2020 Volume 43 Issue 5 Pages 839-847
2020 Volume 43 Issue 5 Pages 839-847
Ethenzamide (ETZ), an antipyretic analgesic categorized as a non-steroidal anti-inflammatory drug (NSAID), is widely used as an OTC drug in combination with other NSAIDs. However, its site of action and mechanism underlying its analgesic action have not yet been fully elucidated. In this study, we performed in vitro pharmacological assays to identify the mechanism underlying the analgesic action of ETZ, and also conducted the rat formalin test to investigate its analgesic effect and site of action. Of the 85 receptors, ion channels, transporters and enzymes tested, we found that ETZ binds to the 5-hydroxytryptamine (5HT)2B receptor in concentration-dependent manner with modest inhibitory effects on monoamine oxidase-A and transient potential vanilloid 1 channel. The 5HT2B receptor antagonist activity of ETZ was also confirmed in a cellular functional assay. Furthermore, the drug exerted no inhibitory effects on cycrooxygenase-1 and -2. In the rat formalin test, oral administration of ETZ significantly reduced the nociceptive responses of the second phase and also the number of c-Fos-expressing cells in the spinal dorsal horn, in a dose-dependent manner. Moreover, intrathecal administration of ETZ significantly reduced the nociceptive responses. These results suggest that the analgesic effect of ETZ is exerted at least in the spinal cord, and the effect would be attributed to multiple mechanisms of action including 5HT2B receptor blockade.
Ethenzamide (ETZ) is an antipyretic analgesic categorized as a non-steroidal anti-inflammatory drug (NSAID), and is widely used as an OTC drug in combination with other NSAIDs, such as aspirin and ibuprofen, in Japan. In our open-label trials conducted in 1990, “NARON ACE®,” which contained two analgesics, ETZ and ibuprofen, was demonstrated to be very effective for providing relief from several types of pains, including headache.1) Despite ETZ having been used for a long time as an OTC analgesic drug, there has been little research on the mechanism or site of action of ETZ. The synergic analgesic effects of ETZ and the cyclooxygenase (COX) inhibitor ibuprofen, as observed in our previous pharmacological study,2) may indicate that the mechanisms of action of the two drugs do not overlap, but it still remains to be elucidated whether ETZ exerts its analgesic activity via COX inhibition or another mechanism(s). Only one previous study of spinal reflex potentials in the cat has indicated that the site of analgesic action of ETZ may be receptors expressed in the spinal cord.3)
The spinal cord is the major site of integration of somatosensory information, and has an important role in the processing of several types of pain.4) A number of animal studies have suggested that peripheral inflammation causes sensitization of not only peripheral neurons, but also of the central spinal neurons, resulting in persistent pain and hyperalgesia.5–8) This central spinal sensitization has been demonstrated to be suppressed by analgesics such as pregabalin9) and sumatriptan,10) which are used to treat neuropathic pain and migraine, respectively. Many conditions characterized by frequent or persistent pain, including in patients suffering from headache, are thought to be initiated by dysregulation of the primary sensory neurons, resulting in central sensitization within the spinal cord network.11) Thus, analgesics which normalize this hyperexcitable spinal activity could be promising candidates for the treatment of several types of pain.4)
The formalin test has been used as a model of persistent inflammatory pain involving central spinal sensitization.8,12) In rats, the nociceptive responses associated with localized inflammation of the hindpaw caused by formalin injection typically consist of lifting, biting/licking and flinching of the paw, expressed in a biphasic pattern.12,13) From a mechanistic perspective, the first phase of the nociceptive responses occurs within seconds of the formalin injection as a direct consequence of chemical stimulation of the peripherally localized Transient Receptor Potential Ankyrin 1 (TRPA1)-containing nociceptors.14) Then, after a quiescence period of several minutes, during which the first-phase nociceptive responses disappear, a second persistent phase occurs, as a result of increased excitability of the nociceptive spinal neurons caused by intense activation of the peripherally nociceptors.8,15) Many inflammatory mediators mediate the second-phase nociceptive responses,8,16–18) which are known to be inhibited by NSAIDs,19,20) although the mechanism and site of analgesic action of ETZ in the formalin test have not yet been clarified.
In this study, to explore the mechanism of action of ETZ, we performed in vitro pharmacological assays covering nociception-related targets, including receptors, ion channels, transporters, and enzymes. Next, we evaluated the effects of systemic administration of ETZ on the nociceptive responses and number of spinal c-Fos-expressing cells in the rat formalin test. Lastly, in order to clarify whether the site of action of ETZ is actually in the spinal cord, the effects of intrathecal administration of ETZ on the nociceptive responses were also evaluated.
ETZ was purchased from SHIZUOKA COFFEIN (Shizuoka, Japan).
AnimalsMale Sprague-Dawley rats were purchased from Charles River Japan (Kanagawa, Japan). The rats were all housed under conditions of controlled temperature (23 ± 3°C), humidity (50 ± 20%) and lighting (lights on from 07:00 to 19:00 h), then used for the study when they became 8 weeks old. All the rats were given access to food and tap water ad libitum. All the experimental procedures were conducted with the approval of the Animal Care Committee at Taisho Pharmaceutical Co., Ltd., in accordance with the company’s guidelines for the Care and Use of Laboratory Animals.
In Vitro Binding, Cellular Functional, and Enzyme AssaysThe following binding, cellular functional, and enzyme assays were conducted by Cerep (Poitiers, France), using established validated assays and standard automated techniques. For the initial screen, ETZ was solubilized in buffer prior to each assay and tested at a single concentration (10 µM) in a single experiment. Each assay included a positive control. The results were expressed as a percent inhibition of control specific binding, agonist response, and activity obtained in the presence of ETZ, respectively. A higher than 50% inhibition was considered as a positive finding, warranting additional testing. A positive assay was followed up with a competition and functional study.
The radioligand binding (competition study) and cellular functional (to determine the agonist/antagonist activity) assays for 5-hydroxytryptamine (5HT)2B receptor were also performed by Cerep. In the radioligand binding assay, five concentrations of ETZ over five orders of magnitude were examined in experiments conducted in duplicate, to determine the IC50 (concentration of ETZ exerting half-maximal inhibition of control specific binding). The IC50 value was determined by non-linear regression analysis of the competition curves generated with the mean replicate values using Hill equation curve fitting. This analysis was performed using a software developed at Cerep (Hill software). In the cellular functional (agonist or antagonist) assays, concentrations of ETZ over three orders of magnitude were examined in experiments conducted in duplicate. The results are expressed as percentages relative to the control specific agonist response ((measured specific response/control specific agonist response) × 100) and as percent inhibition of the control specific agonist response (100 − ((measured specific response/control specific agonist response) × 100)) obtained in the presence of ETZ.
The assay conditions and controls employed in this study (target, tissue or cell source, radioligand, drug used to determine non-specific binding or substrate, and the positive control) are shown in Tables 1–3.
| Assay | Source | Ligand | Non-specific binding | Reference |
|---|---|---|---|---|
| Receptor | ||||
| A1 | Human recombinant CHO cells | [3H]CCPA | CPA | CPA |
| A2A | Human recombinant HEK-293 cells | [3H]CGS 21680 | NECA | NECA |
| A2B | Human recombinant HEK-293 cells | [3H]MRS 1754 | NECA | NECA |
| A3 | Human recombinant HEK-293 cells | [125I]AB-MECA | IB-MECA | IB-MECA |
| B1 | Human recombinant CHO cells | [3H]desArg10-KD | desArg9[Leu8]-BK | desArg10-KD |
| B2 | Human recombinant CHO cells | [3H]Bradykinin | Bradykinin | NPC 567 |
| CGRP | Human recombinant CHO cells | [125I]hCGRPα | hCGRPα | hCGRPα |
| CB2 | Human recombinant CHO cells | [3H]WIN 55212-2 | WIN 55212-2 | WIN 55212-2 |
| CCK1 | Human recombinant CHO cells | [125I]CCK-8s | CCK-8s | CCK-8s |
| CRF1 | Human recombinant CHO cells | [125I]Sauvagine | Sauvagine | Sauvagine |
| D1 | Human recombinant CHO cells | [3H]SCH 23390 | SCH 23390 | SCH 23390 |
| D2S | Human recombinant HEK-293 cells | [3H]Methylspiperone | (+)Butaclamol | (+)Butaclamol |
| D2L | Human recombinant HEK-293 cells | [3H]Methylspiperone | Butaclamol | Butaclamol |
| D3 | Human recombinant CHO cells | [3H]7-OH-DPAT | (+)Butaclamol | 7-OH-DPAT |
| D4.4 | Human recombinant CHO cells | [3H]Methylspiperone | (+)Butaclamol | Clozapine |
| D5 | Human recombinant GH4 cells | [3H]Dopamine | SCH 23390 | Dopamine |
| GABA | Rat cerebral cortex | [3H]GABA | GABA | GABA |
| GABAA1 | Human recombinant CHO cells | [3H]Muscimol | Muscimol | Muscimol |
| GABAB(1b) | Human recombinant CHO cells | [3H]CGP 54626 | CGP 52432 | CGP 54626 |
| AMPA | Rat cerebral cortex | [3H]AMPA | L-Glutamate | L-Glutamate |
| Kainate | Rat cerebral cortex | [3H]Kainic acid | L-Glutamate | Kainic acid |
| NMDA | Rat cerebral cortex | [3H]CGP 39653 | L-Glutamate | CGS 19755 |
| Glycine strychnine sensitive | Rat spinal cord | [3H]Strychnine | Strychnine | Strychnine |
| Glycine strychnine insensitive | Rat cerebral cortex | [3H]MDL 105,519 | Glycine | Glycine |
| CXCR2 (IL-8B) | Human recombinant HEK-293 cells | [125I]IL-8 | IL-8 | IL-8 |
| CXCR4 | Human recombinant CHO cells | [125I]SDF-1α | SDF-1α | SDF-1α |
| CCR1 | Human recombinant HEK-293 cells | [125I]MIP-1α | MIP-1α | MIP-1α |
| TNF-α | U-937 cells | [125I]TNF-α | TNF-α | TNF-α |
| CCR3 | Human recombinant K562 cells | [125I]Eotaxin | Eotaxin | Eotaxin |
| CCR2 | Human recombinant HEK-293 cells | [125I]MCP-1 | MCP-1 | MCP-1 |
| H1 | Human recombinant HEK-293 cells | [3H]Pyrilamine | Pyrilamine | Pyrilamine |
| H2 | Human recombinant CHO cells | [125I]APT | Tiotidine | Cimetidine |
| H3 | Rat cerebral cortex | [3H]Nα-Me-histamine | (R)α-Me-histamine | (R)α-Me-histamine |
| H4 | Human recombinant HEK-293 cells | [3H]Histamine | Imetit | Imetit |
| BLT1 (LTB4) | Human recombinant CHO cells | [3H]LTB4 | LTB4 | LTB4 |
| CysLT1 (LTD4) | Human recombinant CHO cells | [3H]LTD4 | LTD4 | LTD4 |
| MT1 (ML1A) | Human recombinant CHO cells | [125I]2-Iodomelatonin | Melatonin | Melatonin |
| MT2 (ML1B) | Human recombinant CHO cells | [125I]2-Iodomelatonin | Melatonin | Melatonin |
| MT3 (ML2) | Hamster brain | [125I]2-Iodomelatonin | Melatonin | Melatonin |
| NK1 | U-373MG cells | [125I]BH-SP | [Sar9,Met(O2)11]-SP | [Sar9,Met(O2)11]-SP |
| NK2 | Human recombinant CHO cells | [125I]NKA | [Nleu10]-NKA(4–10) | [Nleu10]-NKA(4–10) |
| NK3 | Human recombinant CHO cells | [125I][MePhe7]-NKB | SB 222200 | [MePhe7]-NKB |
| N Neuronal α4β2 | SH-SY5Y cells | [3H]Cytisine | Nicotine | Nicotine |
| N Neuronal α7 | SH-SY5Y cells | [125I]α-Bungarotoxin | α-Bungarotoxin | α-Bungarotoxin |
| Opioid (non-selective) | Rat cerebral cortex | [3H]Naloxone | Naloxone | Naloxone |
| Opioid δ2 (DOP) | Human recombinant CHO cells | [3H]DADLE | Naltrexone | DPDPE |
| Opioid κ (KOP) | Rat recombinant CHO cells | [3H]U 69593 | Naloxone | U 50488 |
| Opioid μ (MOP) | Human recombinant HEK-293 cells | [3H]DAMGO | Naloxone | DAMGO |
| NOP (ORL1) | Human recombinant HEK-293 cells | [3H]Nociceptin | Nociceptin | Nociceptin |
| DP1 | Human recombinant 1321N1 cells | [3H]PGD2 | BW245C | BW245C |
| EP1 | Human recombinant HEK-293 cells | [3H]PGE2 | PGE2 | PGE2 |
| EP2 | Human recombinant HEK-293 cells | [3H]PGE2 | PGE2 | PGE2 |
| EP4 | Human recombinant HEK-293 cells | [3H]PGE2 | PGE2 | PGE2 |
| FP | Human recombinant HEK-293 cells | [3H]PGF2α | Cloprostenol | PGF2α |
| IP (PGI2) | Human recombinant HEK-293 cells | [3H]Iloprost | Iloprost | Iloprost |
| PAR2 | Human recombinant Hela cells | [3H]2-Fuoryl-LIGRL-NH2 | 2-Fuoryl-LIGRL-NH2 | SLIGRL-NH2 |
| P2Y | Rat cerebral cortex | [35S]dATPαS | dATPαS | dATPαS |
| 5HT (non-selective) | Rat cerebral cortex | [3H]Serotonin | Serotonin | Serotonin |
| 5HT1A | Human recombinant HEK-293 cells | [3H]8-OH-DPAT | 8-OH-DPAT | 8-OH-DPAT |
| 5HT1B | Rat cerebral cortex | [125I]CYP (+30 µM Isoproterenol) | Serotonin | Serotonin |
| 5HT1D | Rat recombinant CHO cells | [3H]Serotonin | Serotonin | Serotonin |
| 5HT2A | Human recombinant HEK-293 cells | [125I](±)DOI | (±)DOI | (±)DOI |
| 5HT2B | Human recombinant CHO cells | [125I](±)DOI | (±)DOI | (±)DOI |
| 5HT2C | Human recombinant HEK-293 cells | [125I](±)DOI | (±)DOI | (±)DOI |
| 5HT4e | Human recombinant CHO cells | [3H]GR 113808 | Serotonin | Serotonin |
| 5HT5a | Human recombinant HEK-293 cells | [3H]LSD | Serotonin | Serotonin |
| 5HT6 | Human recombinant CHO cells | [3H]LSD | Serotonin | Serotonin |
| 5HT7 | Human recombinant CHO cells | [3H]LSD | Serotonin | Serotonin |
| Ion channels | ||||
| BZD | Rat cerebral cortex | [3H]Flunitrazepam | Diazepam | Diazepam |
| PCP | Rat cerebral cortex | [3H]TCP | MK 801 | MK 801 |
| P2X | Rat urinary bladder | [3H]α,β-MeATP | α,β-MeATP | α,β-MeATP |
| Cl− Channel | Rat cerebral cortex | [35S]TBPS | Picrotoxinin | Picrotoxinin |
| Transporters | ||||
| Norepinephrine | Human recombinant CHO cells | [3H]Nisoxetine | Desipramine | Protriptyline |
| Dopamine | Human recombinant CHO cells | [3H]BTCP | BTCP | BTCP |
| 5-HT | Human recombinant CHO cells | [3H]Imipramine | Imipramine | Imipramine |
| Other enzymes | ||||
| MAO-A | Rat cerebral cortex | [3H]Ro 41-1049 | Clorgyline | Clorgyline |
| Assay | Source | Stimulus | Measured component | Reference |
|---|---|---|---|---|
| Receptors | ||||
| 5HT2B (agonist effect) | Human recombinant CHO cells | None | IP1 | Serotonin |
| 5HT2B (antagonist effect) | Human recombinant CHO cells | Serotonin | IP1 | SB 206553 |
| Ion channels | ||||
| TRPM8 (antagonist effect) | Human recombinant | Icilin | Intracellular[Ca2+] | BCTC |
| TRPV3 (antagonist effect) | Human recombinant HEK-293 cells | 2-APB | Intracellular[Ca2+] | Ruthenium red |
| TRPV1 (antagonist effect) | Human recombinant CHO cells | Capsaicin | Intracellular[Ca2+] | Capsazepine |
| Assay | Source | Substrate/Stimulus/Tracer | Measured component | Reference |
|---|---|---|---|---|
| Kinases | ||||
| ERK1 | Human recombinant E.coli | ATP + Ulight- CFFKNIVTPRTPPPSQGK-amide | Phospho-Ulight- CFFKNIVTPRTPPPSQGK-amide | Staurosporine |
| ERK2 (P42mapk) | Human recombinant E.coli | ATP + Ulight- CFFKNIVTPRTPPPSQGK-amide | Phospho-Ulight- CFFKNIVTPRTPPPSQGK-amide | Staurosporine |
| ERK5 (MAPK7) | Human recombinant | ATP + Ulight- CFFKNIVTPRTPPPSQGK-amide | Phospho-Ulight- CFFKNIVTPRTPPPSQGK-amide | Ro-318220 |
| Other enzymes | ||||
| COX1 | Human recombinant Sf9 cells | Arachidonic acid | PGE2 | Diclofenac |
| COX2 | Human recombinant Sf9 cells | Arachidonic acid | PGE2 | NS398 |
| Inducible NOS | Mouse recombinant (E. coli) | L-Arginine | NO2− | 1400W |
Under isoflurane anesthesia, a polyethylene catheter (SP10; inner diameter: 0.28 mm; outer diameter: 0.61 mm; Natsume Seisakusho, Tokyo, Japan) was inserted through the atlantooccipital membrane into the lumbar enlargement of the spinal cord (close to the L3–L5 segments) of the rats. After the surgery, the rats were housed individually and allowed to recover for 7 d, until the experiments were started. Rats that developed paralysis of their hindpaws after the surgery were excluded from the subsequent experiments. Catheter placement was verified by the observation of hindlimb paralysis induced by intrathecal administration of lidocaine (2%, 10 µL) through the catheter. Rats exhibiting lidocaine-induced transient paralysis of their hindpaws were used for the following experiments.
Formalin TestIn this study, the second-phase nociceptive responses in the formalin test were evaluated. The measurement of the formalin-induced nociceptive behaviors was started 10 min after intraplantar injection of 1% (v/v) formalin at a volume of 50 µL into the right hindpaw. Elevation, licking, biting, or shaking of the injected paw was defined as a nociceptive response. The total time spent exhibiting these nociceptive responses were recorded with a hand-held stop-watch for a period of 50 min. For the oral ETZ experiment, 100–400 mg/kg of ETZ suspended in 5% (w/v) arabic gum solution was administered orally 30 min before the intraplantar formalin injection at 5 mL/kg. For the intrathecal ETZ experiment, 25 µg/20 µL (7.5 mM)/site of ETZ dissolved in 2% (v/v) dimethyl sulfoxide (DMSO) solution was intrathecally administered 20 min before the intraplantar formalin injection.
Spinal Tissue Preparation and ImmunohistochemistryVehicle or ETZ (400 mg/kg) was administered orally to the rats, and 30 min after, saline or 1% (v/v) formalin was administered by intraplantar injection into the right hindpaw. The rats were anesthetized with isoflurane and necropsied 60 min after the intraplantar injection, and the lumbosacral enlargement (L3–L5) of the spinal cord was removed. The spinal cords were fixed in 4% paraformaldehyde and decalcified using the ethylenediaminetetraacetic acid (EDTA)-decalcification protocol (Wako Pure Chemical Industries, Ltd., Osaka, Japan). After fixation and decalcification, three sections of the lumbosacral enlargement (L3–L5) of the spinal cord were trimmed, embedded in one paraffin block, and processed by the conventional method for immunohistochemical analysis. In addition, three slices (4-µm-thick) 100 µm apart were cut from each paraffin block of the lumbar enlargement of the spinal cord and the total of nine cross sections per rat were subjected to immunohistochemical analysis. Immunohistochemical staining for c-Fos was conducted as follows. After deparaffinization of the tissue sections, heat-induced epitope retrieval was performed in a 0.01 M citrate buffer for 40 min. The specimens were then reacted with the primary antibody for c-Fos (ab208942, Abcam, Cambridge, MA, U.S.A.) overnight at 4°C. Then, the secondary antibody, biotin-conjugated anti-mouse immunoglobulin G (BA-2001, Vector Laboratories, Burlingale, CA, U.S.A.) was applied for 1 h at room temperature. The slides were exposed to H2O2 for 30 min to quench endogenous peroxidase. Thereafter, the slices were incubated in an avidin-biotin complex solution (ABC Elite STANDARD kit, Vector Laboratories). Finally, the slices were visualized with 3-amino-9-ethylcarbazole (AEC STAINING KIT, Sigma-Aldrich, St. Louis, MO, U.S.A.). The slides were also imaged and analyzed under the ScanScope XT system (Leica Microsystems, Wetzlar, Germany). Morphometric analysis for c-Fos immunoreactive cells was performed in the superficial laminae (spinal cord layer I and layer II) of the ipsilateral (right side) spinal dorsal horn. The total number of c-Fos positive cells in three slices per rat were calculated as individual data.
Data AnalysisData obtained from in vitro pharmacological assays conducted once or in duplicate are presented as raw or mean data, respectively. The results of the formalin test (10 to 12 rats per group) and the immunohistochemical data (5 rats per group) are presented as means ± standard error of the mean (S.E.M.). For the results of the formalin test, statistical analyses were performed using Steel test and Student’s t-test. For the results of the immunohistochemical study, statistical analyses were performed using the F-test, followed by Student’s t-test or Aspin–Welch’s t-test. p < 0.05 was considered as being indicative of statistical significance.
ETZ was initially tested at a single concentration of 10 µM in 85 screening assays, as listed in Materials and Methods (Tables 1–3). The results of the initial screening are shown in Table 4. Of the 85 receptors, ion channels, transporters and enzymes tested, ETZ was found to exert significant (greater than 50% inhibition) inhibitory effect only on the 5HT2B receptor (Table 4). Then, a radioligand binding competition study of ETZ for the 5HT2B receptor was performed. This study revealed that ETZ displaced [125I](±)DOI from human recombinant 5HT2B receptors expressed in the Chinese hamster ovary (CHO) cells in a dose-dependent manner. The competition curves for ETZ at the 5HT2B receptor are shown in Fig. 1. The IC50 of ETZ for the 5HT2B receptor was 2.78 µM (0.46 µg/mL) (Fig. 1).
| Target | % Inhibition of control | Target | % Inhibition of control |
|---|---|---|---|
| A1 | −5 | N Neuronal α7 | 3 |
| A2A | 15 | Opioid (non-selective) | −4 |
| A2B | 21 | Opioid δ2 (DOP) | 2 |
| A3 | −15 | Opioid κ (KOP) | 3 |
| B1 | −9 | Opioid μ (MOP) | 14 |
| B2 | −9 | NOP (ORL1) | 10 |
| CGRP | −36 | DP1 | 1 |
| CB2 | −1 | EP1 | −1 |
| CCK1 | −11 | EP2 | 9 |
| CRF1 | 10 | EP4 | −13 |
| D1 | −7 | FP | −7 |
| D2S | 7 | IP (PGI2) | 1 |
| D2L | −8 | PAR2 | −3 |
| D3 | 4 | P2Y | 2 |
| D4.4 | −3 | 5HT | −21 |
| D5 | −16 | 5HT1A | −20 |
| GABA | 2 | 5HT1B | −7 |
| GABAA1 | −19 | 5HT1D | 10 |
| GABAB(1b) | 4 | 5HT2A | 17 |
| AMPA | 8 | 5HT2B | 67 |
| Kainate | −3 | 5HT2C | 0 |
| NMDA | −1 | 5HT4e | 5 |
| Glycine (strychnine sensitive) | −17 | 5HT5a | −9 |
| Glycine (strychnine insensitive) | 17 | 5HT6 | −5 |
| CXCR2 (IL-8B) | −28 | 5HT7 | −2 |
| CXCR4 | −29 | BZD | 3 |
| CCR1 | −5 | PCP | 7 |
| TNF-α | 7 | P2X | −3 |
| CCR3 | −8 | Cl− Channel | −1 |
| CCR2 | −9 | Norepinephrine transporter | −15 |
| H1 | 0 | Dopamine transporter | 19 |
| H2 | −25 | 5HT transporter | 14 |
| H3 | −23 | MAO-A | 40 |
| H4 | −1 | TRPM8 | −17 |
| BLT1 (LTB4) | −23 | TRPV3 | −8 |
| CysLT1 (LTD4) | 4 | TRPV1 | 25 |
| MT1 (ML1A) | 14 | ERK1 | 1 |
| MT2 (ML1B) | −1 | ERK2 (P42mapk) | −10 |
| MT3 (ML2) | −22 | ERK5 (MAPK7) | −2 |
| NK1 | 14 | COX1 | −6 |
| NK2 | 11 | COX2 | 5 |
| NK3 | −16 | Inducible NOS | −3 |
| N Neuronal α4β2 | 16 |

Each data point is the mean of duplicate data. The IC50 value was determined by non-linear regression analysis of the competition curves generated using the mean replicate values using Hill equation curve fitting.
Following the findings of the radioligand binding competition study, we conducted this experiment to determine whether ETZ exerted agonist or antagonist activity on the 5HT2B receptor (Table 2). In CHO cells expressing human recombinant 5HT2B receptors (Table 2), ETZ at 1 (0.165 µg/mL), 10 (1.65 µg/mL), and 100 µM (16.5 µg/mL) produced 7.8, 20.1, and 71.9% inhibition, respectively, of the 5HT-evoked inositol phosphate formation; at the same concentrations, no stimulatory effect of ETZ on inositol phosphate production (data not shown) was observed, indicating the absence of agonist activity of ETZ for the 5HT2B receptor.
Targets of ETZ Other than 5HT2B ReceptorIn the screening assays, ETZ showed modest (greater than 25% inhibition) inhibitory effects on the monoamine oxidase-A and transient potential vaniolloid 1 channel (Table 4).
Oral Administration of ETZ Reduced Formalin-Induced Nociceptive Behaviors in the Rat Formalin Test and Also the Count of c-Fos-Positive Cells in Spinal CordOral administration of ETZ reduced the second-phase nociceptive behaviors in the rat formalin test in a dose-dependent manner (Fig. 2a). The results of immunohistochemical analysis revealed that the number of c-Fos-positive cells in the superficial laminae of the spinal dorsal horn was significantly increased in the formalin-injected animals (vehicle group) as compared to the saline-injected animals (normal group) (Fig. 2b). Furthermore, in the animals administered ETZ at 400 mg/kg (ETZ group), the number of c-Fos-positive cells in the superficial laminae of the spinal dorsal horn induced by formalin injection was significantly decreased as compared to that in the vehicle-treated animals (vehicle group) (Figs. 2b, c).

(a) The total length of time spent by the animals exhibiting the second-phase nociceptive behaviors (10–60 min post formalin injection) in the rat formalin test was evaluated. Data represent the means ± S.E.M. (n = 11–12). ** p < 0.01, *** p < 0.001 versus vehicle group, Steel test. (b) The number/mm2 of c-Fos-positive cells in the ipsilateral superficial (laminae I–II) dorsal horn in normal control, vehicle-treated and ETZ (400 mg/kg)-treated animals. Data represent the means ± S.E.M. (n = 5). ** p < 0.01 versus normal control group, Aspin–Welch’s t-test. # p < 0.05 versus vehicle group, Student’s t-test. (c) Immunohistochemical staining for c-Fos in the ipsilateral superficial (laminae I–II) lumbar dorsal horn in the vehicle- and ETZ-treated animals. Scale bar = 200 µm.
Intrathecal administration of ETZ at 25 µg/20 µL/site significantly reduced the second-phase nociceptive behaviors of the animals in the rat formalin test (Fig. 3).
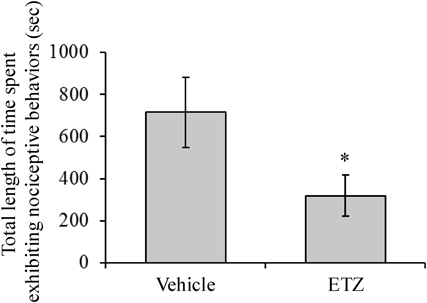
The total length of time spent by the animals exhibiting the second-phase nociceptive behaviors (10–60 min post formalin injection) in the rat formalin test was evaluated. ETZ was administered at 25 µg/20 µL/site. Data represent the means ± S.E.M. (n = 10–12). * p < 0.05 versus vehicle-treated group, Student’s t-test.
In our previous report, we showed that the combination of ETZ plus ibuprofen (a non-selective COX inhibitor) exerted synergistic analgesic effect in the acetic acid-induced writhing test in mice,2) perhaps suggesting the COX-independent analgesic action of ETZ. About 40 years ago, Matsui demonstrated that intraduodenal administration of ETZ attenuated the spinal reflex potentials in cats,3) suggesting that the site of action for the analgesic effect of ETZ might be the spinal cord. Since then, however, the analgesic mechanism and site of action of ETZ have never been investigated.
In the present study, for the first time, we provide evidence shows that ETZ exerts its analgesic effects at the spinal cord via multiple mechanisms of action including blockade of 5HT2B receptor. We found that ETZ showed concentration-dependent affinity for the 5HT2B receptor (Fig. 1) with modest inhibitory effects on the monoamine oxidase-A (MAO-A) and transient potential vanilloid 1 (TRPV1) channel (Table 4). We also confirmed that ETZ exerts 5HT2B receptor antagonist activity (see Results). In addition, as assumed previously, ETZ showed no COX-1 or COX-2 inhibitory activity (Table 4). Furthermore, in the rat formalin test, oral administration of ETZ reduced the second-phase nociceptive responses that are known to be related to central spinal sensitization (Fig. 2a), and also reduced the number of c-Fos-expressing cells in the spinal dorsal horn (Figs. 2b, c). In addition, we demonstrated that direct administration of ETZ into the spinal cord also suppressed the second-phase of formalin-induced nociceptive behaviors (Fig. 3). From these results, we consider it highly likely that ETZ modulates nociceptive processing at the level of the spinal cord via the mediation of multiple mechanisms of action including the blockade of 5HT2B receptor.
The 5HT2B receptor has been reported to be expressed in several tissues, including the spinal cord21,22) and dorsal root ganglion (DRG) neurons,21) and its participation in nociceptive processing and hyperalgesia at these sites is well known. Consistent with our findings, spinal administration of the 5HT2B receptor antagonist RS-127445 has also been shown to prevent the nociceptive responses in rat the formalin test.23) Similarly, in another study, spinal administration of RS-127445 prevented the development and maintenance of the long-lasting hyperalgesia induced by formalin injection into the rat hindpaw.24) Furthermore, in the spinal nerve ligation model of neuropathic pain in rats, thermal or mechanical allodynia was shown to be attenuated by spinal 5HT2B receptor inhibition induced by the 5HT2B receptor antagonists RS-127445,25) LY-266097,25) and SB204741.26) As a mechanistic perspective, Aira et al. proposed convergent synaptic input from a descending serotonergic varicosity and a primary C afferent fiber onto neurons at spinal superficial dorsal horn, and that activation of postsynaptic 5HT2B receptor at the superficial dorsal horn by increased activity of descending serotonergic pathway would lead to dorsal horn hyperexcitablity to input from primary afferent fibers in the spinal nerve ligation model of neuropathic pain.26) Thus, we assumed that ETZ would block the 5HT2B receptor localized on the neurons at superficial dorsal horn, and then suppress the formalin-evoked hyperexcitability to input from primary afferent fibers in this study. Indeed, it was demonstrated that formalin-evoked c-Fos (marker of neuronal activity)-positive cells at the spinal superficial dorsal horn was decreased by ETZ (Figs. 2b, c). Taken together, these findings lend support to our finding that ETZ exerts analgesic action by preventing central spinal sensitization via inhibition of the 5HT2B receptor expressed in the spinal cord.
In this study, we found that ETZ exhibited affinity for the 5HT2B receptor even in the micromolar range (Fig. 1), which was much weaker than that of other newly developed ligands.27–29) Although there are no reports of detailed pharmacokinetic studies, a previous study had shown that micromolar concentrations of ETZ were detected in the blood of rats after oral ETZ administration even at lower doses than those used in our study.30) Thus, the dose of ETZ administered in this study was probably sufficient for the drug to exert 5HT2B receptor inhibitory effect in several tissues.
Since ETZ also showed modest (greater than 25%) inhibitory effects on the MAO-A and TRPV1 in the screening assay (Table 4), these enzyme and channel could contribute to the analgesic effect of ETZ in the formalin test. In agreement with our results, phenelzine (anidepressant possessing MAO inhibiting effect) prevented the formalin induced nociceptive behavior via modulation of neural responses at the spinal dorsal horn.31,32) Further, iod-resiniferatoxin (specific TRPV1 antagonist) given intrathecally also decreased flinching responses in the rat formalin test.33) Taken together, although the inhibitory effects were modest compared to that on the 5HT2B receptor, MAO-A and TRPV1 were also considered to be participated in the analgesic action of ETZ at the spinal cord.
While we identified the spinal cord as the possible site of action of ETZ by direct application, further study is needed to clarify other possible sites of action. Indeed, intrathecal application of adequately high concentration (7.5 mM) of ETZ exerted lesser analgesic effect rather than systemic administration (Figs. 2a, 3), suggests that there would be any other site of action. In regard to the 5HT2B receptor, the local peripheral injection of RS-127445 (selective 5HT2B receptor antagonist) prevented the local pronociceptive effect of 5HT2 receptor agonist,23) and the 5HT2B receptor at DRG has been reported to involved in the 5HT-induced mechanical hyperalgesia.21) These reports imply a local peripheral and DRG as possible sites of analgesic action of ETZ via 5HT2B receptor. Regarding the TRPV1, peripheral ipsilateral treatment with capsazepine (TRPV1 antagonist) prevented formalin-induced hyperalgesia,34) suggest that local peripheral TRPV1 also could be participated in the analgesic action of ETZ. At present, it is not clear how much each site contributes to the effect, but the analgesic effect of systemically administered ETZ is considered to be the sum of the effects at these various sites.
Further, whereas we have reported that over-the-counter drug “NARON ACE®,” which contained ETZ, exerted very good clinical performance for headache symptoms,1) there are no studies focused on the pathogenesis of headache. For example, it has been suggested that in the rat model of migraine, the 5HT2B receptor localized on the vascular endothelial cells of the dura matter has an important role in meningeal neurogenic inflammation.35–37) Furthermore, the 5HT2B receptor expressed in the trigeminal ganglionic cells of mice21) could be assumed to have a role in the pain processing of trigeminal afferents. Thus, these structures known to be relevant to migraine pain could also be possible targets of action of ETZ, and precise investigations focused on these structures may be expected to clarify the molecular basis for the clinical efficacy of ETZ.
In summary, we provide evidence suggesting that the analgesic effect of ETZ in the rat formalin test was mediated by multiple mechanisms of action including the 5HT2B receptor blockade at the spinal cord, and propose appropriate utilization of ETZ for several types of pain in which the hyperalgesia is known to arise from central spinal sensitization.
The authors declare no conflict of interest.