2020 年 43 巻 4 号 p. 682-687
2020 年 43 巻 4 号 p. 682-687
We previously showed that adhesive aggregates were formed when levofloxacin hydrate tablets and lansoprazole orally disintegrating (OD) tablets were suspended in water in the clinical context. In this study, we have clarified the factors causing aggregate formation, focusing on the role of pharmaceutical additives and electrostatic interaction. Co-suspension of enteric-coated proton pump inhibitor (PPI) esomeprazole magnesium hydrate with levofloxacin resulted in aggregate formation, whereas the non-enteric-coated PPI vonoprazan fumarate did not. A comparison of pharmaceutical additive in the two PPIs highlighted polysorbate 80 and methacrylic acid copolymer LD as candidates causing aggregation. When these pharmaceutical additives were added to levofloxacin, only methacrylic acid copolymer LD induced aggregate formation. Since levofloxacin is zwitterionic, we examined another zwitterionic ingredient, ampicillin sodium, and found that it also formed aggregates with methacrylic acid copolymer LD, while benzylpenicillin sodium, which is not zwitterionic, did not form aggregates. When we next examined a series of zwitterionic quinolone antimicrobial drugs, we found that ofloxacin, which is highly soluble, formed aggregates with lansoprazole OD tablets, whereas poorly soluble quinolone antimicrobial drugs did not form aggregates. Further, although cefepime hydrochloride and cephalexin did not form aggregates with methacrylic acid copolymer LD in tap water, aggregates were formed when a suspension of cefepime hydrochloride or cephalexin with methacrylic acid copolymer LD was adjusted to pH 7.0. Our results indicate that electrostatic interaction between zwitterionic ingredients and methacrylic acid copolymer LD can result in aggregate formation under conditions where the drug and methacrylic acid copolymer LD are both sufficiently soluble.
Enteral nutrition tubes are used to administer drugs to patients who cannot take them orally. For enteral administration, tablets are generally crushed or powdered, then suspended in water, or are suspended in hot water (55°C) without crushing.1–3) However, it is often not clear whether administration of multiple drugs in this way may lead to blockage of the nutritional tube or decreased activity of active ingredient (s), and in addition, other formulated components may cause problems. For example, dosage forms incorporating macrogol 6000 as a lubricant are coagulated by hot water, and drugs containing hydroxypropyl methylcellulose do not readily disintegrate in salt water.4) In addition, change of pH by concomitantly administered drugs or chelation of the active ingredient by metal ions can reduce of the bioavailability of the active ingredient.5–11)
In our hospital, we have found that adhesive aggregates are formed when crushed levofloxacin hydrate tablets and lansoprazole orally disintegrating (OD) tablets are suspended simultaneously in tap water at room temperature, although we did not examine the mechanism in detail.12) This issue is important, because blockages could require the insertion of a new gastrostomy tube or nasotracheal tube, or make it difficult to administer precise dosages of active ingredient. It is well established that drugs can interact electrostatically with pharmaceutical additives,13,14) but there has been no report that such interaction during simultaneous suspension could impact on clinical practice. Because levofloxacin exists as a zwitterion in aqueous solution, we considered that its aggregation might involve electrostatic interaction. Therefore, we examined in detail the mechanism of aggregate formation of levofloxacin and lansoprazole OD tablets, focusing on pharmaceutical additives and electrostatic interaction. We identified methacrylic acid copolymer LD as a suspect component, and examined its aggregate formation with other zwitterionic drugs, in addition to levofloxacin.
Pharmaceuticals were obtained from the following sources: levofloxacin hydrate (Cravit®) tablet 500 mg (Daiichi Sankyo Co., Ltd., Tokyo, Japan), lansoprazole (Takepron®) OD tablet 15 mg (Takeda Co., Ltd., Osaka, Japan), lansoprazole capsule 15 mg (Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan), esomeprazole magnesium hydrate (Nexium®) capsule 10 mg (AstraZeneca Co., Ltd., Osaka, Japan), vonoprazan fumarate (Takecab®) tablet 10 mg (Takeda Co., Ltd.), tegafur uracil (UFT E®) combination granule T 100 mg (Taiyo Pharmaceutical Co., Ltd., Tokyo, Japan), tamsulosin hydrochloride OD tablet 0.2 mg (Sawai Pharmaceutical Co., Ltd., Osaka, Japan), sitafloxacin hydrate (Gracevit®) tablet (Daiichi Sankyo Co., Ltd.), ciprofloxacin hydrochloride hydrate tablet 20 mg (Nichi-Iko Pharmaceutical Co., Ltd.), garenoxacin mesylate hydrate (Geninax®) tablet 200 mg (Astellas Pharma Inc., Tokyo, Japan), tosufloxacin tosilate hydrate tablet 150 mg (Sawai Pharmaceutical Co., Ltd.), cefalexin capsule 250 mg (Towa Pharmaceutical Co., Ltd., Osaka, Japan), meropenem hydrate for i.v. infusion 0.5 g (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) and cefepime dihydrochloride for intravenous 1 g (Sandoz Novartis, Tokyo, Japan).
In addition, chemicals and reagents were obtained from the following sources: levofloxacin (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), lansoprazole (Wako Pure Chemical Corporation, Osaka, Japan), polysorbate 80 (Wako Pure Chemical Corporation), benzylpenicillin sodium (Wako Pure Chemical Corporation), ampicillin sodium (Wako Pure Chemical Corporation), and ofloxacin (Wako Pure Chemical Corporation). Methacrylic acid copolymer LD was kindly provided by Sanyo Chemical Industries, Ltd. (Kyoto, Japan). All other reagents were of special grade.
Aggregate Formation in Co-suspensions of Levofloxacin Hydrate Tablets and Proton Pump Inhibitors (PPIs)One levofloxacin hydrate tablet 500 mg was crushed in a mortar and suspended in 30 mL of tap water at room temperature. After 5 min, one lansoprazole OD tablet, one decapsulated generic lansoprazole capsule, one decapsulated esomeprazole magnesium hydrate capsule, or one crushed vonoplazane fumarate tablet was added to the levofloxacin suspension and mixed. After 5, 15, and 30 min, the presence or absence of aggregation and the shapes of aggregates were observed. All experiments were carried out at room temperature (25°C).
Aggregate Formation in Co-suspensions of Levofloxacin and Pharmaceutical Additives Contained in the PPI FormulationsTable 1 lists all the pharmaceutical additives described in the interview forms of levofloxacin hydrate tablets (Daiichi Sankyo Co., Ltd., February, 2014 revision, 11th edition), original lansoprazole OD tablets (Takeda Co., Ltd., August, 2015 revision, 16th edition), generic lansoprazole capsules (Nichi-Iko Pharmaceutical Co., Ltd., January, 2017 revision, 16th edition), esomeprazole magnesium hydrate capsules (Astellas Pharma Inc., February, 2016 revision, 10th edition) and vonoprazane fumarate tablets (Takeda Co., Ltd., March, 2016 revision, 7th edition). Polysorbate 80 and methacrylic acid copolymer LD were included in the original lansoprazole OD tablets, generic lansoprazole capsules and esomeprazole magnesium hydrate capsules, which formed aggregates with levofloxacin, but were not included in vonoprazane fumarate tablets, which did not form aggregates with levofloxacin. Therefore, we next added 100 mg of methacrylic acid copolymer LD, 2.3 mg of polysorbate 80, or 15 mg of lansoprazole to 500 mg of levofloxacin suspension in the same manner as before. The amounts used corresponded to those contained in one tablet of original lansoprazole OD.15)
| Lansoprazole OD tablet 15 mg (original)a) | Lansoprazole capsule 15 mg (generic)b) | Esomeprazole magnesium capsule 20 mgc) | Vonoprazan fumarate tablet 10 mgd) | Levofloxacin hydrate tablet 500 mge) |
|---|---|---|---|---|
| Polysorbate80 | Polysorbate80 | Polysorbate80 | Croscarmellose sodium | Carmellose |
| Methacrylate acid copolymer LD | Methacrylate acid copolymer LD | Methacrylate acid copolymer LD | Crystal cellulose | Carnauba wax |
| Anhydrous citric acid | Corn starch | Citric acid triethyl | D-Mannitol | Crystal cellulose |
| Aspartame | D-Mannitol | Hydroxypropyl cellulose | Fumaric acid | Hydroxypropyl cellulose |
| Citric acid triethyl | Hypromellose | Hypromellose | Hydroxypropyl cellulose | Hypromellose |
| Crospovidone | Macrogol6000 | Monostearic acid glycerin | Hypromellose | Macrogol 6000 |
| Crystal cellulose | Meglumine | Sucrose starch spherical granule | Macrogol 6000 | Sodium stearyl fumarate |
| D-Mannitol | Sucrose | Stearic acid magnesium | Stearic acid magnesium | Talc |
| Hydroxypropyl cellulose | Sodium lauryl sulfate | Talc | Titanium oxide | Titanium oxide |
| Hypromellose | Talc | Yellow iron sesquioxide | Yellow iron sesquioxide | |
| Iron sesquioxide | Titanium oxide | |||
| Twelve other additives |
a) Interview form of original lansoprazole OD tablets (Takeda Co., Ltd., August, 2015 revision, 16th edition), b) interview form of generic lansoprazole capsules (Nichi-Iko Pharmaceutical Co., Ltd., January, 2017 revision, 16th edition), c) interview form of esomeprazole magnesium hydrate capsules (Astellas Pharma Inc., February, 2016 revision, 10th edition), d) interview form of vonoprazane fumarate tablets (Takeda Co., Ltd., March, 2016 revision, 7th edition), e) interview form of levofloxacin hydrate tablets (Daiichi Sankyo Co., Ltd., February, 2014 revision, 11th edition).
Five hundred milligram of levofloxacin was suspended in 30 mL of tap water. After 5 min, a tamsulosin hydrochloride OD tablet or 1 package of tegafur uracil combination granules was added and the mixture was observed.
Aggregate Formation between Various Drugs and Methacrylate Acid Copolymer LDAmpicillin Sodium and Benzylpenicillin SodiumTwo hundred and fifty mg of benzylpenicillin sodium (a non-zwitterionic drug) or 250 mg ampicillin sodium (a zwitterionic drug) was dissolved in 15 mL Tris hydrochloric acid buffer solution (pH 7.0). After 5 min, 50 mg methacrylate acid copolymer LD was added, and the mixture was observed.
Zwitterionic Quinolone Antibacterial DrugsOne sitafloxacin hydrate tablet 50 mg, ciprofloxacin hydrochloride hydrate tablet 200 mg, garenoxacin mesilate hydrate tablet 200 mg, tosufloxacin tosilate hydrate tablet 150 mg or 100 mg of ofloxacin, each representing the single-dose amount for adults, was suspended in tap water, and adjusted the pH to 7.0 with 1 M NaOH, then 1 tablet of original lansoprazole OD was added, and the mixture was observed.
Zwitterionic Beta-Lactam Derivative Antibacterial DrugsFive hundred milligram of meropenem hydrate, 250 mg of cephalexin capsule, or 500 mg of cefepime hydrochloride was suspended in super-pure water, then 50 mg of methacrylic acid copolymer LD was added, and the suspension was observed for 30 min. In addition, 1 M NaOH was added to cephalexin capsule suspension and cefepime hydrate solution to adjust the pH to 7.0, and then 50 mg of methacrylic acid copolymer LD was added.
When an original OD tablet or generic capsule of lansoprazole was dissolved in a suspension of a levofloxacin hydrate tablet, aggregates were formed, as observed in the previous study. When an esomeprazole magnesium hydrate capsule was added to a suspension of levofloxacin, adhesive aggregates were also observed, and did not disaggregate even upon thorough mixing (Fig. 1A). On the other hand, no aggregates were formed when a vonoprazane fumarate tablet was added to a suspension of levofloxacin.
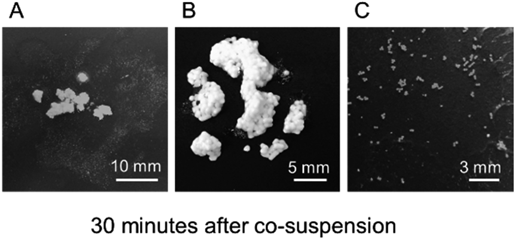
Aggregate formation was observed 30 min after adding (A) esomeprazole magnesium hydrate capsules, (B) tegafur uracil combination granules or (C) tamsulosin hydrochloride OD tablets to a levofloxacin hydrate tablet suspension.
Although a co-suspension of levofloxacin and polysorbate 80 did not form aggregates even after 30 min, a co-suspension of methacrylic acid copolymer LD and levofloxacin aggregated immediately and the aggregates were stable for at least 30 min. No aggregates were formed in a co-suspension of lansoprazole and levofloxacin.
Aggregate Formation in Co-suspensions of Levofloxacin and Drugs Whose Formulations Include Methacrylic Acid Copolymer LDCo-suspensions of levofloxacin with tegafur uracil combination granules or tamsulosin hydrochloride OD tablets, which contain methacrylic acid copolymer LD, formed aggregates within 10 min, and these were stable for at least 30 min (Figs. 1B, C).
Aggregate Formation with Zwitterionic Drugs and Methacrylate Acid Copolymer LDAmpicillin Sodium and Benzylpenicillin SodiumA suspension of ampicillin sodium (a zwitterion) with methacrylic acid copolymer LD in Tris hydrochloric acid buffer solution (pH 7.0) formed aggregates within 5 min, while benzylpenicillin sodium (a non zwitterion) formed no aggregates even after 30 min (Fig. 2).
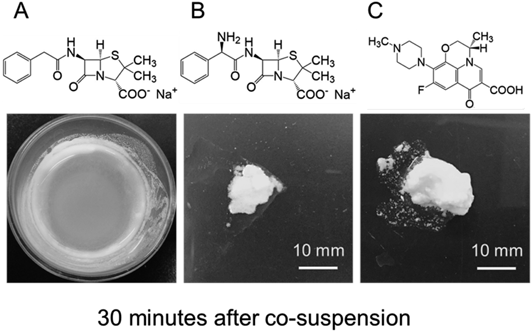
Aggregate formation was observed 30 min after adding methacrylic acid copolymer LD to (A) benzylpenicillin sodium solution, (B) ampicillin sodium solution or (C) levofloxacin solution.
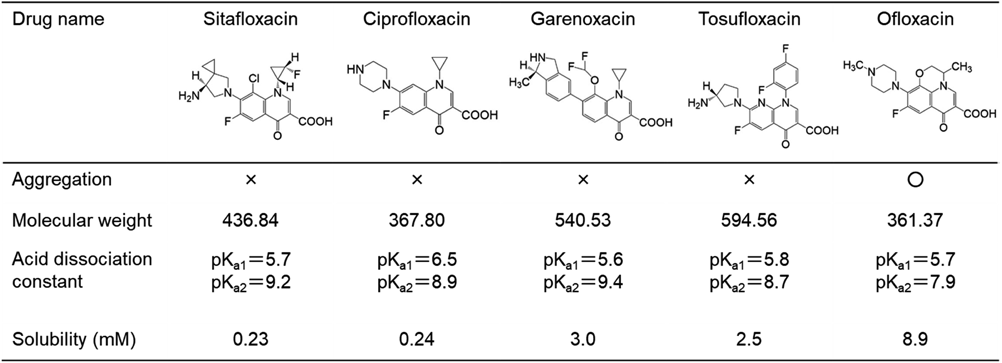
Next, we examined suspensions of lansoprazole OD tablets, which contain methacrylic acid copolymer LD, with sitafloxacin hydrate, ciprofloxacin hydrochloride hydrate, garenoxacin mesilate hydrate, tosufloxacin tosilate hydrate and ofloxacin. Sitafloxacin hydrate, ciprofloxacin hydrochloride hydrate, garenoxacin mesylate hydrate and tosufloxacin tosilate, which are not soluble in tap water, formed no aggregates within 30 min. On the other hand, ofloxacin, which was completely dissolved in tap water, did form aggregates with lansoprazole OD tablets (Fig. 3).
Aggregate formation was observed 30 min after adding methacrylic acid copolymer LD to a sitafloxacin hydrate tablet suspension, ciprofloxacin hydrochloride tablet suspension, garenoxacin mesylate hydrate tablet suspension, tosufloxacin tosilate hydrate tablet suspension or ofloxacin solution. Solubility values at pH 7.0 were cited from ref. 25).
When meropenem hydrate was dissolved in super-pure water and methacrylic acid copolymer LD was suspended in this solution, aggregates were observed after 5 min. On the other hand, no aggregates were seen with cefepime hydrochloride or cephalexin. However, when the pH of the suspensions of cefepime hydrochloride and cephalexin with methacrylic acid copolymer LD was adjusted to 7.0, aggregation occurred within 5 min (Fig. 4).

Aggregate formation was observed 30 min after adding methacrylic acid copolymer LD to meropenem hydrate solution, cefepime dihydrochloride solution or cefalexin capsule suspension. Results for cefepime and cefalexin capsule are shown before and after pH adjustment (left, before; right, after).
The aim of this work was to establish the mechanism of aggregate formation in a co-suspension of levofloxacin hydrate tablets and lansoprazole OD tablets.12) These experiments were conducted at the temperature of 25°C, because in clinical practice, the crushed tablets are usually suspended in water at room temperature (about 25°C) for administration to patients. First, we found that a co-suspension of an esomeprazole magnesium hydrate capsule (enteric-coated) with a levofloxacin hydrate tablet formed aggregates, whereas a suspension of a vonoprazan fumarate tablet (non-enteric-coated) did not. This suggested that the aggregating factor was a pharmaceutical additive, not the PPIs themselves. Comparison of the pharmaceutical additives in the above drug formulations identified polysorbate 80 and methacrylic acid copolymer LD as candidate aggregators, and further studies with other PPIs showed that methacrylic acid copolymer LD was the responsible factor for aggregation in the lansoprazole OD tablet. Methacrylic acid copolymer LD is widely used for enteric coating or sustained release coating.15,16) To confirm the role of methacrylic acid copolymer LD, we examined other drugs. Aggregates were formed in co-suspensions of levofloxacin with tegafur uracil combination granules and tamsulosin hydrochloride OD tablets, which also contain methacrylic acid copolymer LD. However, crushed aspirin enteric-coated tablets, which contain methacrylic acid copolymer LD as enteric firm coating, did not form aggregates with levofloxacin (date not shown). This result may mean that the amount of methacrylic acid copolymer LD or the coating method is important for aggregate formation.
Some polymer pharmaceutical additives are reported to interact electrostatically with other compounds.17–22) Salamanca et al. reported that ampicillin trihydrate, a zwitterionic ingredient, formed a complex with amino-alkyl methacrylate, another polymer pharmaceutical additive, via electrostatic interaction.23) Since levofloxacin has two pKs (pKa1 6.11 and pKa2 8.18), it would form a zwitterion under neutral conditions,24) so we considered that levofloxacin and methacrylic acid copolymer LD might aggregate via electrostatic interaction. In this study, we confirmed that ampicillin sodium formed aggregates with methacrylic acid copolymer LD, whereas benzylpenicillin sodium, which cannot form a zwitterion since it lacks the amino group of ampicillin sodium, did not. Furthermore, in order to clarify the involvement of zwitterionic character in the mechanism of aggregate formation, we examined other zwitterionic drugs, sitafloxacin hydrochloride hydrate, ciprofloxacin hydrochloride hydrate, garenoxacin mesilate hydrate, tosufloxacin tosilate hydrate and ofloxacin, and found that among them, only ofloxacin formed aggregates. However, the non-aggregate-forming drugs are all poorly soluble: sitafloxacin hydrate 0.23 mM in water, ciprofloxacin hydrochloride hydrate 0.24 mM in 0.05 M phosphate buffer (pH 7.0), garenoxacin mesilate hydrate 3.0 mM in water, tosufloxacin tosilate hydrate 2.5 mM in Mcllvaine buffer (pH 7.0), as compared with ofloxacin, 8.9 mM in 0.05 M phosphate buffer (pH 7.0) at 25°C.25) With regard to the combination of levofloxacin and lansoprazole OD tablets, aggregation was observed when the concentration of levofloxacin was 8 mM or more, but not when it was less than 5.4 mM (date not shown). Thus, the dissolved concentration of zwitterionic drug is also critical for aggregate formation.
Therefore, we next examined more soluble zwitterionic drugs: meropenem hydrate (pKa1 2.9, pKa2 7.4), cefepime hydrochloride (pKa1 1.11 (carboxyl group), pKa2 3.00 (thiazole amine group)) and cephalexin (pKa1 3.65, pKa2 7.14).26,27) The co-suspension of meropenem hydrate and methacrylic acid copolymer LD formed aggregates, while the other two did not. However, methacrylic acid copolymer LD dissolves at pH 5.5 or higher, and the pH values of the solutions of meropenem hydrate, cephalexin and cefepime hydrochloride in super-pure water were 7.7, 5.0, and 4.4, respectively. When we adjusted the pH to 7.0 with sodium hydroxide, both cefepime hydrochloride and cephalexin formed aggregates with methacrylic acid copolymer LD. These results suggested that the earlier failure to form aggregates was due to the insolubility of methacrylic acid copolymer LD in the original solutions. From these results, we considered that aggregates were formed from methacrylic copolymer LD and zwitterionic ingredients. We also examined whether other polymers, methacrylic acid copolymer type L and methacrylic copolymer type S which consist with methacrylic acid and methyl methacrylate, were aggregated with the zwitterionic ingredients. Although aggregation did occur with meropenem hydrate aqueous solution and levofloxacin aqueous, these aggregates were more fragile than those formed with methacrylic acid copolymer LD (data not shown). Thus, the degree of aggregation seems to depend on the precise structure of the copolymer.
In conclusion, our results indicate that electrostatic interaction causes the aggregation observed in a co-suspension of levofloxacin hydrate tablets and lansoprazole OD tablets. The aggregation occurs at pH over 5.5, where methacrylic acid copolymer LD is soluble,28) and requires a sufficiently soluble zwitterionic active ingredient. Polymers are widely used for improvement of solubility and stability of drugs29–31) and also in drug delivery systems.32–44) Although drug-pharmaceutical additive and pharmaceutical additive-pharmaceutical additive interactions are taken into account during drug development, our results suggest that the effect of mixing with other drugs in the clinical context should also be carefully considered.
The authors declare no conflict of interest.