2020 Volume 43 Issue 6 Pages 938-945
2020 Volume 43 Issue 6 Pages 938-945
The neurodevelopmental toxicity of isoflurane has been proved by many studies, which makes it essential to explore the underline mechanisms and search for protective agents to attenuate its neurotoxcity. Accumulating evidence showed that L-theanine had neuroprotective effects on injured neurons and the developing brain. The present study was designed to investigate whether L-theanine could attenuate isoflurane-induced damage in neural stem cells and cognitive impairment in young mice, and to discuss the role of protein kinase B (Akt)–glycogen synthase kinase 3β (GSK-3β) signaling pathway in this process. Multipotential neural stem cells (NSCs) and C57BL/6J mice were treated with either gas mixture, isoflurane, or L-theanine 30 min prior to isoflurane exposure, respectively. NSC viability was detected by CCK-8 assay. NSC proliferation and apoptosis were assessed by immunofluorescence and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay, respectively. The levels of cleaved caspase-3 and phosphorylated (p)-Akt and p-GSK-3β in NSCs were tested by Western blotting. Cognitive function of mice was tested by Morris Water Maze at postnatal day (P) 30–35. The results indicated that isoflurane exposure inhibited NSC viability and proliferation, promoted NSC apoptosis as well as increased caspase-3 activation and down-regulated the expressions of p-Akt and p-GSK-3β in NSCs, and that isoflurane exposure on neonatal mice would induce late cognitive impairment. Pretreatment with L-theanine could attenuate isoflurane-caused damage in NSCs and cognitive deficits in young mice. Addinonally, the protective effects of L-theanine on isoflurane-injured NSCs could be reversed by Akt inhibitor Triciribine. Our data showed that pretreatment with L-theanine eliminated the NSC damage and cognitive impairment induced by isoflurane exposure, and that the neuroprotective effect of L-theanine was associated with the Akt–GSK-3β signaling pathway.
The developmental neurotoxicity of general anesthetics has been widely concerned in recent years. A lot of clinical retrospective studies showed that children who experience multiple exposures to anesthesia before 3 years old were more prone to learning and memory disabilities in their adulthood.1,2) As a typical volatile anesthetic, isoflurane is commonly used in anesthesia practice because of its excellent sedative and analgesic effect. However, it is still not a perfect anesthetic in pediatric clinic. Many studies have shown that isoflurane inhibited neurogenesis, induced neuroapoptosis and neural damage which would ultimately lead to long-term neurocognitive and memory dysfunction in the developing brain.3–7) These findings make anesthetists start to reevaluate the application of isoflurane in children, and to explore the mechanisms and potential protective measures for isoflurane-induced neurocognitive deficits.
L-Theanine is a unique amino acid in tea leaves and is known to affect central nervous system, such as sedation, anti-anxiety and modulation of neurotransmitter activities.8–11) Recent studies showed that L-theanine had neuroprotective effects on injured neurons and could improve learning and memory functions in the developing rat brain.12–15) As a natural glutamate analog, L-theanine’s neuroprotective effect is proved to be mainly mediated by its antagonistic action on glutamate receptors, such as α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic-acid (AMPA), kainate and N-methyl-D-aspartate (NMDA) receptors,16,17) and regulation on the release and neurotransmission of glutamate.9,18–21) In addition, L-theanine also showed its neuroprotective effect on environmental toxins-induced neuronal cell death and cognitive impairments by increasing the down-regulation of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF),22) as well as reducing the macromolecular oxidative damage.15,23,24) However, the underline mechanisms of the neuroprotective effect of L-theanine are still not fully investigated, and there is lack of data on neural stem cells (NSCs).
Glycogen synthase kinase 3β (GSK-3β), a serine/threonine kinase, is highly expressed in the developing brain and can be phosphorylated at serine 9 by Akt (protein kinase B). It has been reported that activation of the Akt–GSK-3β signaling pathway is involved in the protection of several brain injury models such as traumatic brain injury, Alzheimer’s disease, ischemia/reperfusion-induced myocardial apoptosis, and anesthetic-induced neurotoxcity.25–28) As it remains unclear whether and how L-theanine attenuates isoflurane-induced neurotoxicity in NSCs, in the present study we investigated the neuroprotective effects of L-theanine on isoflurane-caused injury in NSCs in vitro and cognitive impairment in young mice, and proposed that Akt–GSK-3β might be involved in this process.
All experiments and procedures were approved by the Animal Ethics Committee of Nanchang University and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize suffering.
Neuronal cells were obtained from the hippocampus of 16–18-d-old embryonic mice by a caesarean section for pregnant C57BL/6J mice. After enzymatically digested with accutase (GIBCO, Los Angeles, CA, U.S.A.), the cell pellets were re-suspended in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F12 (DEME/F12, GIBCO) medium with 20 ng/mL of recombinant human basic fibroblast growth factor (bFGF, GIBCO) and recombinant human epidermal growth factor (EGF, GIBCO), 2% N2 and 1% B27 (GIBCO), and 100 U/mL streptomycin and penicillin (GIBCO). The cells were plated at a density of 2 × 105 cells/mL and the cultures were maintained at 37°C in a humidified 95% air–5% CO2 atmosphere. The primary cultured cells were grown in suspension and every 3 d a half of culture medium was changed. When incubated for 7 d, the neurospheres were collection and dissociation by accutase, then the suspended cells were passaged at a density of 2 × 105 cells/mL. This is defined as first passage and then the cells were passaged every 7 d in the same way. After the second passage, the cells were cultured in basal medium with 10% fetal bovine serum (FBS) to allow differentiation. After 7 d culture, abundant nestin-positive cells proliferated in neurospheres with some β-tubulin III or glial fibrillary acidic protein (GFAP)-positive cells, suggesting the characteristic of the NSCs preparation.
Experimental ProtocolAfter the second passage, the primary cultured NSCs were randomly divided into four groups: control group (Con), isoflurane group (Iso), L-theanine pretreatment group (LT + Iso), and inhibitor group (Triciribine + LT + Iso). Triciribine was purchased from Selleck (U.S.A.). After cultured for 2 d when the cells were enriched in cultures, the treatments were implemented. The cells in control group were incubated in a gas mixture (5% CO2, 21% O2, and 74% N2), while isoflurane group were exposed by 3.4% isoflurane in the gas mixture for 6 h; and the cells in LT + Iso group were pretreated with 100 µM L-theanime 30 min prior to isoflurane anesthesia and L-theanine was maintained in the culture throughout the isoflurane exposure. The cells in Triciribine + LT + Iso group were first pretreated with 10 µM Triciribine for 1 h, next with 100 µM L-theanime for 30 min following isoflurane exposure for 6 h.
After all the drug treatments, the identification of NSCs was detected by the Nestin immunofluorescence staining. NSC viability was tested by CCK-8 assay; NSC proliferation rate and apoptosis rate were examined by 5-bromo-2′-deoxyuridine (BrdU) and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) immunofluorescence method, respectively. The expressions of cleaved caspase-3, phosphorylated (p)-Akt and p-GSK-3β were detected by Western blotting method.
NSC IdentificationAfter the second passage, the cell cultures were dissociated and seeded at a density of 2 × 104 cells/mL in 24-well plates pre-coated with 100 µg/mL poly-L-lysine and incubated with “complete medium” for more than 24 h. After the cells attached to the plates and enriched in monolayer, we used Nestin immunofluorescence staining to characterize the cultured cells. Specifically, the cells were first fixed with 4% paraformaldehyde (PFA) for 15 min and placed in 0.3% Triton X-100 for 10 min. After treated with blocking buffer (10% goat serum) for 1 h, cells were incubated with primary anti-Nestin antibody (1 : 500; Millipore, MA, U.S.A.) overnight at 4°C. Then the samples were incubated with secondary antibody [tetramethylrhodamine isothiocyanate (TRITC)-conjugated immunoglobulin G (IgG)] for 2 h in room temperature, and the nuclei was stained by D4′,6′-diamidion-2′-phenylindole (DAPI) (1 : 1000; Abcam, U.S.A.). For NSC differentiation assessment, the cells were cultured in basal medium with 10% FBS to allow differentiation. At the end of 7 d, cells were fixed and stained by anti-β-tubilin III (Sigma-Aldrich Inc., St. Louis, MO, U.S.A.) antibody and anti-GFAP antibody (Sigma-Aldrich Inc.), respectively. A laser fluorescence microscope (Olympus, BX51, Japan) was used to capture the images.
Measurement of Cell ViabilityCell viability was detected by the CCK8 assay (cell counting kit, Dojindo Molecular Technologies, MD, U.S.A.). Briefly, after the second passage, the cells from passage 1 were dissociated and seeded on 96-well plates at a density of 5 × 103 cells per well. Before the end of isoflurane exposure, 10 µL CCK8 solution was added to each well and the plates were incubated for 4 h at 37°C. The optical density (OD) was assessed with a reader at the wavelength of 450 nm. At least three independent experiments were conducted for each assay.
BrdU Immuofluorescence StainingAfter the second passage, the cell cultures from passage 1 were dissociated and seeded at a density of 2 × 104 cells/mL in 24-well plates precoated with 100 µg/mL poly-L-lysine. At the last 4 h of isoflurane treatment, NSCs were exposed to 10 µg/L of BrdU. Then 12 h after isoflurane exposure, the treated cells were fixed in 4% PFA and then placed in 0.3% Triton X-100 for immunofluorescence staining. After blocking for 1 h, the primary anti-BrdU antibody (1 : 1000; Abcam) was used to detect proliferating cells. Immunoreactive cells were captured and analyzed by fluorescence microscope. BrdU positive cells were counted blindly in 6 randomly selected sections per well. At least three independent experiments were performed for data collection.
TUNEL AssayThe cells were labeled by the TUNEL assay to detect NSC apoptosis according to the instruction of in situ Cell Death Detection Kit (Roche Inc. Roche, Mannheim, Germany). In brief, the cells were seeded on 24-well plates and fixed at 12 h after isoflurane exposure. During experiments, the samples were protected from light and DAPI was used to stain nuclei. Data were collected from at least three independent experiments.
Western Blot AnalysisFollowing exposure to isoflurane and/or L-theanine, the cells from the eight wells in the center of each plate (one plate represented one group) were collected and lysed with radio-immunoprecipitation assay (RIPA) lysis buffer (20 mM Tris–HCl, 150 mM NaCl, 1 mM Na2 ethylenediamine tetraacetic acid (EDTA), 1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin; Cell Signaling Technology, U.S.A.) containing 1 mM phenylmethanesulfonyl fluoride (PMSF, Cell Signaling Technology)). Protein concentrations in resulting lysates were tested by BCA protein assay. Then equal amounts (40 µg) of the resulting cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel and the separated proteins were transferred to polyvinylidene fluoride membranes. Then the membranes were incubated with blocking buffer for 2 h at room temperature and incubated in primary antibodies overnight at 4°C. The primary antibodies include: rabbit monoclonal anti-caspase-3 antibody (17/34 kDa, 1 : 1000, Cell Signaling Technology), rabbit monoclonal anti-phosphorylated-Akt (phosphorylation within the carboxy terminus at Ser473, 1 : 1000, Cell Signaling Technology), rabbit monoclonal anti-phosphorylated GSK-3β (phosphorylation within the carboxy terminus at Ser9, 1 : 1000, Cell Signaling Technology), rabbit monoclonal anti-Akt (1 : 1000, Cell Signaling Technology), anti-GSK-3β (1 : 1000, Cell Signaling Technology) and mouse monoclonal anti-β-actin (internal standard, 1 : 10000, Cell Signaling Technology). The next day, blots were incubated with secondary antibody (anti-rabbit/mouse horseradish peroxidase-conjugated IgG, 1 : 5000; Santa Cruz, CA, U.S.A.) for 2 h at room temperature. The signals were exposed to X-ray film and detected by analysis system. At least three independent experiments were performed and quantified as OD values according to their controls.
Mice Anesthesia and TreatmentThe neonatal mice were randomly assigned into the control group (Con), isoflurane group (Iso) or L-theanine treatment group (LT + Iso). The mice in isoflurane group were exposed with 3.4% isoflurane (in a gas mixture of 60% oxygen balanced with nitrogen) 2 h daily from postnatal day (P)6 to P8 and then had the Morris Water Maze (MWM) test from P28 to 35. The mice in L-theanine received 10 mg/kg L-theanine intraperitoneal injection 30 min before isoflurane exposure. This concentration of L-theanine has been proved to be neuroprotective to cognitive fucntion.12–14) The control condition was oxygen (60% oxygen and balanced with nitrogen) with an equal rate of flow in a chamber which was similar to the anesthesia chamber. The induction flow rate of fresh gas was 2.5 L/min from the start up to 5 min and then 1 L/min with the rest to maintain anesthesia. The mice were kept in an anesthesia chamber with a warming pad at the bottom to maintain the rectal temperature of each mouse around 37°C (±0.5°C). The concentrations of isoflurane and oxygen were continuously monitored by using a gas analyzer (Drager Inc., German) during the anesthesia. After the exposures, the mice were transferred to their cages and fed to P28 for the MWM test.
MWMMWM test were conducted to test the cognitive function of isoflurane-exposed mice. Briefly, a round steel tank (120 cm in diameter; 60 cm in height) filled with warm water was divided into four quadrants equally in MWM. The surface of water was 1.0 cm over the level of a platform (10 cm in diameter) which was placed in the center of the third quadrant. The temperature of water was kept around 22°C. The water was made opaque by using milk powder. We tested the P30 mice for the daily training of the MWM for 5 consecutive days (P30 to P34) with four trials daily and then performed the probe test on P35. The mice were tested for swimming one day in advance and allowed to swim freely in the pool for 120 s and given 15 s to stay on the platform after finding the target. In the 5′ days training, the time for mice to reach the platform (escape latency) and swimming speed were recorded by a video installed above the pool. In probe test on P35, the counts of the mice moved across the original area of the removed platform (platform crossing times) was recorded to assess the cognitive function. During the experiments, each mouse got dry under a heat lamp and returned to the holding cage after every trial.
Statistical AnalysisThe in vitro data were presented as means ± standard error of the mean (S.E.M.) and analyzed by one-way ANOVA, followed by post-hoc Duncan’s test using the SPSS 20.0 program and prism 6 software. Two-way ANOVA with repeated measurement and post-hoc analysis were used to evaluate the difference of escape latency and swimming speed of the mice during the MWM test. Tukey–Kramer method was used to compare the difference in the platform crossing times of the mice among different groups. Statistically significant difference was set at p < 0.05.
Nestin is considered as a marker of NSC. To identify the cells, we firstly examined the Nestin expressions in the cultured cells. The results suggested that most of the cells were Nestin-positive both in suspension and adherent culture (97.8 ± 1.3%, Fig. 1A) 2 d after the second passage. After 7 d differentiation, the cultured cells also expressed glial fibrillary acidic protein (GFAP), a specific maker for astrocytes (68.4 ± 2.4%, Fig. 1B, arrowhead), and β-tubulin Ш, a specific maker for neuron (13.8 ± 1.7%, Fig. 1B, arrow). These results indicated that the cells in this experiment were NSCs.
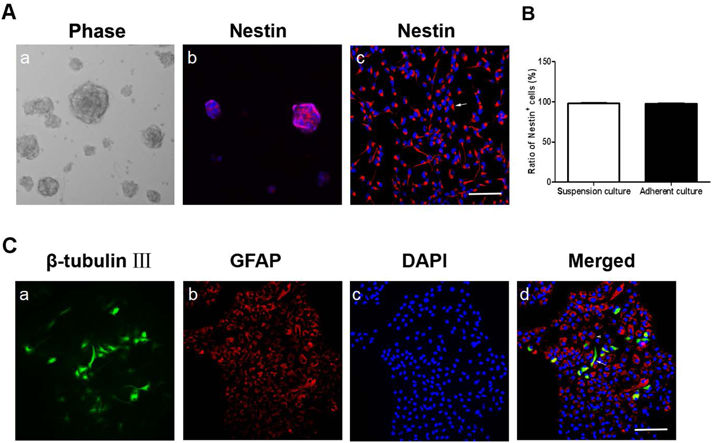
(A) Representative images of neurospheres and Nestin expressions in suspension and adherent culture. (a) The image of neurospheres under light microscope. (b, c) Nestin immnostaining in suspension and adherent culture respectively. The cells were stained by NSC marker Nestin (red) and the nuclei were stained with DAPI (blue). (d) The comparison of Nestin-positive cells in neurospheres and adherent culture. (B) Representative immunofluroscence images of cells after 7 d′ differentiation culture. (a–d) The cells differentiated into neurons and astrocytes, which were stained by β-tublin III (green, arrow) and GFAP (red, arrowhead), respectively. The nuclei were counterstained by DAPI (blue). Scale bar = 50 µm. Data were showed as mean ± S.E.M. and at least three independent experiments were conducted. (Color figure can be accessed in the online version.)
After the identification, we first explored the effect of isoflurane on NSC viability using CCK8 kit. Compared with control, isoflurane exposure (3.4%, 6 h) significantly inhibited NSC viability in a time-dependent way, and minimal survival of 58.5% occurred at 12 h after isoflurane treatment. When pretreatment with L-theanine (100 µM) for 30 min before isoflurane exposure, the survival rate of NSC in LT + Iso group was 93.2 ± 1.9%, and there is no significant difference on NSC viability between the control group and LT + Iso group, which suggested that L-theanine pretreatment could reverse the isoflurane-decreased NSC viability (Fig. 2).
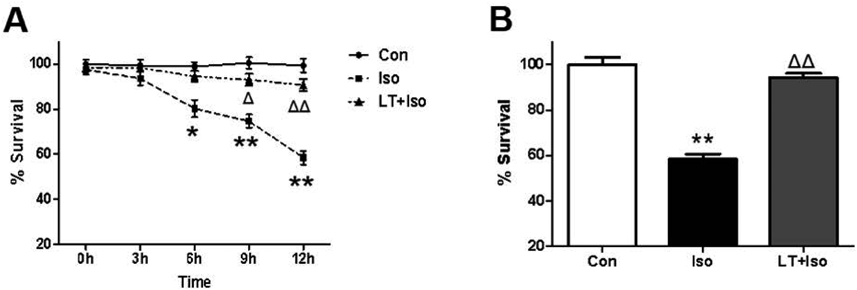
(A) Cell survival compared among NSCs in control group (Con), Isoflurane exposure group (Iso) and L-theanine pretreatment group (LT + Iso) for 3–12 h. At each time point, the OD value of CCK8 assay was normalized to the corresponding controls (B) Cell viability compared among different groups at 12 h after isoflurane exposure. Data were showed as mean ± S.E.M. and collected from three independent experiments. ** p < 0.01 vs. Con; ΔΔ p < 0.01 vs. Iso.
NSC proliferation rate was detected by BrdU immunofluorescence staining. As shown in Fig. 3, the BrdU positive cells in isoflurane group were remarkably decreased when compared to the control group. On the contrary, L-theanine pretreatment significantly increased the NSC proliferation rate after isoflurane exposure. In addition, there is no significant difference on the rate of BrdU-positive cells between the control group and LT + Iso group. These results indicating that L-theanine pretreatment could reverse the NSC proliferation inhibition caused by isoflurane exposure (Figs. 3A, B).
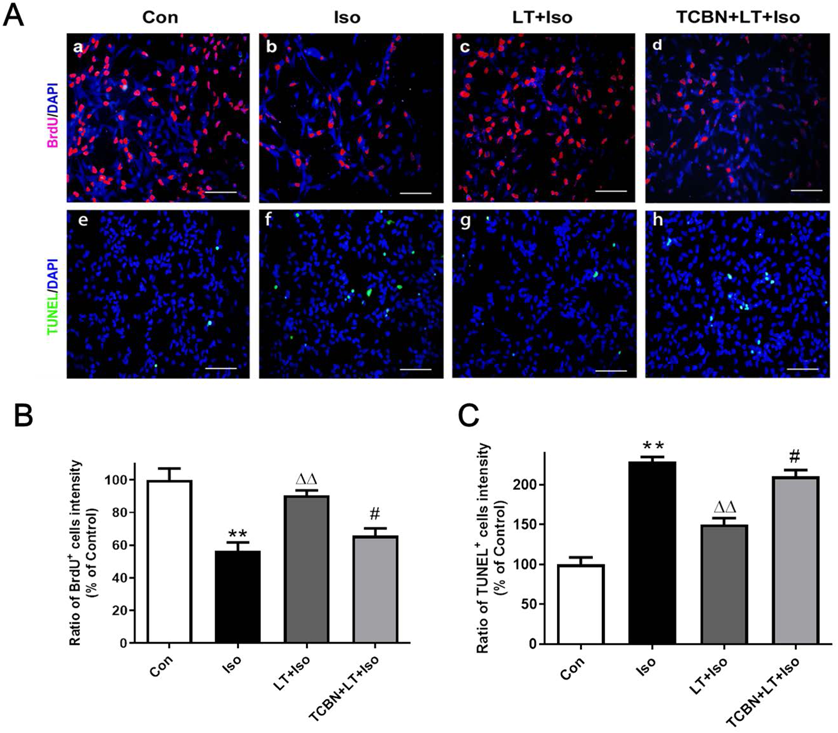
(A) Representative immunostaining images of BrdU and TUNEL positive cells in the different groups. NSCs were stained for BrdU (red) and TUNEL (green). The nuclei was counterstained by DAPI (blue). Scale bar = 50 µm. (B) Quantification analysis of BrdU immunostaining. (C) Quantification analysis of TUNEL immunostaining. Data were showed as mean ± S.E.M. Data were collected from three independent experiments. ** p < 0.01 vs. Con; ΔΔ p < 0.01 vs. Iso; # p < 0.05, vs. LT + Iso. (Color figure can be accessed in the online version.)
TUNEL assay were used to determine NSC apoptosis rate. When compared with control group, isoflurane exposure increased TUNEL positive cells, while L-theanine pretreatment decreased TUNEL positive cells in NSCs exposed to isoflurane. However, the TUNEL positive cells in LT + Iso group was still increased when compared to the control group (Figs. 3A, C). Besides, cleaved caspase-3 is often associated with apoptosis. Western blot analysis showed that isoflurane exposure increased cleaved caspase-3 levels in NSCs, while L-theanine treatment decreased the cleaved caspase-3 levels in isoflurane-exposed NSCs. Additionally, when compared to the control group the cleaved caspase-3 expressions in LT + Iso group was still increased. Taken together, these data suggested that L-theanine could alleviate isoflurane exposure induced increased NSC apoptosis but could not fully eliminate the effect of isoflurane on NSC apoptosis (Figs. 4A, B).
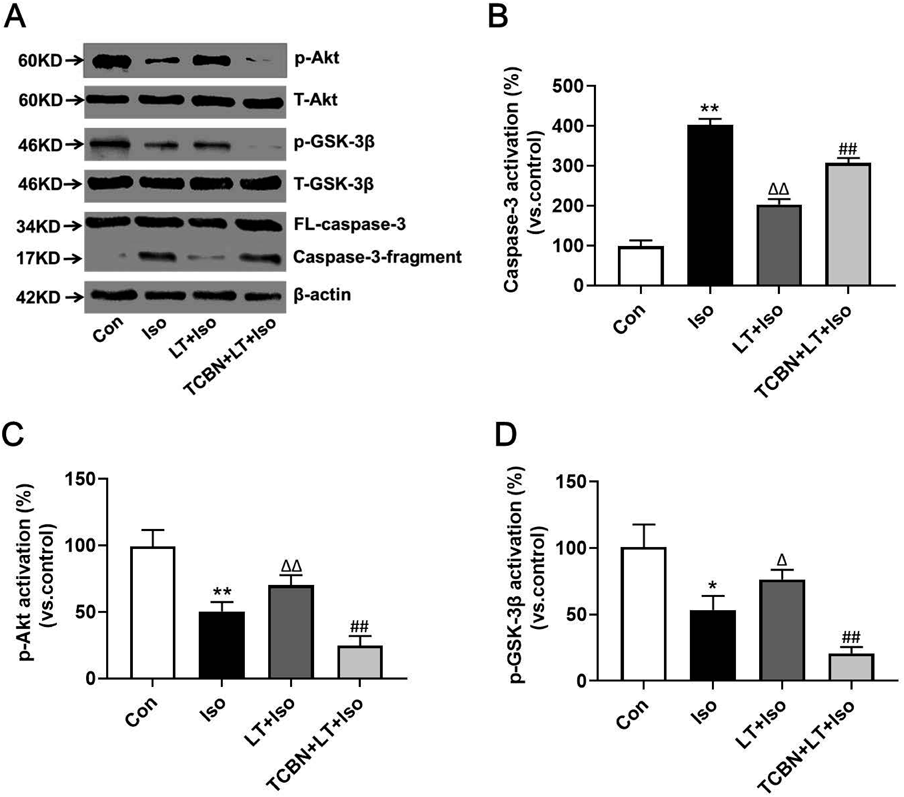
(A) Typical blots of related proteins in different groups. β-Actin was used as an internal control. (B) Effect of different treatments on cleaved caspase-3 expressions in NSCs. (C) Effect of different treatments on p-Akt expressions in NSCs. (D) Effect of different treatments on p-GSK3β expressions in NSCs. The expressions of cleaved caspase-3, p-Akt and p-GSK-3β were normalized to β-actin. The data from three independent experiments were expressed as the ratio to values of the corresponding controls and were showed as mean ± S.E.M. * p < 0.05, ** p < 0.01 vs. Con; Δ p < 0.05, ΔΔ p < 0.01 vs. Iso; ## p < 0.01 vs. LT + Iso.
To figure out whether Akt–GSK-3β pathway was involved in the neuroprotective effects of L-theanine on isoflurane-injured NSCs, we incubated the cells with Akt inhibitor Triciribine in vitro. The results of Western blot showed that NSCs exposed to isoflurane (3.4%, 6 h) lead to decreased p-Akt and p-GSK-3β expressions, while pretreatment with L-theanine reversed the effects of isoflurane exposure in p-Akt and p-GSK-3β levels. There is no significant difference in p-Akt and p-GSK-3β expressions between control group and LT + Iso group. Additionally, Akt inhibitor Triciribine decreased the expressions of p-Akt and p-GSK-3β when compared with the LT + Iso group. Triciribine also reversed the protective effects of L-theanine on NSC proliferation, apoptosis and caspase-3 activation in isoflurane-injured NSCs (Figs. 3, 4B). There is no significant difference in the total amount of Akt and GSK-3β among different groups (Figs. 4A, C, D). Taken together, our results indicated that Akt–GSK-3β pathway maybe is involved in the neuroprotective effects of L-theanine against isoflurane-caused injury.
L-Theanine Mitigated the Isoflurane-Induced Cognitive Impairment in Young MiceFinally, we assessed the effect of L-theanine on learning and memory functions in isoflurane-exposed young mice. Neonatal mice received the isoflurane exposure from P6 to 8 and were tested in the MWM from P30 to P35 as demonstrated in Fig. 5A. There was no significant difference in the swimming speed among different groups as shown in Fig. 5B (F = 5.390; p > 0.05). Two-way ANOVA with repeated measurement showed that 3.4% isoflurane 2 h daily for 3 d increased the escape latency on training days 3–5 (F = 1.930; p < 0.05). However, when compared to the isoflurane group, the escape latency was decreased in the L-theanine treatment group on both trail days 4 and 5 (F = 1.56; p < 0.05) (Fig. 5C). Comparison of the platform crossing times showed that isoflurane anesthesia decreased the platform crossing times as compared to the control group. In contrast, compared with the isoflurane group, mice in the L-theanine group had more platform crossing times. Collectively, these results showed that isoflurane exposure in neonatal mice induced long-term cognitive impairment in adult and that pretreatment with L-theanine mitigated this defect.
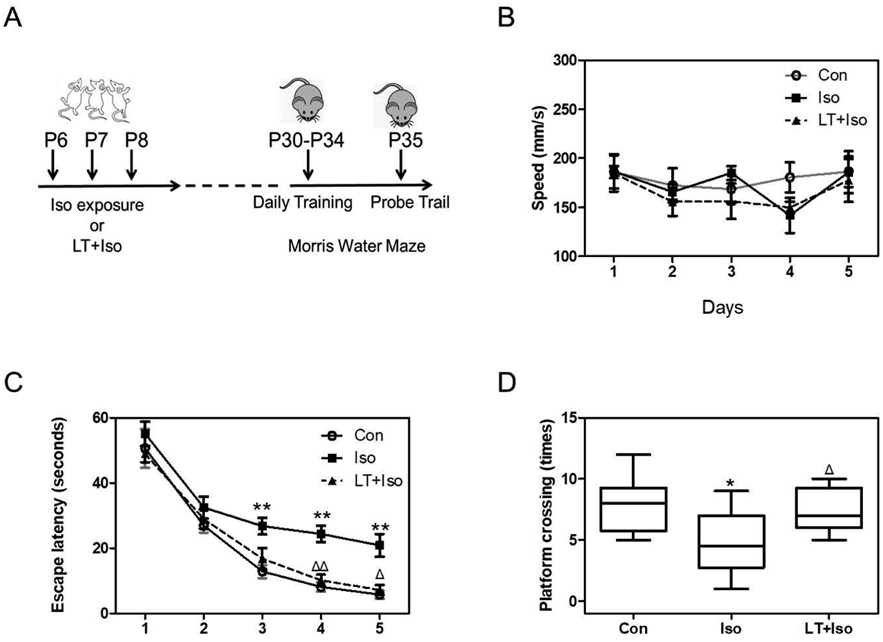
(A) The diagram timeline of the behaviour studies. We treated the neonatal mice with 3.4% isoflurane 2 h daily from postnatal day (P) 6 to P8. The daily training of Morris Water Maze was conducted from P30 to 34; the probe test was performed on P35. (B) Comparison of swimming speed of mice showed that there was no significant difference in mice swimming among different groups. (C) Comparison of escape latency of mice among different groups in the daily training of MWM. (D) Comparison of platform crossing times among different groups in the probe test of MWM. All data were presented as mean ± S.E.M. ** p < 0.01 vs. Con; ΔΔ p < 0.01, Δ p < 0.05 vs. Iso.
In the present study, we first demonstrated the neurotoxcity of isoflurane on NSCs. Our results showed that isoflurane inhibited NSC viability and proliferation as well as promoted NSC apoptosis. Then we further explored the neuroprotective effect of L-theanine on NSCs by reversed the changes caused by isoflurane and investigated its possible mechanism associated with Akt–GSK-3β pathway. In addition, we also proved that pretreatment with L-theanine improved isoflurane-caused cognitive impairment in young mice.
Isoflurane is a widely used clinical inhalational anesthetic, especially in pediatric surgery. In the past decades, there has been mounting and convincing evidence from animal models showed that isoflurane is neurotoxic to the developing brain. Several studies showed that isoflurane could induce neuron apoptosis in the developing brain, leading to long-term cognitive dysfunction.11–15) Specifically, it was demonstrated that isoflurane could inhibit neurogenesis in both adult and neonatal rat brain.13) These findings promote researchers to investigate the underline mechanisms and search for potential protective strategies for isoflurane-induced neurotoxicity. However, there is still lack of data on isoflurane-induced NSC injury. In the present study, we showed that isoflurane exposure (3.4%, 6 h) inhibited neurogenesis by decreasing NSC proliferation and viability as well as increasing NSC apoptosis, which is consistent with the previous studies.28,29) Additionally, we also demonstrated that 3 consecutive day’s isoflurane exposure on neonatal mice induced late cognitive deficits in adult, which supported the previous studies.11–15) The 3.4% isoflurane is a clinically relevant concentration and the anesthesia with 3.4% isoflurane 2 h daily for 3 consecutive days from P6 to 8 mimics the multiple exposures of anesthesia in clinic. Note that the brain growth spurt reaches the peak at postnatal day 7 in rodent, we therefore performed the isoflurane exposure from P6 to P8 in the present study.
Glutamate is the most abundant excitatory neurotransmitter in the nervous system, playing an important role in synaptogenesis, neuron apoptosis, regulation of growth cones, and cognitive function in the brain.30,31) It has been proved that anesthetics’ neurotoxcity is closely associated with the excitatory neurotoxicity of glutamate. In vitro study, isoflurane inhibited the produce and release of glutamate in nerve terminals, increased the uptake of glutamate in astrocytes and neurons, caused glutamate transmission inhibition and ultimately leading to neurotoxicity.32–35) L-Theanine is a structural analog of excitatory neurotransmitter glutamate and glutamine, which could bind to glutamate receptors and block the reuptake of glutamate, and thus inhibited the excitatory neurotoxicity of glutamate in brain.9,10,21) By inhibiting glutamine transporters, L-theanine protected neurons and astroglia from excitatory neurotoxicity of glutamate in rat brain.8,9) However, the neuroprotective effect of L-theanine on central neural system is still not fully understood, especially on NSC and its underline mechanism. L-Theanine is able to cross the blood–brain barrier, which becomes its advantage in neuroprotection. Our results showed that L-theanine attenuated isoflurane-induced NSC injury, increased NSC viability and proliferation, decreased NSC apoptosis, and improved learning and memory function in young mice, which maybe is associated with its inhibition of glutamate excitatory neurotoxicity caused by isoflurane exposure.
As a main anabolism pathway in cellular signal transduction, Akt–GSK-3β signaling pathway plays an importantly mediated role in cell survival, proliferation, apoptosis and metabolism.36) Akt–GSK-3β pathway is involved in isoflurane-induced neurogenesis inhibition and cognitive impairment.28) There are also similar studies on other anesthetics such sevoflurane and ketamine.27,37,38) Besides, Akt–GSK-3β signaling pathway mediated the neuroprotection against glutamate-induced neuronal damage.39,40) Based on these researches, we hypothesized that L-theanine provided neuroprotective effects on isoflurane-injured NSCs possibly by activating Akt–GSK-3β signaling pathway. To examine this hypothesis, we first observed the differences in the expressions of p-Akt and p-GSK-3β in NSCs among different groups. The results showed L-theanine reversed isoflurane-induced down regulation of p-Akt and p-GSK-3β in NSCs, as long as isoflurane-induced NSC neurotoxicty. Then we found that Akt inhibitor Triciribine could reverse the neuroprotective effects of L-theanine on isoflurane-injured NSCs. All these observations indicated that Akt–GSK-3β signaling pathway was possibly involved in the neuroprotective effects of L-theanine on isoflurane-exposed NSCs. However, whether the increase in p-Akt and p-GSK-3β is merely and directly due to L-theanine treatment is not clear, and whether L-theanine increased activity of Akt–GSK-3β or it inhibited the suppression of Akt–GSK-3β activity needs to be further studied.
In summary, we have demonstrated L-theanine pretreatment provides neuroprotection against isoflurane-induced injury in NSCs and cognitive impairment in young mice possibly by activating Akt–GSK-3β pathway. Our results provide a potential protective strategy for isoflurane-induced neurotoxicity in pediatric anesthesia. Nevertheless, there are still limitations in this study. Firstly, we did not detect NSC differentiation or migration in this study as our priority was to investigate whether L-theanine could prevent NSC from isoflurane exposure-induced injury. The further study should be done to investigate whether isoflurane could change NSC differentiation or migration and whether L-theanine could alleviate these effects on NSC in vitro. Secondly, it is necessary to conduct investigations on the effect of L-theanine alone on NSCs and figure out the optimum concentration of L-theanine on isoflurane-injuried NSCs. In addition, there is lack of in vivo data and as for the molecular mechanism of L-theanine’s neuroprotective effect on isoflurane’s neurotoxicity, further experimental studies should be conducted.
This study was supported by Grants from the National Natural Science Foundation of China (no. 81660328), the Project of Jiangxi Provincial Department of Science and Technology (no. 220181BAB205060).
The authors declare no conflict of interest.