2020 Volume 43 Issue 8 Pages 1159-1171
2020 Volume 43 Issue 8 Pages 1159-1171
Regulating synaptic formation and transmission is critical for the physiology and pathology of psychiatric disorders. The adenosine A2A receptor subtype has attracted widespread attention as a key regulator of neuropsychiatric activity, neuroprotection and injury. In this study, we systematically investigated the regulatory effects of a novel A2A receptor agonist, PSB-0777, on the expression of synaptic proteins and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors (AMPA receptors) at the cellular level in a time- and dose-dependent manner. After 30 min of high-dose PSB-0777 stimulation, the expression of Synapsin-1 (Syn-1), postsynaptic density protein 95 (PSD95), and AMPA receptors and the number of synapses were rapidly and significantly increased in rat primary cortical neurons compared with the control. Sustained elevation was found in the low and medium-dose groups after 24 h and 3 d of treatment. In contrast, after stimulation with PSB-0777 for 3 consecutive days, the expression of Syn-1 was decreased, and PSD95, AMPA receptors and the number of synapses were no longer increased in the high-dose group. Our study focuses on the detailed and systematic regulation of synaptic proteins and AMPA receptors by an A2A receptor agonist, PSB-0777, which may result in both beneficial and detrimental effects on neurotransmission and neuroprotection and may contribute to the pathophysiology of psychiatric disorders related to A2A receptors. These experimental data may contribute to understanding of the mechanisms for neuroprotective and therapeutic effect of A2A receptor agonists.
Adenosine and its different types of adenosine receptors are closely related to various physiological effects and play important roles in maintaining the steady state of the nervous system.1,2) Adenosine receptors are divided into four subtypes: A1, A2A, A2B, and A3 Receptors.3) Under different adenosine concentrations and different physiological or pathological conditions, different receptor subtypes are activated. Under physiological conditions, adenosine can activate the higher affinity A1 and A2A receptors, whereas under conditions of pathology (such as hypoxia/ischemia), the adenosine concentration becomes elevated and can stimulate the lower affinity A3 and A2B receptors. Adenosine mostly operates through the inhibitory function of A1 receptor and facilitation function of A2A receptors.4) In the central nervous system (CNS), A2A receptors are mainly distributed in areas involved in the control of motivation, executive functioning or learning and memory such as the striatum, hippocampus and cortex, and they have been believe to play a crucial role in many physiological processes.5–7) Through regulating the release of neurotransmitters and the action of neuromodulators, A2A receptors can affect the action of synapses, neuroinflammation, and homeostasis in the nervous system.4) They may be involved in the pathophysiology of neurological and psychiatric diseases such as Alzheimer’s disease and mental disorders.8) It has been reported that A2A receptors can play a neuroprotective role by regulating brain derived neurotrophic factor (BDNF).9) Due to the important regulatory role of the A2A receptor, various promising A2A receptor agonists have been developed and evaluated therapeutic applications in animal models for mental disease. Therapeutic use of A2A receptor agonists has been suggested for autism-spectrum disorders (ASDs) and schizophrenia (SCZ).10)
PSB-0777 is a novel A2A receptor agonist, which was originally reported by El-Tayeb et al.11) and colleagues as a promising drug for the treatment of inflammatory intestinal diseases and expected to be devoid of cardiovascular adverse effects. Antonioli et al.12) also demonstrated that the A2A receptor agonist PSB-0777 may serve as a novel anti-inflammatory drug, attenuating injury and relieving inflammation. The results showed that PSB-0777 is a potent agonist of A2A receptor and has few off-target activities.11–14) Carlin et al. found that PSB-0777 caused dose-dependent hypothermia and hypoactivity, which was abolished in A2A receptor knockout mice.14) Another study showed that PSB-0777 is an inhibitor of ADP-induced platelet aggregation when acting alone and is non-cytotoxic to cells, thus indicating a potential role of A2A receptors for therapeutic use in cardioprotection.15) The effects and mechanism of A2A receptor in the CNS are still vague and remain controversial.4) It may play different roles in different brain regions. PSB-0777 is a novel A2A receptor agonist with good selectivity for the A2A receptor, making it a good tool for molecular research investigating A2A receptor and potentially promising prospects in related therapeutic applications.
Numerous studies have demonstrated that synaptic formation, transmission and function exert significant roles in neuronal function and that synaptic dysfunction contributes to several psychiatric disorders.16–18) Synaptic dysfunction include the loss of glutamate receptors, shrinkage and simplification of dendrites, reduction of spine synapse density, and diminished glutamatergic neurotransmission in many brain regions including prefrontal cortex and hippocampus.19–21) A2A receptors can modulate synaptic activity by regulating glutamate and dopamine neurotransmission or interacting with signalling molecules such as BDNF.22,23) By regulating neuromodulator, activation of A2A receptors can regulate the recruitment and functions of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors (AMPA receptors) and N-methyl-D-aspartic acid receptor (NMDA receptors) and further induce and regulate long-term potentiation (LTP) in hippocampus.24–27)
Various lines of evidence suggest that changes in Synapsin-1 (Syn-1), postsynaptic density protein 95 (PSD95) and AMPA receptors are closely related to synaptic formation, synaptic plasticity and synaptic transmission maintenance.28,29) As previously reported, enhancement of GluR1 Ser845 phosphorylation may facilitate excitatory transmission by anchoring the AMPA subunit GluR1 to excitatory synapses.30,31) And research has revealed that PSD95 can accompany AMPA receptors and help to refine the temporal and spatial modulation of their expression and function.32) In mature synapses, overexpression of PSD95 can promote dendrite maturation and regulate synaptic plasticity.33) Syn-1, PSD95 and AMPA receptors are associated with ASDs via synaptic pathways,34) but sufficient research on how A2A receptor agonists regulate these synaptic proteins is lacking. In addition, as a subtype of vesicular glutamate transporters (Vgluts), Vglut1 contributes to the probability of exocytotic release of glutamate in excitatory neurons, which is closely related to neuronal excitability and potential plasticity.35,36) However, further studies are required to determine the effects of A2A receptor agonists on Vglut1 expression.
Although the physiological and behavioural correlates of adenosine A2A receptor signalling have been widely studied using a combination of pharmacological and genetic tools, the detailed effects of A2A receptor agonists on synapse formation, transmission and function in cortical neuron still lack thorough and systematical research. Regarding these issues, no systematic and comprehensive studies have examined the influence of A2A receptor agonists on synaptic formation and transmission at different times and with different dose courses at the cellular level. Therefore, we designed a series of experiments to systematically investigate the regulatory effects of the novel A2A receptor agonist PSB-0777 on synaptic proteins (Syn-1, PSD95, Vglut1) and AMPA receptors, which are necessary and crucial for synaptic formation and transmission in cultured primary cerebral cortical neurons. By applying different treatments of PSB-0777 (10, 20, or 100 nM) for very acute (30 min), sub-acute (24 h) and chronic (3 consecutive days) durations and employing Western blotting and immunocytochemistry techniques, we explored the effects of PSB-0777 on synapse formation and transmission and whether this synaptic regulation induced by PSB-0777 was time- and dose-dependent.
All experimental procedures involving animals were carried out according to the Guide for the Care and Use of Laboratory Animals (international standard book number (ISBN) 0-309-05377-3) and were approved by the Institutional Animal Care and Use Committee of the School of Medicine, Yunnan University. Timed pregnant Sprague-Dawley rats were obtained from Kunming Medical University (Kunming, Yunnan, China).
Cell Culture PreparationNeuronal primary cortical cultures were prepared according to previous reports with minor modifications.37) In brief, pregnant rats were anaesthetized on the 17th or 18th embryonic day (E17 and E18) with diethyl ether. Foetuses were extracted, and cortical tissue was dissected in Hank’s balanced salt solution (Gibco, Carlsbad, CA, U.S.A.). The dissected tissue was prepared by digestion with 0.125% trypsin (Gibco). Then, the cells were counted and seeded at appropriate densities on poly-D-lysine (PDL) precoated multiwell plates in plating medium [Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) with 10% foetal bovine serum (Gibco)] for 24 h. After one day in vitro (1 DIV), the plating medium was replaced with serum-free Neurobasal medium (Gibco) supplemented with B27 (Invitrogen, Carlsbad, CA, U.S.A.) and Glutamax (Gibco). The cultures were kept in a 5% CO2 atmosphere and 95% humidity incubator maintained at 37°C. Subsequent half medium replacement was performed every 3 d.
Drug TreatmentAt 10 DIV, cultured neurons were treated with the adenosine receptor A2A agonist PSB-0777 (TOCRIS Bioscience, R&D, U.S.A.). The cultures were randomly divided into control groups and PSB0777 experimental groups. The experimental groups were treated with low (10 nM), medium (20 nM) and high (100 nM) doses of PSB-0777 for 30 min (acute), 24 h (sub-acute) or 3 consecutive days (long-term). The experimental concentrations of the adenosine receptor A2A agonist PSB-0777 were determined according to previous studies.9,38) To confirm the effect of A2A receptor on synaptic proteins, an antagonist of A2A receptor (50 nM of ZM241385) was pre-treated 1 h before PSB-0777 treatment.9)
Western BlottingRat primary cortical neuron cells were harvested after drug treatment, and proteins were extracted via the addition of ice-cold RIPA buffer (Beyotime, Beijing, China) containing protease inhibitor (Roche Applied Science, Penzberg, Germany) and phosphatase inhibitor (Roche Applied Science). After quantification, the samples were denatured and electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, CA, U.S.A.) and then blotted onto nitrocellulose membranes (Millipore, MA, U.S.A.) for immunoblotting. Following blocking [1 h at room temperature in Tris-buffered saline containing 0.125% Tween-20 (TBS-T) and 1% bovine serum albumin (Sigma-Aldrich)], the membranes were probed with primary antibodies in TBS-T buffer containing 5% bovine serum albumin at 4°C overnight. The following antibodies were used: A2A receptor (1 : 1000, Abcam, U.K.), Synapsin-1 (1 : 2000, Abcam), PSD95 (1 : 1000, Cell Signaling Technology, U.S.A.), GluR1 (1 : 500, Santa Cruz, U.S.A.), GluR2 (1 : 500, Santa Cruz), GluR1 ser845 (1 : 1000, Abcam) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used as a loading control (1 : 5000, Thermo Fisher, U.S.A.). Membranes were washed three times with TBS-T, followed by incubation with horseradish peroxidase-conjugated donkey anti-rabbit (1 : 1000, Invitrogen) or goat anti-mouse (1 : 5000, Thermo Fisher) secondary antibodies at room temperature for 1 h. After three washes with TBS-T, the resulting protein bands were visualized using an enhanced chemiluminescence (ECL) system (Millipore). For quantification, the optical density of each band was analysed using Image-Pro Plus Version 6.0 software (Media Cybernetics, U.S.A.).
Immunofluorescence AnalysisE17-E18 pregnant rats were sacrificed to obtain neuronal primary cortical cultures. At 10–12 DIV, neurons on coverslips were treated with the A2A receptor agonist. After treatment, coverslips with growing cells in 24-well plates were briefly rinsed with phosphate buffered saline (PBS). The neurons growing on the coverslips were fixed in 4% (w/v) paraformaldehyde (PFA) for 20 min at room temperature and then blocked with 5% normal goat serum and 0.4% Triton X-100 in PBS for 1 h at room temperature. Subsequently, diluted primary antibodies from different species were added dropwise to the neurons on the coverslips. Following incubation at 4°C overnight, the coverslips were washed with PBS-0.04% Tween-20 (PBST), and the residual solution was blotted with absorbent papers. Neurons were then reacted with diluted Alexa 488- (1 : 300, Jackson Labs, U.S.A.) and Alexa 594- (1 : 300, Jackson Labs) conjugated secondary antibodies for 1 h at room temperature, followed by three washes. The cells on the coverslips were mounted on glass slides with the anti-fade mounting medium containing 4′-6-diamidino-2-phenylindole (DAPI) (Beyotime), and images were acquired immediately using a laser-scanning confocal microscope (FV i100; Olympus, Tokyo, Japan). All images were obtained under the same conditions, including the laser output strength, exposure time, and gain. Neurons were randomly photographed, and five or more images were captured for each coverslip.
The primary antibodies used were as follows: anti-A2A (1 : 100, Abcam), anti-PSD95 (1 : 100, Cell Signaling Technology), anti-Vglut1 (1 : 100, Thermo Fisher), and anti-AMPA GluR1 (1 : 100, Cell Signaling Technology).
Data and Statistical AnalysisObservers were blinded to the grouping and experimental design during data collection and analysis. Statistical analyses were performed with SPSS 21.0 software (SPSS, Inc., Chicago, IL, U.S.A.). All results are expressed as the mean ± standard error of the mean (S.E.M.). All data were evaluated using one-way ANOVA, and significant differences between groups were analysed using the least significant difference (LSD) post hoc test for Western blot analysis and the post hoc Tukey test for immunofluorescence analysis. The level of significance was set at p < 0.05, and statistical significance was defined as * p < 0.05, ** p < 0.01, or *** p < 0.001 for the post hoc LSD test and #p < 0.05, ##p < 0.01, or ### p < 0.001 for the post hoc Tukey test.
We first tested the effect of the A2A receptor agonist on A2A receptor expression. We used PSB-0777 ammonium salt, which is a potent adenosine A2A receptor agonist that exhibits subtype selectivity for A2A receptors over A1, A2B, and A3 receptors.11,13,14) The rat cultured primary cortical neurons were treated with low (10 nM), medium (20 nM), or high (100 nM) doses of PSB-0777 for 30 min (acute), 24 h (sub-acute) or 3 consecutive days (long-term), and the expression level of A2A receptors was determined by Western blotting and immunofluorescence analysis.
The results of the Western blot analyses showed that after 30 min, A2A receptor (medium dose: 280.1 ± 66.7%, p < 0.05; high dose: 478.7 ± 170.5%, p < 0.001) expression levels were rapidly increased in the medium- and high-dose PSB-0777 groups compared with the control group (Figs. 1A, B). Treatment with PSB-0777 for 24 h markedly augmented A2A receptor levels (low dose: 166.5 ± 35.4%, p < 0.01; medium dose: 194.3 ± 31.3%, p < 0.001; high dose: 146.5 ± 20.3%, p < 0.05) (Figs. 1A, C). And 3 d of PSB-0777 treatment (10 and 20 nM) still elevated A2A receptor levels (low dose: 268.8 ± 42.0%, p < 0.001; medium dose: 200.6 ± 31.8%, p < 0.001; Figs. 1A, D).

Primary cultured cortical neurons (10–12 DIV) were treated with a low (10 nM), medium (20 nM) or high (100 nM) dose of PSB-0777 for 30 min, 24 h or 3 d, and then Western blot (WB) analysis of proteins was performed. Data were combined from 5–6 independent experiments and analysed by one-way ANOVA (n = 20–24, post hoc LSD test, * p < 0.05, ** p < 0.01, *** p < 0.001) and presented as the mean ± standard error of the mean (S.E.M.). (A, B) A2A receptors were more intense after 30 min of 20 and 100 nM PSB-0777 exposure, as shown by immunoblotting. (A, C) PSB-0777 increased the expression of A2A receptors at 24 h. (A, D) The expression of A2A receptors increased after 3 d of 10 and 20 nM PSB-0777 treatment.
Next, we performed an immunofluorescence experiment to further verify these effects of PSB-0777. We measured the immunofluorescence staining intensity of A2A receptors (red) using confocal microscopy. The results were similar to the Western blot results. We found that in cortical cultures incubated with PSB-0777 for 30 min, the medium and high doses of PSB-0777 rapidly elevated the A2A receptor fluorescence intensity (medium dose: 219.1 ± 14.8%, p < 0.001; high dose: 280.3 ± 41.2%, p < 0.001) compared with the control group (Figs. 2A, D). Treatment with PSB-0777 for 24 h increased A2A receptor levels (low dose: 233.3 ± 16.6%, p < 0.001; medium dose: 294.9 ± 15.6%, p < 0.001; high dose: 140.7 ± 15.3%, p < 0.01) (Figs. 2B, E), while treatment with 10 and 20 nM PS0777 for 3 d elevated A2A receptor levels (low dose: 213.4 ± 18.6%, p < 0.001; medium dose: 208.7 ± 8.7%, p < 0.001); however, the high dose of PSB-0777 did not increase A2A levels (Figs. 2C, F). These result revealed that PSB-0777 regulated A2A receptor expression in a dose- and time-dependent manner.

Cultured cortical neurons were exposed to PSB-0777 for 30 min, 24 h or 3 d. Immunofluorescence (IF) staining of primary cultured neurons with anti-A2A receptor (red) antibody and DAPI (blue) was performed. Data were combined from 5 independent experiments and analysed by one-way ANOVA (n = 100–105, post hoc Tukey test, #p < 0.05, ##p < 0.01, ###p < 0.001) and presented as the mean ± S.E.M. Scale bars, 5 µm. (A, D) A2A receptors were increased after 30 min of 20 and 100 nM PSB-0777 exposure. (B, E) After 24 h, PSB-0777 increased the expression of A2A receptors. (C, F) The expression of A2A receptors increased after 3 d of low and medium-dose PSB-0777 treatment. (Color figure can be accessed in the online version.)
To determine whether the A2A receptor agonist PSB-0777 could regulate synaptic protein expression, we treated cultured primary cortical neurons with PSB-0777 for 30 min, 24 h or 3 d, as previously mentioned, and the expression levels of Syn-1 and PSD95 were determined by Western blot analysis.
We found that after 30 min of treatment, 100 nM PSB-0777 quickly increased the Syn-1 (208.1 ± 54.5%, p < 0.001) levels; both the medium and high doses of PSB-0777 rapidly elevated PSD95 levels (medium dose: 340.7 ± 168.8%, p < 0.01; high dose: 150.0%, p < 0.001) compared with the control (Figs. 3A, B). In the 24-h treatment groups, both the low and medium doses of PSB-0777 significantly elevated Syn-1 (low dose: 145.5 ± 13.9%, p < 0.05; medium dose: 176.2 ± 30.2%, p < 0.01) and PSD 95 expression levels (low dose: 174.2 ± 30.4%, p < 0.05; medium dose: 202.0 ± 71.7%, p < 0.01), and Syn-1 (161.6 ± 47.6%, p < 0.01) levels were also elevated in the high-dose group (Figs. 3C, D). Furthermore, after stimulation for 3 d, the low and medium dose of PSB-0777 continuously increased the expression of Syn-1 (low dose: 154.1 ± 32.8%, p < 0.01; medium dose: 133.7 ± 25.8%, p < 0.05) and PSD 95 (low dose: 200.7 ± 41%, p < 0.05; medium dose: 216.5 ± 63.5%, p < 0.05), while the high dose of PSB-0777 decreased Syn-1 (63.2 ± 22.3%, p < 0.05) expression (Figs. 3E, F), revealing the dose- and time-dependent regulation of pre- and postsynaptic marker proteins by PSB-0777.

Cultured cortical neurons were treated with PSB-0777 (10, 20 or 100 nM) for 30 min, 24 h or 3 d, or pretreated with 50 nM of ZM241385 (an A2A receptor antagonist) for 1 h before PSB-0777 application. The proteins were then extracted. The levels of the pre-synaptic marker Syn-1 and the post-synaptic marker PSD95 were measured by Western blotting. Data were combined from 5–8 independent experiments and analysed by one-way ANOVA and presented as the mean ± S.E.M. (n = 20–32, post hoc LSD test, * p < 0.05, ** p < 0.01, *** p < 0.001). (A, B) The expression levels of Syn-1 were significantly and rapidly elevated after stimulation with 100 nM PSB-0777 for 30 min; moreover, PSD95 levels quickly increased in both the 20 and 100 nM groups. (C, D) After 24 h of PSB-0777 treatment, the expression of Syn-1 was markedly augmented, and PSD95 levels were also elevated in both the 10 and 20 nM groups. (E, F) The expression levels of Syn-1 and PSD95 were higher after 10 and 20 nM PSB-0777 treatment for 3 d, while the Syn-1 level was decreased in the 100 nM group. (G, H) After 30 min of 100 nM PSB-0777 stimulation, the expression levels of Syn-1 and PSD95 were increased, an effect that was prevented by ZM241385. (I, J) The increased expression levels of Syn-1 and PSD95 by 24 h of treatment with 20 nM PSB-0777 were abolished by ZM241385.
We used ZM241385 (an antagonist of A2A receptor) to determine the specificity of A2A receptor involvement. Pretreatment with ZM241385 prevented the increase in expression of Syn-1 (PSB0777 + ZM241385: 101.4 ± 50.9%, p < 0.01) and PSD95 (PSB0777 + ZM241385: 85.9 ± 61.8%, p < 0.001) induced by 30 min of treatment with 100 nM PSB-0777 (Figs. 3G, H). Similar blockade by ZM241385 was found after 24 h of treatment with 20 nM PSB-0777 (Figs. 3I, J). These findings suggested that PSB-0777 could regulate the expression of Syn-1 and PSD95 via activation of the A2A receptor.
Time- and Dose-Dependent Effects of PSB-0777 on Synapse FormationWe subsequently determined whether PSB0777 could regulate synapse formation. PSD95 is located in the postsynaptic membrane, and it is a key scaffolding protein indicative of the formation of synapses and can be used as a marker of the postsynaptic membrane.39) An immunofluorescence assay was performed with an anti-PSD95 antibody after treatment with PSB-0777 for 30 min, 24 h or 3 d to determine the number of PSD95 clusters per 100 µm2 of dendrites in primary cortical neurons. The recordings showed that the medium and high dose of PSB-0777 rapidly promoted synapse formation (medium dose: 12.3 ± 0.6, p < 0.01; high dose: 13.7 ± 1.1, p < 0.001 compared to the control: 10.2 ± 0.6 synapses/100 µm2) within 30 min (Figs. 4A, B). The enhancement was also observed in the 24-hour PSB-0777 treatment; the low-dose (14.5 ± 1.1, p < 0.01) and medium-dose (16.1 ± 1.8, p < 0.001) groups showed a strong elevation compared with the control group (11.4 ± 1.2; Figs. 4C, D). After long-term PSB-0777 treatment for 3 d, the cluster density of PSD95 was still upregulated in the 10 nM (12.6 ± 1.9, p < 0.05) and 20 nM (14.0 ± 0.7, p < 0.001) groups, but not in the 100 nM group compared with the control (10.1 ± 1.3, Figs. 4E, F). Pretreatment with ZM241385 inhibited the increased cluster density of PSD95 (PSB0777 + ZM241385: 11.4 ± 1.0, p < 0.001 compared to PSB-0777: 15.3 ± 1.4) induced by 24 h of treatment with 20 nM PSB-0777 (Figs. 4G, H).

Cultured cortical neurons were exposed to PSB-0777 for 30 min, 24 h, or 3 d, or pretreated with ZM241385 before PSB-0777 application to determine the specificity of A2A receptor involvement. The cells were then fixed, permeabilized and immunostained with anti-PSD95 (green, 1 : 100) antibodies, and the nuclei were stained with DAPI. Data were combined from 5-6 independent experiments. Scale bars, 5 µm. After normalization to the control, the data were analysed by one-way ANOVA and presented as the mean ± S.E.M. (n = 213–304, post hoc Tukey test, # p < 0.05, ## p < 0.01, ### p < 0.001 vs. control). White arrows highlight PSD95 clusters in a dendrite. (A) After 30 min of treatment with both the medium and high dose of PSB-0777, the cluster density of PSD95 in dendrites was much greater than in the control. (C, E) The cluster density of PSD95 in dendrites was much greater for both the low and medium dose groups after 24 h and 3 d of PSB-0777 treatment. (B, D, F) Quantification of the PSD95 cluster density in dendrites after 30 min, 24 h, and 3 d of PSB-0777 treatment. (G, H) The A2A receptor antagonist ZM241385 inhibited the increased number of PSD95 puncta induced by 24 h of treatment with 20 nM PSB-0777. (Color figure can be accessed in the online version.)
Expression of AMPA receptors is important and necessary for excitatory synaptic neurotransmission,31,40–42) and the enhancement of GluR1 Ser845 phosphorylation can facilitate excitatory transmission.30,43–45) Therefore, we subsequently explored the effects of PSB-0777 on AMPA receptor expression. We treated cultured primary cortical neurons with low, medium and high doses of PSB-0777 as previously described, and the levels of the AMPA receptor subunits GluR1 and GluR2 and phosphorylation of GluR1 at Ser845 were determined by Western blot analysis.
The data showed that, after acute treatment with a high dose of PSB-0777, GluR1 (319.3 ± 80.7%, p < 0.01), Phos-GluR1 Ser845 (268.7 ± 97%, p < 0.01) and GluR2 (229.5 ± 34.7%, p < 0.001) expression levels were prominently augmented (Figs. 5A, B). After 24 h, the low and medium doses of PSB-0777 significantly enhanced the expression of Phos-GluR1 Ser845 (low dose: 188.9 ± 38.0%, p < 0.05; medium dose: 300.7 ± 94.3%, p < 0.001) and GluR2 (low dose: 213.7 ± 55.1%, p < 0.01; medium dose: 207.9 ± 66.9%, p < 0.001); GluR1 expression levels were also significantly increased (medium dose: 263.6 ± 67.5%, p < 0.01; high dose: 215.4 ± 98.7%, p < 0.05) in the medium and high-dose groups (Figs. 5C, D). Similar increases in GluR1 (low dose: 188.7 ± 33.9%, p < 0.01; medium dose: 209.0 ± 42.0%, p < 0.01), Phos-GluR1 Ser845 (low dose: 168.5 ± 48%, p < 0.05; medium dose: 172.5 ± 68.9%, p < 0.05) and GluR2 (low dose: 168.8 ± 40.6%, p < 0.05; medium dose: 176.2 ± 45.0%, p < 0.01) levels were observed after 3 consecutive days of low and medium doses of PSB-0777 stimulation compared with the control (Figs. 5E, F). Additionally, the Phos-GluR1 Ser845 and GluR2 levels were decreased in the high-dose group, although no significant difference was found. We used ZM241385 to confirm the involvement of A2A receptor. After 24 h of treatment with 20 nM PSB-0777, the expression levels of AMPA receptors were elevated, and this effect was abolished by ZM241385 (for GluR1-Ser845, PSB0777 + ZM241385: 115.6 ± 28.4%, p < 0.001; for GluR1, PSB0777 + ZM241385: 116.9 ± 50.3%, p < 0.05; for GluR2, PSB0777 + ZM241385: 88.4 ± 39.9%, p < 0.01, Figs. 5G, H).

Cortical neurons were treated with low, medium and high doses of PSB-0777 for 30 min, 24 h, or 3 d, or pretreated with ZM241385 before PSB-0777 application. The proteins were then extracted for Western blot analysis. GluR1, GluR2 and Phos-GluR1 Ser845 expression levels were determined. Data are expressed as the percentage of the control from 4–7 independent experiments and were analysed by one-way ANOVA and presented as the mean ± S.E.M. (n = 16–28, post hoc LSD test, * p < 0.05, ** p < 0.01, *** p < 0.001). (A, B) The expression levels of GluR1, Phos-GluR1 Ser845 and GluR2 were significantly augmented after high-dose PSB-0777 treatment for 30 min. (C, D) The expression levels of GluR1 were enhanced after 24 h of exposure to a medium or high-dose PSB-0777. Simultaneously, Phos-GluR1 Ser845 and GluR2 expression levels were substantially enhanced after exposure to both low and medium-dose PSB0777 for 24 h compared with the control. (E, F) After 3 d of PSB-0777 treatment, the expression levels of GluR1, Phos-GluR1 Ser845 and GluR2 were still upregulated in the low and medium-dose groups compared with the control. (G, H) After 24 h of 20 nM PSB-0777 stimulation, the expression levels of GluR1, Phos-GluR1 Ser845 and GluR2 were increased, an effect that was prevented by ZM241385.
Previous studies have shown that enhancement of the AMPA subunit GluR1, which anchors the receptor at excitatory synapses, may facilitate excitatory transmission and neuronal function.30,31) We performed double immunofluorescence labelling of GluR1 and PSD95 to explore the formation of AMPA-containing synapses by PSB-0777 treatment. The data showed that the medium and high dose of PSB-0777 elevated double-stained AMPA GluR1-containing synapses (medium dose: 168.2 ± 23.0%, p < 0.05; high dose: 297.7 ± 54.4%, p < 0.01) in primary cortical neurons at 30 min (Figs. 6A, B). Similarly, after 24 h of PSB-0777 exposure, AMPA-containing synapses (low dose: 171.0 ± 18.8%, p < 0.01; medium dose: 215.3 ± 32%, p < 0.001; high dose: 182.9 ± 35.0%, p < 0.05) were increased compared with the control (Figs. 6C, D). In addition, these increases could last up to 3 d for low- and medium-dose PSB-0777 stimulation (low dose: 236.3 ± 76.2%, p < 0.01; medium dose: 234.3 ± 27.1%, p < 0.01; Figs. 6E, F). And ZM241385 inhibited these increases induced by 24 h of treatment with 20 nM PSB-0777 (Figs. 6G, H). These results demonstrated that the A2A receptor agonist PSB-0777 facilitated the transfer of the AMPA subunit GluR1 to synapses, which might regulate synaptic transmission and function depending on the time and dose.

Double immunostaining was performed on cultured cortical neurons with anti-PSD95 (green) and anti-GluR1 (red) antibodies after PSB-0777 exposure. Data were combined from 5–6 independent experiments, analysed by one-way ANOVA and presented as the mean ± S.E.M. (n = 100–120, post hoc Tukey test, # p < 0.05, ## p < 0.01, ### p < 0.001). Yellow arrows indicate GluR1-containing synapses (GluR1 + PSD95 puncta), and white arrows indicate normal synapses. Scale bars, 5 µm. (A) After 30 min of exposure to the medium and high dose and (C) after 24 h of PSB-0777 treatment, the number of GluR1-positve synapses was strikingly augmented, (E) and this effect was increased after 3 d of low- and medium-dose PSB-0777 treatment. (B, D, F) Quantitative analysis of GluR1-containing synapses in cortical neurons after 30 min, 24 h and 3 d of PSB-0777 treatment. (G, H) ZM241385 inhibited the increased GluR1-positve synapses induced by 24 h of treatment with 20 nM PSB-0777. (Color figure can be accessed in the online version.)
Previous studies have illuminated a crucial role of excitatory glutamate synapses in synaptic plasticity and synaptic transmission.46) Altered glutamate signalling is related to some psychiatric disorders,47) and the A2A receptor acts as a neuroregulator that can mediate the glutamatergic signalling pathway.24) Thus, we continued to explore the formation of glutamatergic synapses by double immunofluorescence staining with anti-Vglut1 (an excitatory glutamatergic neuronal marker)/anti-PSD95 antibodies after treatment with PSB-0777 for 30 min, 24 h, or 3 d. Quantitative analysis of colocalized puncta of Vglut1/PSD95 revealed an elevation of Vglut1-positive glutamatergic synapses in a time- and dose-dependent manner. The data showed that the medium and high doses of PSB-0777 significantly augmented Vglut1-positive glutamatergic synapses (medium dose: 198.1 ± 23.0%, p < 0.01; high dose: 307.8 ± 36.4%, p < 0.001) in primary cortical neurons at 30 min (Figs. 7A, 7B). After 24 h of 10 nM and 20 nM PSB-0777 exposure, the Vglut1/PSD95 (low dose: 209.2 ± 22.1%, p < 0.001; medium dose: 359.1 ± 25.7%, p < 0.001) positive colocalization markedly increased (Figs. 7C, D). After 3 d of incubation with 10 nM and 20 nM PSB-0777, the Vglut1-positive glutamatergic synapses (low dose: 215 ± 16.1%, p < 0.001; medium dose: 267.2 ± 17.0%, p < 0.001) continued to increase (Figs. 7E, F). However, the high dose of PSB-0777 did not further increase Vglut-positive glutamatergic synapses.
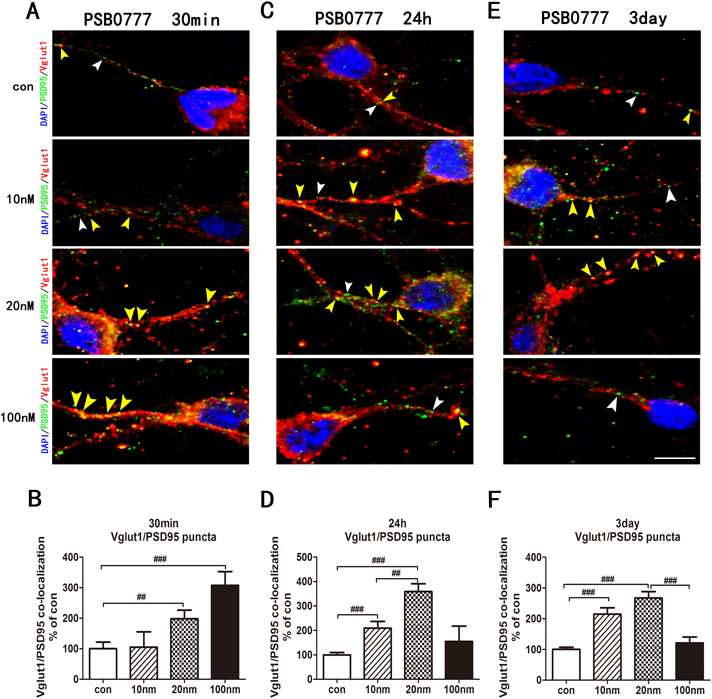
Double immunofluorescence staining was performed on cultured cortical neurons with anti-Vglut1 (red)/anti-PSD95 (green) antibodies after PSB-0777 (10, 20, 100 nM) exposure. Data were combined from 5 independent experiments, analysed by one-way ANOVA and presented as the mean ± S.E.M. (n = 107–112, post hoc Tukey test, # p < 0.05, ## p < 0.01, ### p < 0.001). Yellow arrows indicate Vglut1 localized in glutamatergic synapses (Vglut1 + PSD95 puncta), and white arrows indicate normal synapses. Scale bars, 5 µm. (A) The number of Vglut1-positive glutamatergic synapses was upregulated in the medium- and high-dose groups after 30 min of PSB-0777 stimulation. (C, E) A similar enhancement was found in both the low- and high-dose groups after 24 h and 3 d of A2A agonist stimulation. (B, D, F) Quantification of Vglut1-positive glutamatergic synapses after 30 min, 24 h and 3 d of PSB-0777 treatment. (Color figure can be accessed in the online version.)
Due to its wide distribution, the adenosine receptor is involved in multiple and complex regulatory mechanisms, as well as a large variety of physiologic and pathologic events. Adenosine agonist treatment has been shown to have good therapeutic application prospects.48) For example, A1 receptor agonists are involved in the control of asthma, neuropathic pain, metabolic diseases and inflammations49,50); A2A receptor agonists are suggested for therapy in ASDs, SCZ, diabetic neuropathic pain, wound healing and inflammation.10,48,50) A2B receptor agonists are under preclinical scrutiny for potential treatment of cardiac ischemia.51) The A3 receptor may have potential utility for cancer therapy.50,51) Over the past decades, studies have shown that adenosine receptors and their agonists may play crucial intermediary roles by regulating the release of neurotransmitters and the action of neuromodulators to control the viability of synapses, neuroinflammation, mitochondrial function, and cytoskeletal dynamics.4) Regardless, the role of adenosine receptor and the involved regulatory mechanism of their agonists in the CNS are large unknown and await further investigation. PSB-0777 is a novel A2A receptor agonist that exhibits good selectivity for A2A receptor, which has been proposed as a novel anti-inflammatory drug. This is the first study to demonstrate that the novel A2A receptor agonist PSB0777 has a regulatory effect on synapse-associated proteins.
In this study, we used a primary cortical neuronal culture system to observe the synaptic proteins and AMPA receptors that are related to synaptic formation, transmission and function. After acute (30 min), sub-acute (24 h), and long-term (chronic, 3 consecutive days) treatment with an A2A receptor agonist PSB-0777, we found the following: 1) After acute treatment (30 min) with a high dose of PSB-0777, a rapid and significant increase in Syn-1, PSD95, GluR1, GluR1 ser845, and GluR2 levels occurred. Moreover, high-dose PSB-0777 treatment augmented the formation of glutamatergic synapses in primary cortical neurons. 2) Similar results were found for sub-acute (24 h) treatment, for which a medium dose of PSB-0777 significantly enhanced Syn-1, PSD95, GluR1, GluR1 ser845, and GluR2 expression. Additionally, the effects on glutamatergic synapses showed a similar upregulation. 3) After treatment for 3 consecutive (long-term) days, low and medium-dose PSB-0777 still elevated the expression of these synaptic proteins and receptors. A high dose of PSB-0777 decreased Syn-1 expression. The elevation of PSD95, GluR1, GluR1 ser845, and GluR2 levels in the high-dose groups, as well as the increased formation of glutamatergic synapses, were not sustained. 4) These regulatory effects of PSB-0777 on synapse-associated proteins were abolished by the A2A receptor antagonist ZM241385, which indicated that as a novel A2A receptor agonist, PSB0777 indeed exerts it effects through activation of the A2A receptor. In summary, PSB-0777 regulated the expression of synapse-associated proteins in a time- and dose-specific manner and further influenced synaptic formation, transmission and activity (Figs. 1–7).
The A2A Receptor Agonist PSB-0777 Modulates the Expression of Syn-1 and PSD95 in a Time- and Dose-Specific MannerActivation of the A2A receptor exerts diverse effects on multiple cellular processes depending on its concentration and treatment duration. The A2A receptor has different effects in different cells and domains of the nervous system. Thus, activation of the A2A receptor in the CNS may has a complex effect of neuroprotection or aggravation of nerve injury.52–54) The action of A2A receptors modulates neuronal activity by regulating synaptic transmission and plasticity,22,55,56) and synaptic dysfunction is closely related to the pathophysiology of several mental disorders, including ASDs, schizophrenia, and post-traumatic stress disorder.57–60) However, relevant studies are still incomplete, the involved mechanisms and molecules are largely unknown. Syn-1 promotes a rapid aggregation of synaptic vesicles (SV) and directly affects SV stability, the aggregation state and the regulation of SV pools within nerve terminals, and further regulate the neurotransmitter release.61) Syn-1 play a crucial role in plasticity regulation of synapse, and it recognized as the synaptic markers and reflects the density and distribution of synapses.62) PSD95 is necessary for the activity and stability of synapses. It is involved in regulating the number of synapses and the formation of synaptic connections, to maintain the synaptic plasticity.63,64) Both Syn-1 and PSD95 are closely related to the occurrence, development and treatment of psychiatric disorders. ASDs represent a synaptic defect disease, and the deficiency of Syn-1 and PSD95 is associated with ASD development.34) Here we focused on the use of cultured primary cortical neurons to investigate the detailed impact of PSB-0777 on the expression of synaptic proteins and the time- and dose-specific effects. We found that treatment with PSB-0777 for a short and medium duration (30 min, 24 h) significantly enhanced Syn-1 and PSD95 levels as well as the formation of synapses, which could last up to 3 d in response to low- and medium-dose PSB-0777 stimulation (Figs. 3, 4), suggesting a rapid and continuous positive regulation, which has a neuroprotective effect in cortical neurons. In contrast, a high dose of long-term (3 d) exposure to PSB-0777 eliminated synaptic proteins elevation. These regulation effects of PSB-0777 on PSD95 and Syn-1 were inhibited by A2A receptor antagonist. The activation of A2A receptors activate the protein kinase A (PKA), cAMP response element binding protein (CREB)/BDNF/TrkB, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and calcium/calmodulin-dependent protein kinase II (CaMKII)/nuclear factor-kappaB (NFκB) signalling pathway, which may regulate the expression of Syn-1 and PSD95, the specific underlying mechanism requires further research.9,48,65–70)
PSB-0777 Modulates the Expression of AMPA Receptors and Their Synaptic DistributionVarious lines of evidence suggest that changes in the expression, properties and functional postsynaptic abundance of AMPA receptors contribute to two main forms of excitatory synaptic plasticity–LTP and long term depression (LTD.).28,29,71) In addition, AMPAR phosphorylation is an important posttranslational modification that modulates the membrane expression of these receptors, various channel properties, and synaptic plasticity.72) Additionally, AMPA receptor dysfunction is associated with ASDs via synaptic pathways.34) The expression and function of AMPA can be regulated by kinases including PKA, ERK1/2 and CaMKIIα, and activation of the A2A receptor plays a role through the PKA and ERK pathways.48,65–67) We found that the 30-min acute treatment with a high dose of PSB-0777 augmented GluR1, GluR2 and GluR1 Ser845 levels. The formation of GluR1-containing synapses was noticeably increased, indicating a rapid enhancement of AMPA activity at the synapses. A similar elevation was found for the 24-hour (medium dose group) and 3-d (low and medium dose group) treatment (Figs. 5, 6). However, a 3-consecutive-day exposure to a high concentration of PSB-0777 did not elevate AMPA receptor expression or the formation of GluR1-containing synapses. Additionally, these regulatory effects of PSB-0777 were inhibited by an A2A receptor antagonist. PSD95 has been reported to drive the maturation of glutamatergic synapses. Additionally, PSD95 overexpression increases the proportion of AMPAR-expressing synapses and AMPAR-mediated synaptic transmission.33,73,74) Here, we found that after A2A receptor agonist treatment, the expression trends of PSD95 and GluR1 Ser845 were consistent with those of AMPA receptors, indicating that after A2A receptor activation, PSD95 and GluR1 Ser845 might modulate the AMPA receptor expression and synaptic distribution to regulate synaptic function.
PSB-0777 Modulates Excitatory Synaptic TransmissionDisrupted excitatory synapse function is a common underlying neuropathy of neuropsychiatric disorders. Disrupted glutamatergic synapse function has been found in ASD and SCZ.42,47,57,75) Glutamate is the major excitatory transmitter. To maintain synaptic efficacy, recycled SVs are refilled with glutamate by Vgluts. Vglut1 is a predominant subtype of Vgluts. It exhibit pathway-specific and target-specific expression in glutamatergic neural circuits in the CNS.35,76) It is noteworthy that the Vglut1 level is an indicator of glutamatergic neuronal activity, the probability of transmitter release and the potential for plasticity in excitatory neurons.35,36,77,78) The A2A receptor signalling pathway is involved in the regulation of glutamatergic synapses; however, the specific mechanism is still indistinct.4) In this study, we double-stained primary neurons for Vglut1/PSD95 to assess the expression of Vglut1 in glutamatergic excitatory synapses. We found that the A2A receptor agonist PSB-0777 could regulate Vglut-positive excitatory glutamatergic synapses in an inverted U-shaped manner depending on the time and dose (Fig. 7).
A Time- and Dose-Specific Mechanism of PSB-0777 in Neuroregulation and NeuroprotectionA2A receptors play a very complex regulatory role in synapses. Our study indicated that the regulation of PSB-0777 in synapses occurs in a time- and dose-dependent manner. High levels and short-term (30 min) or medium levels and medium-term (24 h) treatment with PSB-0777 rapidly and continuously strengthened synaptic formation and transmission, which indicated a neuroprotective role. This protective mechanism could last up to 3 d (long-term) by treatment with low and medium-dose PSB-0777. However, exposure to high levels for a long time may result in the negative regulation of synaptic formation and transmission. Our results suggested that appropriate dose and time treatment of the novel A2A receptor agonist PSB-0777 had protective effects on cortical neurons and might be helpful to illuminate new therapeutic ideas for related psychiatric disorders such as ASDs and SCZ.
In the current study, we showed that appropriate levels of a novel A2A receptor agonist, PSB-0777, could enhance synaptic proteins and AMPA receptors, but exposure to high levels for a long time attenuated these gains, demonstrating time- and dose-dependent regulation by PSB-0777 in cultured primary cortical neurons. In summary, we systematically characterized the regulatory effects of PSB-0777 on synaptic proteins and AMPA receptors, which are related to synapse formation, synaptic transmission and synaptic activity, specifically glutamatergic synapse formation and regulation in cortical neurons. We elucidated the exact regulation of the A2A receptor agonist PSB-0777 at different concentrations and for different treatment times, revealing a potential relationship between A2A receptors and the regulation of synaptic formation and function. In particular, we focused on primary cortical neurons, a pure research system that precisely and directly illuminates the effects of the A2A receptor agonist on synaptic regulation. The knowledge gained from such studies will be instrumental in understanding the protection or dysfunction potentially exerted by A2A receptors by regulating synaptic formation and function. The results suggest that A2A receptors play a crucial role in neurotransmission, neuroprotection and the pathology of psychiatric disorders. Additionally, appropriate use of the A2A receptor agonist has neuroprotective effects, which may further provide a scientific basis and new ideas for therapy in related psychiatric disorders.
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (Grant No. 81860499) and the Academic New Artist of Distinction for Doctoral Post Graduate in Yunnan Province (C176230200).
The authors declare no conflict of interest.