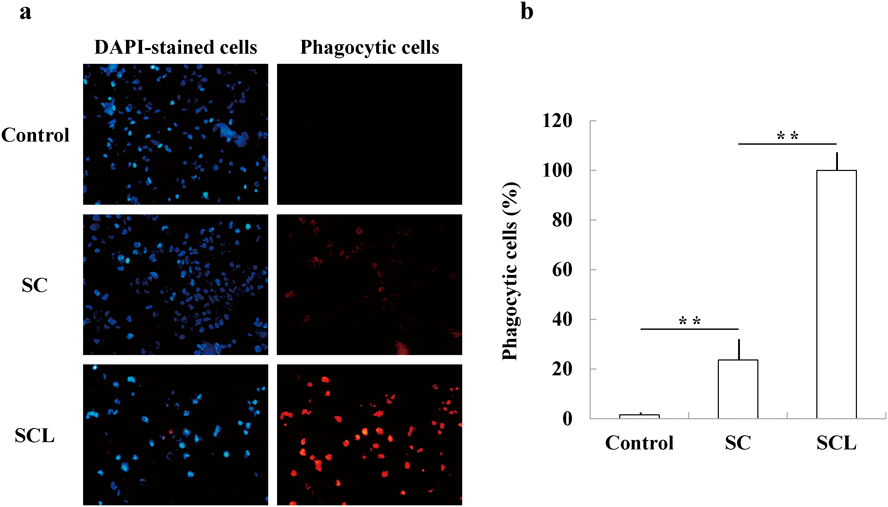
(a) Photographs (×400) of DAPI-stained cells and phagocytic cell uptake of pH-sensitive fluorescent dye-labeled E. coli in the same field. (b) Phagocytic cells (%) that took up E. coli in the control, SC, or SCL group. Significant differences in values between the control and SC groups and between the SC and SCL groups are shown as ** p < 0.01.
To clarify how β(1-3)-glucan in SC and SCL is involved in enhancing coagulation-related factor gene expression and phagocytosis of human monocytes, the effect of dSC and dSCL on monocyte F3 mRNA expression and E. coli phagocytosis was determined. As described above, β(1-3)-glucan concentrations in dSC and dSCL samples were less than 5% of those in SC and SCL. Figure 6a shows that the stimulatory effect of dSC and dSCL on F3 mRNA expression in monocytes was significantly (p < 0.01) reduced to approximately 30% and 35%, respectively, compared to the effect of SC and SCL. Figures 6b and 6c further show that the effect of dSCL on E. coli monocyte phagocytosis was significantly (p < 0.01) reduced to approximately 30% compared to the effect of SCL.

(a) The effect of dSC compared to that of SC and the effect of dSCL compared to that of SCL on F3 mRNA expression (%) in monocytes. F3 mRNA expression level in the presence of SC or SCL is tentatively shown as 100%. Significant differences in values between the SC and dSC groups and between the SCL and dSCL groups are shown as ** p < 0.01. (b) Photographs (×400) demonstrating the effects of SCL and dSCL on monocyte phagocytosis of fluorescent dye-labeled E. coli. (c) The relative effect of SCL and dSCL on monocyte phagocytosis, determined from the data shown in (b). The amount of phagocytic cells in the presence of SCL is tentatively shown as 100%. Significant differences in values between the SCL group and dSCL group are shown as ** p < 0.01.
In general, β-glucans include some of the most abundant forms of polysaccharides found inside the cell walls of both bacteria and fungi. All β-glucans are glucose polymers linked by a 1-3 linear β-glycosidic chain core that differ in length and branching structure. The branches from the core of the β-glucan glycoside chain are very diverse, but the two main groups are composed of 1-4 and 1-6 glycoside chains. These branching assignments appear to be species-specific. For example, β-glucans of fungi have 1-6 side branches.22) It is known that β(1-3)- and β(1-6)-glucans of fungi specifically stimulate the innate immune system. The binding of these β-glucans to the receptor initiates and regulates the subsequent innate immune response.23,24) Therefore, ingestion of SC (Hanabiratake) with high β(1-3)-glucan content may stimulate the innate immune system similarly to fungal β-glucans.
Since ancient times, plant-based lactic acid bacteria have been used to produce various fermented foods such as pickles and other products containing lactic acid. Thus, lactic acid bacteria are known to be important for gut microbiota and health maintenance. Accordingly, we hypothesized that SCL, which is SC fermented with plant-based lactic acid bacteria, would possess the beneficial effects of both SC and lactic acid bacteria.
In the present study, we examined the effects of SC and SCL on the innate immune system of mice in vivo, and on human peripheral blood monocytes in vitro. We first determined the numbers of monocytes/macrophages, neutrophils, and NK cells accumulated in the epithelial villi of the jejunum (which is involved in intestinal innate immunity) and spleen (which reflects the body’s immune function) of mice orally administered SC, SCL, mineral water, or L suspension. The expression levels of a chemokine receptor and a hematopoietic cell signaling factor were also examined in these organs. Analysis of immune-related cells in the jejunum and spleen revealed a significant increase in the number of macrophages, neutrophils, NK cells, CCR2-expressing cells, and phospho-Syk-expressing cells in the jejunum epithelial villi and spleen of mice administered SC or SCL compared to observations in mice administered mineral water or L suspension. Furthermore, the numbers of macrophages, neutrophils, NK cells, CCR2-expressing cells, and phospho-Syk-expressing cells in the epithelium of the jejunum were significantly higher in the SCL group administered 2.5 mg than in the SC group administered the same dose. Similarly, in the spleen, the accumulation of CCR2-expressing cells and phospho-Syk-expressing cells was also significantly higher in the 2.5 mg SCL group than in the 2.5 mg SC group. In addition, we examined changes in the expression of various factor genes in monocytes/macrophages obtained from the peritoneal lavage fluid of mice administered SC or SCL orally. The data suggested that oral administration of SCL significantly enhanced the innate immunity in both the abdominal cavity and blood through dose-dependent increases in coagulation- and inflammation-related factor mRNAs F3 and Tnfα in the monocytes/macrophages of the mouse peritoneal cavity.
We next determined the direct effect of SC or SCL on the expression of genes of coagulation- and inflammation-related factors and those of innate immunity-related cytokines in cultured human monocytes, and compared it with that of mineral water or supernatant of L suspension. The data indicated a dose-dependent increase in the mRNA expression levels of innate immunity-related factors of human monocytes/macrophages in vitro following incubation with SC or SCL, but not with the supernatant of L suspension. Additionally, expression levels of all these factors were markedly higher in the SCL treatment group than in the SC treatment group.
To understand why the effects of SC solution and SCL suspension on the innate immunity stimulating activity differed although the same amount of dried SC was used to prepare the SC and SCL samples, we determined the concentration of β(1-3)-glucan in hot water extracts of SC and SCL and found that the concentration of β(1-3)-glucan in SCL suspension was about 70-fold higher than that in SC solution. A previous report indicated that β(1-3)-glucan promotes the proliferation of lactic acid bacteria,25) suggesting that the increased lactic acid bacteria in the presence of β(1-3)-glucan may enhance the solubilization of β(1-3)-glucan and other components, such as α-mannan and lipopolysaccharide, from the cells of SC. Therefore, the higher concentrations of solubilized β(1-3)-glucan and other components in the SCL sample than in the SC sample may have caused the strong expression of innate immunity-related genes and activation of the innate immunity.
In addition, we performed experiments using pH-sensitive fluorescent dye-labeled E. coli for monocyte phagocytosis to determine whether the strong effect of SCL on the expression of innate immunity-related genes in human monocytes actually leads to enhanced monocyte phagocytosis. The results clearly showed that SCL significantly enhances monocyte phagocytosis over SC. Furthermore, the effects of β-glucanase-digested SC and SCL, in which 95% or more of β(1-3)-glucan was lost, on F3 mRNA expression and monocyte phagocytosis were evaluated in comparison with SC and SCL. The data suggested that 65–70% of the enhancing effects of SC and SCL on monocyte coagulation-related mRNA expression and phagocytosis was dependent on β(1-3)-glucan, as shown in Fig. 6.
β(1-3)-Glucans are present in various organisms, such as yeast, fungi (including mushrooms), seaweed, and barley. In these organisms, β(1-3)-glucans enhance innate immunity mediated by the receptor dectin-1 (CLEC7A), a type II transmembrane protein found in several types of immune-related cells, including macrophages, neutrophils, and NK cells.22-24) The SCL suspension used in this study contained high concentrations of β(1-3)-glucans and other immune-activating substances, such as α-mannan and lipopolysaccharide.26) β(1-3)-glucans, α-mannan, and lipopolysaccharide (LPS) have been shown to bind to dectin-1 (CLEC7A), dectin-2 (CLEC6A),27) and Toll-like receptors (TLRs),28) respectively, on the epithelial cell surface of the jejunum, stimulating the synthesis of several cytokines. The present study suggested that among the potentiating effects of SC and SCL, 65–70% of the effects depended on β(1-3)-glucan via dectin-1, and 30–35% of the effects depended on other factors, such as α-mannan and LPS, via dectin-2 and TLRs, respectively.
Oral administration of soluble β-glucan preparation from SC was reported to modulate cytokine production in mice.7) Although it is still unclear how mushrooms containing β(1-3)-glucans and other immune-activating substances induce immune cells in the epithelium of the jejunum and in the spleen, the results of the present study indicate that ingestion of SCL may be effective for the prevention of infectious diseases. Furthermore, because mushroom consumption has been reported to be effective against malignant tumors,29) we are currently studying the efficacy of SCL against colorectal cancer and melanoma using a tumor-bearing mouse model.
In conclusion, we have shown that orally administered β(1-3)-glucan-rich SC fermented with lactic acid bacteria (SCL) significantly increases the accumulation of macrophages, neutrophils, and NK cells in the jejunum epithelial villi and spleen of mice. This SCL was also effective in inducing the expression of various coagulation- and inflammation-related molecules in mouse peritoneal monocytes/macrophages. In addition, SCL directly and significantly increased innate immunity-related gene expression and phagocytosis in cultured human monocytes. These findings suggest that oral administration of SCL significantly enhances the innate immune response of the mouse and possibly human small intestine and circulating blood. Thus, SCL administration may contribute to combating various diseases that affect the innate immune response.
We are grateful to Dr. Masaru Shibata (Ohken Co., Ltd., Suzuka, Mie, Japan) who introduced Hanabiratake mushroom as a functional food and discussed the design of the study. We also thank Mr. Kazushi Hiya (Asuka Co., Ltd., and Asuka Health Science Foundation, Iidaka-cho, Matsuzaka, Mie, Japan) who promoted this study. The study was supported in part by Grants-in-Aid for Scientific Research and Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS; no. 16K08633) and Asuka Health Science Foundation.
Authors received samples of SC and SCL, and a contribution to the research expenses in part from Asuka Health Science Foundation.
The online version of this article contains supplementary materials.