2020 Volume 43 Issue 5 Pages 859-863
2020 Volume 43 Issue 5 Pages 859-863
Pathological angiogenesis is a leading cause of blindness in several retinal diseases. The key driving factor inducing pathological angiogenesis is the pronounced hypoxia leading to a marked, increased production of vascular endothelial growth factor (VEGF). The aim of this study was to determine whether the abnormal vascular growth occurs in a manner dependent on the degree of the vascular defects. Vascular defects of two different degrees were created in the retina by subcutaneously treating neonatal rats with the VEGF receptor (VEGFR) tyrosine kinase inhibitor KRN633 on postnatal day (P) 4 and P5 (P4/5) or P7 and P8 (P7/8). The structure of the retinal vasculature changes was examined immunohistochemically. Prevention of vascular growth and regression of some preformed capillaries were observed on the next day, after completion of each treatment (i.e., P6 and P9). The vascular regrowth occurred as a result of eliminating the inhibitory effect on the VEGFR signaling pathway. KRN633 (P4/5)-treated rats exhibited a retinal vasculature with aggressive intravitreal neovascularization on P21. On the other hand, the appearance of tortuous arteries is a representative vascular pathological feature in retinas of KRN633 (P7/8)-treated groups. These results suggest that an interruption of the retinal vascular development at different time points induces different vascular pathological features in the retina. Pharmacological agents targeting the VEGF signaling pathway are useful for creating an abnormal retinal vasculature with various pathological features in order to evaluate the efficacy of anti-angiogenic compounds.
Anti-vascular endothelial growth factor (VEGF) agents are in widespread clinical use for treating vasoproliferative retinal diseases. However, adverse effects associated with blockade of the VEGF signaling pathway have been suggested.1) Therefore, the development of safe and effective drugs for pathological retinal angiogenesis has been the focus of attention in recent years.
Several rodent models have been developed to investigate the mechanisms underlying pathological retinal angiogenesis.2) The oxygen-induced retinopathy (OIR) model has become the most used animal model. The OIR can be induced by exposing neonatal rodents to hyperoxia or by alternating hyperoxia and hypoxia.3–11) The developing retinal blood vessels are highly sensitive not only to hyperoxia but also to the loss of VEGF signals.12,13) Therefore, the exposure of neonatal rodents to either high oxygen concentrations or VEGF receptor (VEGFR) inhibitors impairs retinal vasculature by preventing endothelial cell growth and regressing capillaries, leading to tissue hypoxia.14–17) Severe hypoxia drastically increases retinal tissue VEGF levels, inducing aggressive angiogenesis.
In previous studies, we found that treatment of 7-d-old rats with VEGFR inhibitors for 2 d impairs retinal vasculature by preventing endothelial cell growth and regressing capillaries.15,16) The brief period of interruption of retinal vascular development induces abnormal retinal angiogenesis.15,16) If the severity of the retinal hypoxia is related to the extent of the vascular defects, then abnormal vascular growth will occur in a manner dependent on the degree of the vascular defects. In the present study, we created different degrees of vascular defects and patterns in the retina by treating neonatal rodents with VEGFR inhibitors at different time points during the first postnatal week (postnatal day [P]4 or P7), and examined the structure of the retinal vasculature changes following the completion of the treatment.
All animal procedures have been approved by the Institutional Animal Care and Use Committee of the Kitasato University and handled in accordance with the Association for Vision Research and Ophthalmology Statement.
Experimental ProceduresSprague-Dawley rats (Charles River Breeding Laboratories, Tokyo, Japan), which were pregnant, were maintained on a standard diet (Oriental Yeast, Tokyo, Japan), and tap water ad libitum. Daily inspections were performed to determine the day of birth that was defined as P0.
The retinal vascular development is easily manipulated using pharmacological agents targeting the VEGF signaling pathway such as VEGFR tyrosine kinase inhibitors.14–17) In this study, we used KRN633, a VEGFR tyrosine kinase inhibitor,18) to interrupt the retinal vascular development. KRN633 was synthesized in the laboratory of Dr. Tohru Nagamitsu (Department of Organic Synthesis, Kitasato University School of Pharmaceutical Sciences), and the compound was administered as a 0.5% methylcellulose water suspension.
Our previous studies demonstrated that treatment of 7-d-old rats, which have the vasculature covering approximately 80% of the retinal surface, with KRN633 interrupted radial vascular expansion and reduced capillaries in the vascularized area.16,17) To create different degrees of vascular defects and patterns in the retina, neonatal rats were treated subcutaneously with KRN633 (10 mg/kg) or its vehicle on P4 and P5 (P4/5) or P7 and P8 (P7/8). Control animals were injected with the vehicle (0.5% methylcellulose) alone. On P6 or P9, P14, or P21, rats were deeply anesthetized with sodium pentobarbital (Nacalai Tesque, Kyoto, Japan) and then underwent systemic perfusion with 1% paraformaldehyde in phosphate buffered saline (PBS) via the aorta (Fig. 1A). After the perfusion, eyes were removed and stored in fixative for 24 h at 4°C.

A: Protocol 1, Neonatal rats were treated with either vehicle or KRN633 on postnatal day (P) 4 and P5. Protocol 2, Neonatal rats were treated with either vehicle or KRN633 on P7 and P8. B: A representative fluorescence microscopic image of retinal flat-mount stained for endothelial cells (RECA) on P21 from KRN633 (P4/5)-treated group. In panel a, the freehand line and dotted line indicate the peripheral edge of the retina and the developing edge of the vascular bed, respectively. Higher-magnification of the dotted line boxes in panel a are shown in the right panels b and c, respectively. b: Right panel, the freehand line indicates the tortuous artery segment from the optic nerve to the first branch point. The length of the vessel (b) and the straight dotted line (a) between 2 points were measured. c: Lower panel, clusters of intravitreal blood vessels were shown in white. The area of intravitreal neovascularization and the vascular area in the entire retina were measured. Scale bars: 1000 µm (a), 200 µm (b), 200 µm (c).
In the P4/5 group, eyes were collected on P6 (Control, n = 4 and KRN633, n = 5), P14 (Control, n = 5 and KRN633, n = 5), or P21 (Control, n = 5 and KRN633, n = 6). In the P7/8 group, eyes were collected on P9 (Control, n = 4 and KRN633, n = 5), P14 (Control, n = 4 and KRN633, n = 5), or P21 (Control, n = 4 and KRN633, n = 5). For comparison, normal eyes were collected on P4 (n = 4) and P7 (n = 5) after systemic perfusion and processed as described above.
ImmunohistochemistryImmunohistochemical staining for endothelial cells in the retina was performed as previously reported.15,16) Briefly, the retina was separated from the lens, vitreous, and pigment epithelium, and then incubated in blocking solution (5% normal hamster serum) prepared in PBS containing 0.3% Triton X-100 (PBS-T) for 0.5–1 h. The retina was incubated overnight with a 1 : 800 dilution of mouse monoclonal anti-rat endothelial cell antigen (RECA)-1 antibody (Serotec, Oxford, U.K.) followed by incubation with a 1 : 400 dilution of Cy3-conjugated donkey antibody against mouse immunoglobulins (Jackson Immuno Research, West Grove, PA, U.S.A.). Retinas were rinsed in PBS-T and the retinal flat-mounts were prepared using a mounting medium for fluorescence (Vectashield; Vector Laboratories, Burlingame, CA, U.S.A.). Images were obtained with the fluorescent microscope system BZ-9000 (Keyence, Osaka, Japan).
Quantitative Analysis of the Retinal VasculatureThe abnormalities of the retinal vasculature were assessed according to the methods described in our previous study.15) Briefly, the area of the retinal surface covered by the network of RECA-stained endothelial cells was measured using ImageJ software (http://rsb.info.nih.gov/ij/), and the size of this area relative to that of retinal surface area was determined as a measure of retinal vascularization (Fig. 1Ba). We quantified the tortuosity in the arteries by tracing a line along the tortuous vessel and comparing it to a straight line traced from the vessel origin at the optic nerve to the final branch point (Fig. 1Bb). To quantify the intravitreal neovascularization, areas of blood vessel clusters growing from neuronal layers of the retina into the vitreous cavity, were measured. The area of the intravitreal neovascularization relative to the vascular area was calculated (Fig. 1Bc).
Data AnalysisAll values are presented as the mean ± standard error (S.E.). Statistical comparisons of paired data were performed using Student’s t-test, and multi-group data were evaluated using ANOVA followed by Tukey’s post-hoc test using the Prism6 software (GraphPad Software, San Diego, CA, U.S.A.). p < 0.05 was considered statistically significant.
In the rat retina, the superficial endothelial cell network spreads radially from the optic nerve head toward the periphery in the ganglion cell layer and covers the retinal surface within the first 10 d.19) In this study, at the onset times of the KRN633 treatment (P4 and P7) the endothelial cell network covered approximately 60 and 80% of the retinal surface, respectively (Figs. 2Aa, Ba). The 2-d treatment with KRN633 (10 mg/kg) on P4 and P5 (P4/5) and P7 and P8 (P7/8) prevented radial vascular growth and reduced the capillaries throughout the vasculature on the next day after completion of the treatment (Figs. 2Ab, Bb). The degree of reduction in vascular density in KRN633-treated rats was dependent on the postnatal age: the reduction of vascular density in the P4/5 group (65.3 ± 0.7%, n = 5) was significantly (p < 0.05) larger than that in the P7/8 group (56.9 ± 0.3%, n = 5). Thus, the susceptibility of endothelial cells to the inhibition of VEGFR appeared to alter during the first postnatal week.

A–C: Fluorescence microscopy images of retinal flat-mounts stained for endothelial cells (RECA) from both vehicle- and KRN633-treated groups. The white dotted line in each panel indicates the peripheral edge of the retina. Scale bar: 1000 µm in a (applies to b–d).
Blood vessel growth occurred on P14 in the retinas of both KRN633-treated groups, but the expansion of the vascular network was prevented (Figs. 2Ac, Bc). The progressive intravitreal angiogenesis was observed on P21 in retinas of KRN633 (P4/5)-treated rats (Fig. 2Ad). Arterial tortuosity was evident on P14 and P21 in retinas of KRN633 (P7/8)-treated rats (Figs. 2Bc, Bd). In contrast, the superficial endothelial cell network in vehicle-treated rats almost reached the peripheral edge of the retina by P9 (Fig. 2Cb); further development of the vasculature (i.e., remodeling of vasculature) occurred on P14 and P21 (Figs. 2Cc, Cd).
Quantitative data are summarized in Fig. 3. The size of the vascularized area in KRN633-treated rats was smaller than that in vehicle-treated ones (Fig. 3A). In rats treated with KRN633 (P4/5), the vascularization (i.e., increase in vascularized area) was completely blocked (Fig. 3A). The tortuosity index increased on P14 and P21 in KRN633 (P7/8)-treated rats compared to vehicle-treated ones (Fig. 3B), and the intravitreal neovascularization increased in KRN633 (P4/5)-treated rats (Fig. 3A).
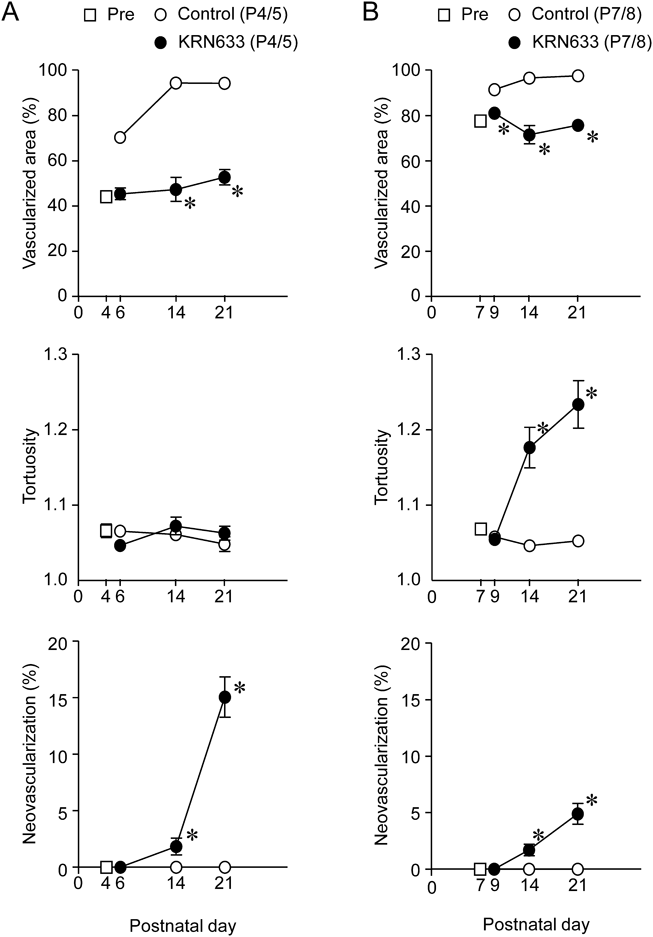
A: The percentage of the retinal area covered by retinal vasculature. B: The tortuosity index. C: The intravitreal neovascularization. n = 4–6. * p < 0.05. vs. the corresponding age-matched control value (vehicle).
In the present study, neonatal rats were treated with the VEGFR inhibitor KRN633 at two different time points (P4 and P7) resulting in the production of different degrees and patterns of vascular defects in the retina. After the completion of each treatment, rats treated with KRN633 on P4 and P5 (P4/5) exhibited a retinal vasculature with aggressive intravitreal neovascularization. In contrast, the appearance of tortuous arteries in retinas of KRN633 (P7/8)-treated groups was the representative vascular pathological feature. Thus, neonatal rats treated with VEGFR inhibitors at different time points exhibited different vascular pathological features in the retina.
Following cessation of the vascular development interruption with VEGFR inhibitors, abnormal vascular growth occurred with a decrease in the inhibitory effects on blood vessels. However, there were differences in the pathological features between KRN633 (P4/5)- and KRN633 (P7/8)-treated groups. The blockade of the VEGF signaling pathway with VEGFR inhibitors and neutralizing antibodies prevented the appearance of tortuous arteries as well as intravitreal neovascularization.20–24) Therefore, the observed vascular abnormalities could be attributed to the increased VEGF production through the retinal hypoxia, due to the insufficient vascular network. If the severity of the retinal hypoxia was related to the extent of the vascular defects, the severity of the vascular abnormalities might be dependent on the amount of functional, perfused blood vessels. Indeed, a dose–response study for KRN633 (1, 5, and 10 mg/kg) indicated that the severity of vascular abnormalities was related to the severity of vascular defects following treatment with VEGFR inhibitors.15) However, a 2-d treatment of neonatal rats with the three doses of KRN633 on P7/8 induced the same vascular pathological features, including tortuous arteries. Thus, the difference in vascular pathological features may not be explained by the severity of the vascular defects alone. The size of the peripheral avascular zone was larger in KRN633 (P4/5)-treated rats than in KRN633 (P7/8)-treated groups. Therefore, the difference in the peripheral avascular size might contribute to the observed difference in vascular pathological features. During the first postnatal week, retinal vasculature develops at a relatively high speed. In this study, we found a significant difference in the susceptibility of retinal capillaries to the inhibition of VEGFR between P4 and P7 rats. This finding was consistent with previous observations12,17) and is associated with the decreased proportion of growing and/or immature blood vessels in the vascular bed. The difference in the level of maturity of retinal blood vessels on the onset times (P4 and P7) of treatment with KRN633 might also be involved.
In the present study, we used systemic administration of KRN633, a VEGFR tyrosine kinase inhibitor, to manipulate the retinal vascular development. This procedure appears to become an easy and reproducible method for creating avascular areas in the central and peripheral retina. KRN633 is a highly selective inhibitor of VEGFR tyrosine kinase; however, it also inhibits other tyrosine kinases, including platelet-derived growth factor receptor.18) We focus on the process of re-vascularization, which occurs following the elimination of the inhibitory effect of KRN633 on the VEGFR signaling pathway; however, potential off-target effects may contribute to the abnormalities in retinal vasculature. Furthermore, systemic administration of anti-angiogenic compounds affects the structure and function of blood vessels in peripheral organs.25,26) Therefore, it will be important that future studies investigate whether selective blockade of retinal VEGF (i.e., intravitreal injection of anti-VEGF antibody) induces similar morphological abnormalities in the retinal vasculature.
In summary, we found that neonatal rats treated with VEGFR inhibitors at different time points exhibited different vascular pathological features in the retina. Similar to OIR models, the hypoxia-related enhancement of VEGF/VEGFR signaling pathway is responsible for abnormal retinal vascular growth.16,17) However, special devices including an oxygen-regulated chamber are unnecessary to induce abnormal retinal vasculature in our models. Only a 2-d drug treatment at different time points in newborn rats could induce abnormal blood vessels with different vascular pathological features. Our models might be useful for studying the mechanisms underlying abnormal retinal angiogenesis and evaluating the efficacy of anti-angiogenic compounds.
This study was supported in part by Grants JSPS KAKENHI Grant numbers 25122712 (to TN) and 26460103 (to TN).
The authors declare no conflict of interest.