2021 Volume 44 Issue 10 Pages 1445-1457
2021 Volume 44 Issue 10 Pages 1445-1457
Oxytocin (OXT) —“the love hormone”— has been involved in the anti-depressant activity of some selective serotonin reuptake inhibitors (SSRIs). The exact mechanism underlying the OXT pathway in depression is not fully clear. This study aimed to investigate the effect of OXT analogue, carbetocin (CBT) and the SSRI, escitalopram (ESCIT) on depressive-like behaviors following maternal separation (MS). It is worthy to mention that intranasal CBT has been approved by U.S. Food and Drug Administration (FDA) for Prader–Willi syndrome. Adolescent Wistar albino maternally-separated rats were given CBT, (100 µg/animal/d via inhalation route), and, ESCIT, (20 mg kg−1, per os ( p.o.)) either alone or in combination for 7 d. Repeated 3-h MS demonstrated increased immobility time in forced swim test (FST) and decreased locomotor activity in open field test. MS elevated plasma level of adrenocortico-trophic hormone (ACTH) but notably reduced plasma OXT, with no effect on hippocampal OXT-R expression. Following MS, hippocampal contents of 5-hydroxytryptamine receptors (5HT1A-R), serotonin transporter (SERT) were increased. CBT and ESCIT corrected the behavioral dysfunction in FST and suppressed the high levels of ACTH. Additionally, both treatments boosted OXT level, reduced 5HT1A-R and normalized SERT contents, which reflects increased availability of serotonin. Finally, CBT markedly ameliorated the histopathological damage induced by MS and suppressed the increased glial fibrillary acidic protein. CBT and ESCIT manage depressive-like behavior by positively affecting serotonergic and oxytocinergic systems. Targeting OXT system —using CBT— ameliorated depressive like behaviors induced by maternal separation most probably via enhancing OXT plasma levels, attenuating hormonal ACTH and restoring the expression of hippocampal oxytocin and serotonin mechanisms.
Adverse early-life experience is among the prevailing anxiety disorders and is associated with emotional and behavioral dys-regulation.1) Mental disorders ranging from anxiety to substance abuse first manifested emerge in childhood, and further progress into teenage years and adolescence.2) Exposure to prolonged maternal separation (MS) might have immediate deleterious effects on brain structure and functions or might manifest as a long-term vulnerability to cognitive deficits later in life.3) Acute stress during early life or exposure to high levels of glucocorticoids (GCs) are known to decrease neurogenesis and may re-program brain plasticity, particularly, in the hippocampus.4)
Oxytocin (OXT) —a neuropeptide hormone— is synthesized in the hypothalamus with widely distributed receptors (OXT-Rs) in limbic forebrain areas. It acts as a neurotransmitter and is involved in emotional and affective behavior.5) OXT plays, also, a substantial role in maturation of neurons during prenatal period and throughout life.6) Symptoms of major depressive disorder (MDD), such as social withdrawal, reduced appetite and cognitive impairment, may all reflect central OXT alterations.7) Importantly, OXT dampen behavioral and social stress reactions through modulation of hypothalamic–pituitary–adrenal (HPA) activity via reducing adrenocorticotrophic hormone (ACTH) and cortisol secretion.8) Carbetocin (CBT), is a long-acting synthetic OXT analogue, which is widely used to prevent or treat postpartum hemorrhage.9) Recently, intranasal CBT has been approved by U.S. Food and Drug Administration (FDA) in Prader–Willi Syndrome as it improves hyperphagia and associated behavioral symptoms.10) Primarily, CBT agonizes peripherally expressed OXT-Rs, but also, hypothesized to influence OXT-Rs in the brain.11) Escitalopram (ESCIT), a highly selective serotonin re-uptake inhibitor (SSRI), represents a first-line option in the management of MDD. Its clinical advantage is based on its relatively rapid onset of action, benign side-effect profile and favorable pharmacokinetics as well as cost-effectiveness.12)
It has been reported that depression is associated with reduced OXT.13) Administration of OXT exhibited anxiolytic effects14) and helped to maintain the antidepressant activity of venlafaxine in rats.15) As previously documented, there is a link between 5-hydroxytryptamine (5-HT) and OXT anatomically and functionally where OXT modulate 5-HT release.16) Previous study showed that administration of the SSRI; citalopram resulted in increased plasma OXT level in rats.17) Accordingly, the present study has been designed to explore the potential possibility of repositioning of the already clinically used intranasal form of the OXT agonist —CBT— on the depressive-like behavior following MS representing an early-life adversity. The SSRI —ESCIT— a frequently clinically used antidepressant, was tested in parallel. The present investigation is the first to study the effect of nebulized intranasal CBT on depression. The study has further focused on the contribution of serotonergic and oxytocin receptor mechanisms in the possible antidepressant activity of CBT as well as involvement of oxytocinergic system in the antidepressant effect of ESCIT.
Escitalopram (ESCIT) was a gift from RAMEDA Pharmaceutical, (6th of October, Cairo, Egypt) and carbetocin (CBT) acetate was purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Me-β-cyclodextrin which was used for preparation of CBT inhalation formulation was obtained, also, from Sigma-Aldrich Co. Ethylenediaminetetraacetic acid (EDTA), Arginine, Sodium Chloride, and Chlorobutanol were procured from Lobachemie, India. All other chemicals used were of analytical grade.
ESCIT was given via oral route as it is the route of administration by which it is normally clinically administered. Nebulized intranasal drug delivery system was chosen as to circumvent the blood brain barrier and provide a rapid and non-invasive method to deliver CBT to the brain. ESCIT was freshly prepared before administration by dissolving it in isotonic saline for oral administration. The concentration was adjusted so that each 50 g body weight received 0.5 mL of the orally administered drug preparation. Freshly prepared solutions of CBT were prepared on daily basis. The inhalation formulation was obtained from an intranasal CBT U.S. patency.18) The solution concentration was adjusted so that each 2 mL contains 100 µg of CBT. CBT formulation consisted of the following inactive ingredients: Me-β-cyclodextrin, EDTA, arginine, sodium chloride, and chlorobutanol.
AnimalsSixty-five Adolescent Wistar albino rats, 32 females and 33 males (n = 13, female = 6–7, male = 6–7 per group) weighing between 50–100 g were included in this study. The pups were offspring of 4 male and 8 female rats. Animals were obtained from the animal house of the National Research Centre (Cairo, Egypt). The animals were acclimatized to the animal facility for 1 week. They were housed in standard polypropylene cages and kept under constant experimental conditions (25 ± 2 °C, relative humidity 55 ± 10% and 12-h light-dark cycle). The rats were fed a normal pellet diet and water ad libitum. The animals were kept for at least, one week for adaptation before being subjected to laboratory experiment. The rats were handled according to the recommendation in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH Publication No. 8023, revised 1978). The protocol was approved by the Ethics Committee for Animal Experimentation at Faculty of Pharmacy, Cairo University, Egypt (Permit No. PT 1805).
Maternal Separation (MS)Rat pups were exposed to the stress of MS for a short period of time (3 h/d) for only a short duration of two weeks. Animals were not subjected to any other type of stress. The date of birth of the litter was designated post-natal day (PND) 0. On PND 2, litters were culled to 13 pups per cage, balanced sex was kept within each cage to ensure equal nourishment during the early postnatal period. Pre-weaned rat pups were separated every day from their mothers starting PND 2 to day 15, for a duration of 3 h per day. The mothers were physically removed from their respective cages, while the pups were left undisturbed, that is to minimize the effect of the handling on the pups, with the exception of once weekly cage cleaning. During the separation period, the pups were housed in a different room, to disallow communication with their mothers by use of ultrasonic vocalizations. After the 3 h, the pups were rejoined with their mothers. Thereafter, on PND 16, all the pups were totally separated from their mothers in different cages.19,20)
Nebulization/InhalationA head-only inhalation exposure chamber was designed and tailored to deliver CBT to rats. The dose (0.1 mg/animal)18) was administered using compressor nebulizer (Model: NE-C29, Omron, Japan) through nebulizing 2 mL of the freshly prepared solutions for 5 min.
Experimental DesignRat pups were classified into 5 groups each consisting of 13 rats (balanced sex was nearly kept within cage). Group I: Non-maternally separated (MS) rats served as control. Group II, III, IV and V: Animals were exposed to MS as denoted above. Starting day 16, treatments were started. Group I and II: rats were orally administered isotonic saline as the vehicle. Group III, IV and V: MS rats received ESCIT at a dose of 20 mg/kg; per os (p.o.)21) and CBT at a dose of 0.1 mg/animal/d via inhalation,18) and a combination of the oral ESCIT dose and inhaled CBT dose, respectively. The doses were selected based on the published literature for ESCIT and CBT. All the treatments were sustained for a duration of 7 d and the last dose of any treatment was given 24 h before the behavioral tests (Fig. 1).

CBT; carbetocin, ESCIT; escitalopram.
On the 24th day, the behavioral tests including open field test (OFT) and forced swim test (FST), were performed one after the other. By the end of behavioral examination, blood samples were collected from the retro-orbital sinus for the determination of OXT and ACTH levels. Blood samples were collected between 9 : 30 and 10 : 30 a.m. in pre-cooled EDTA tubes, kept on crushed ice until centrifugation (20 min at 2000 rpm at 2 °C) and the plasma was stored at −20 °C until subsequent biochemical assessment. Following that, each group was further divided into 2 sets, rats were euthanized by cervical dislocation and brains were immediately excised, rinsed with ice-cold saline.
In one set of animals, hippocampi were promptly dissected out, directly chilled and stored at −80 °C. Hippocampi of first set (n = 8 rats, 4 males and 4 females) were homogenized (10% (w/v)) in phosphate-buffered saline (PBS, pH 7.4) for the estimation of the chosen biochemical parameters including 5-hydroxytryptamine receptors (5HT1A-R), serotonin transporter (SERT), OXT-R contents. In the second set (n = 5 rats), the whole brains were fixed in 10% buffered neutral formalin for the histopathological analysis and immunohistochemical examination of glial fibrillary acidic protein (GFAP). Dead animal bodies were frozen till incinerated in the incinerator of the Faculty of Veterinary Medicine, Cairo University.
Behavioral AssessmentOpen Field Test (OFT)OFT was used to measure locomotion and anxious behaviors.22) The open field apparatus which consisted of a wooden square arena (72 × 72 cm), with a 36-cm high, opaque, white walls. The floor was divided by black lines into 16 equal squares (18 × 18 cm). Each rat was removed from its cage, transported individually into the testing room which was isolated from sound and unintentional interruptions. The animal was placed in the left corner of the open field apparatus, facing the center, then the experimenter left the room. To assess the process of habituation to the novelty of the arena, rats were exposed to the apparatus for 2 consecutive days.
The following 2 parameters were assessed and recorded during the 10 min observation period; the ambulation frequency, denoted as, the total number of squares that the animal crossed with all 4 limbs and the rearing frequency, which is, the number of times the animal stood stretched on its hind limbs with or without forelimb support. After the 10-min test, rats were returned to new cage other than the home cages since re-introduction of the animal to the home cage may modify the behavior of other animals not yet tested. The open field was cleaned between each rat using 70% ethyl alcohol and then permitted to dry to avoid odor cues and/or residues left by rats tested earlier. A video camera was fixed on the top of the box to record the movement and behavior of the rats for later off-line analysis.22)
Forced Swim Test (FST)FST, as a behavioral despair test, was used to assess rats’ responsiveness to stress and evaluate the efficacy of antidepressant drugs or new compounds. A transparent, Plexiglass cylindrical water tank (20 × 47 cm) was used during the experiments. It was filled to a depth of 30 cm with fresh water, where at this depth, no pup could touch bottom with its nose above water. Temperature was adjusted using, a glass mercury thermometer at 24 ± 1 °C. During the test, the recording device was placed at least 30 cm above the cylinder to provide clear image of the complete diameter of the cylinder below. Animals in both separated and non-separated conditions underwent 2 forced-swim trials; the pretest and the 5-min test session. The first training session occurred 24 h following cessation of treatment, viz. on day 23 and lasted for 15 min. The swim test was, then, carried out 24 h after the pretest.23)
The test procedures were based on those described for rats. The animal was placed gently and slowly into the water, avoiding its head from being submerged under the water. The immobility time was recorded for each rat, the pup was judged to be immobile when it ceased struggling and remained floating motionless in the water with minimal movements to keep its nose above water and its hind legs spread so as to maintain balance. Immediately following each forced swim test, the pup was moved to a warm cage dried under a heat lamp, before being returned to the home cage. The re-introduction of the tested animal to the home cage was done after all rats remaining in the cage have performed the swim test. The water tank was emptied and refilled with fresh water after each rat.24)
Histopathological ExaminationBrains (n = 5 rats) from control and treated groups were carefully dissected out and fixed in 10% neutral-buffered formalin. Coronal sections were carried out at the level of the frontal cortex and hippocampus areas. In general, the slices were made just rostral to the neuroanatomic region of interest and the rostral faces of each slice were embedded downward in the tissue cassettes so that they were sectioned first. Serial sections within the co-ordinates for the frontal cortex (Bregma 3.72 to 4.68 and Interaural 12.72 to 13.68) and for the ventral hippocampus (Bregma −5.64 to −4.68 and Interaural 3.36 to 4.32) were selected for tissue staining, according to a stereological atlas of the rat brain25) as they contain all the targeted regions of interest. Generally, all coronal sections were routinely processed and stained with hematoxylin and eosin (H&E).26) The stained sections were blindly examined histopathologically using light microscope (Olympus, Germany). The detected lesions in the H&E-stained sections of frontal cortexes and hippocampi of all rats were scored by determination of the percentage of the lesion frequency in five randomly selected microscopic fields per animal using the following score system; 0 = absence of the lesion in all rats of the group, 1 = 1–10%, 2 = 11–25%, 3 = 26–50%, 4 = 51–75%, 5 = over 75%. The individual score for each animal was determined and then the median score for each group was calculated for various histopathological lesions.27)
Quantification of cell density in various hippocampal subdivisions; cornu ammonis (CA) 1–3 subfields, and dentate gyrus (DG), was performed using a computerized microscopic image analyzer attached to full HD microscopic camera (Leica microsystems, Germany). We calculated the cell density at 40x objective magnification per unit area of the microscopic field (18.8913 mm2).
Neuronal Cell CountParaffin sections of different experimental groups were stained by cresyl violet stain according to method described by Bancroft and Gamble.28) The total number of neuronal cells as well as the number of intact cells were all counted in 5 random microscopic fields at power of X40 in the cerebral cortexes using Lieca Qwin 500 Image Analyzer (Leica, Cambridge, England).
ImmunohistochemistryFor detection of GFAP expression in brain tissue, immunohistochemical technique was performed using avidin–biotin peroxidase (Sigma chemicals Co.).29) Briefly after routine deparaffinization of the obtained various brain sections, incubation with GFAP monoclonal antibody (Dako Corp., M0761, Carpenteria, CA, U.S.A.) was allowed, followed by various reagents supplied for avidin–biotin peroxidase technique (Vactastain ABC peroxidase kit, Vector Laboratories, CA, U.S.A.) method for the detection of the antigen–antibody complex. The expression of GFAP was visualized using chromagen 3,3 -diaminobenzidine tetrahydrochloride (DAB, Sigma Chemical Co.). Quantification of GFAP was carried out by measuring the optical density at 5 randomly chosen fields in each section and averaged using image analysis software (Image J, version 1.46a, NIH, Bethesda, MD, U.S.A.). An experienced investigator blinded to samples has performed all histopathological assessments to avoid any bias.
Enzyme-Linked Immunosorbent Assays (ELISAs)Using rat-specific ELISA kits and according to the manufacturer’s instructions, the following parameters were determined in hippocampal samples; 5HT1A-R (Cusabio Life science, Wuhan, Hubei, China), SERT (Cloud-Clone Corp., Katy, TX, U.S.A.), OXT-R (Cloud-Clone Corp.). Estimation of plasma ACTH (Lifespan biosciences Inc., Seattle, WA, U.S.A.) and OXT (Elabscience, Houston, TX, U.S.A.) was, also, performed. The results were expressed as picograms per gram tissue for all the parameters except picograms per milliliter for ACTH and nanograms per milliliter for OXT.
Statistical AnalysisAll data in the study were examined for normality as well as homogeneity of variance using D’Agostino–Pearson and Bartlett’s tests; respectively. Data that met the assumptions for parametric analysis were analyzed using one-way ANOVA followed by the Tukey’s post-hoc test and were expressed as mean (X) ± standard error (S.E.). The non-parametric data were analyzed and compared using either kruskal wallis or kruskal wallis H test followed by the Mann–Whitney U test and represented as either median ± range or median ± interquartile range (IQR). Data were analyzed using IBM Statistical Package for Social Sciences (SPSS), version 25 (IBM Corp., NY, U.S.A.). The level of significance was accepted at p < 0.05. Graphical representation was created using Microsoft Excel® 2013.
As depicted in Figs. 2a–c, MS-rats spent more time immobile (68.15%) in FST and displayed less crossings (5.5%) and rearing (65.25%) in OFT as compared to non-maternally separated rats. ESCIT and CBT effectively reduced the immobility time as compared to MS group by 1.28 and 1.42 folds, respectively. Adding CBT to ESCIT, abated the immobility time by 1.19 folds as compared to ESCIT treatment only. CBT, alone and in combination with ESCIT, restored the ambulation frequency in OFT. On the other hand, treatment with ESCIT solely, failed to bring about statistically significant improvement in the ambulation frequency as compared to the MS model. The rearing frequency was recovered after the administration of all the treatment regimens.
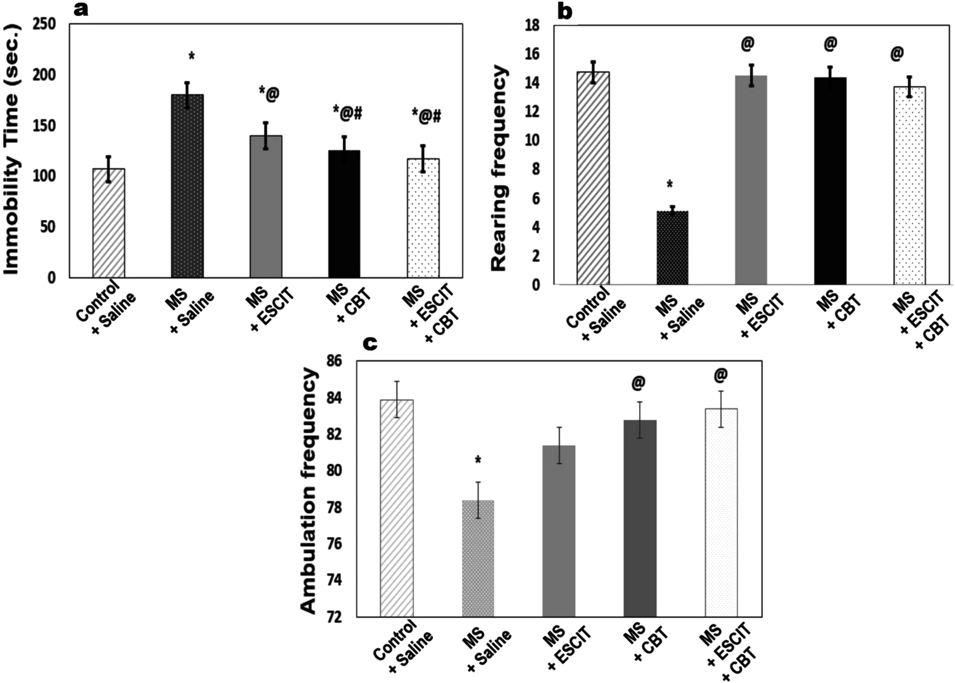
MS; maternal separation, CBT; carbetocin, ESCIT; escitalopram. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as X ± S.E. (n = 8 rats). For a, The F value (4, 35) = 1681.172. For b, the F value (4, 35) = 28.942. For c, the F value (4, 35) = 7.032.
As revealed in Fig. 3a, CBT, ESCIT, either alone or combined, elevated the plasma OXT level that was detected to be decreased (31.38%) following MS. On the other hand, hippocampal content of OXT-R was not affected by MS (Fig. 3b). However, ESCIT altered OXT-R content in MS rats by increasing it by 1.204 folds, in comparison with MS group. There was an unexpected significant elevation in this biomarker after administration of CBT, either alone or in combination with ESCIT, by 118.87 and 147.71%, respectively.

CBT; carbetocin, ESCIT; escitalopram, MS; maternal separation, OXT; oxytocin, OXT-R; oxytocin receptor. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as X ± S.E. (n = 8 rats). For a, The F value (4, 35) = 13.119. For b, the F value (4, 35) = 35.746.
MS-rats experienced stress as evidenced by increased plasma ACTH (136.32%). Nevertheless, CBT and ESCIT hindered this biochemical change by lowering ACTH by 1.54 and 1.43 folds, respectively in comparison to MS group. The combination therapy showed more pronounced amelioration (1.95 folds) of MS-induced elevation in ACTH level as compared to ESCIT-treated group (Fig. 4).

ACTH; Adreno-corticotrophic hormone, CBT; carbetocin, ESCIT; escitalopram, MS; maternal separation. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as X ± S.E. (n = 8 rats). The F value (4, 35) = 54.793.
MS caused notable elevation in hippocampal contents of 5HT1A-R (34.08%) and SERT (59.85%). 5HT1A-R content was corrected after each of ESCIT and CBT, alone by 1.15- and 1.09-folds decrease, respectively, as compared to MS group. The adjunctive therapy of ESCIT and CBT significantly reduced 5HT1A-R by 1.09-fold when compared to ESCIT alone. After the administration of ESCIT and CBT, either alone or combined, there was a sharp decline in SERT content by 1.45, 1.39 and 1.44 folds, respectively (Table 1).
| Parameters/Groups | 5HT1A-R (pg/mg protein) | SERT (pg/mg protein) |
|---|---|---|
| Control + Saline | 149.225 ± 2.43 | 169.587 ± 11.44 |
| MS + Saline | 200.075 ± 1.04* | 271.088 ± 20.21* |
| MS + ESCIT | 173.962 ± 2.20*@ | 186.650 ± 12.82@ |
| MS + CBT | 182.588 ± 4.52*@ | 194.700 ± 13.26@ |
| MS + ESCIT + CBT | 159.263 ± 2.97@# | 187.363 ± 13.41@ |
5HT1A-R; 5-hydroxy tryptamine receptor type 1A, CBT; carbetocin, ESCIT; escitalopram, MS; maternal separation, SERT; serotonin transporter. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as X ± S.E. (n = 8 rats). For a, The F value (4, 35) = 48.095. For b, the F value (4, 35) = 7.463.
Sections of frontal cortexes and hippocampal dentate gyrus (DG) and cornu ammonis subdivisions 1 and 3 (CA1 and CA3) of control non-MS rats showed normal histological structure (Figs. 5a–f). While marked histological alterations were observed in brain sections of MS rats, the cortex showed marked neuronal cells’ degeneration as vacuolar degeneration and necrosis particularly those at layers II. III and IV with neuronophagia and many apoptotic bodies (Figs. 5g, h). Some neurons appeared shrunken and darkly stained with marked proliferation of astrocytes. The hippocampus (Fig. 5i) in those rats showed reduced cell density in the CA1 and CA3, the neurons of which showed nuclear karyorrhexis and necrosis (Figs. 5j, k) with an obvious vacuolation and loss of the pyramidal cell layer of the DG as well as some degeneration and necrosis of the granular cell layer (Fig. 5l). Neuronal degeneration, neuronophagia and many apoptotic bodies were noticed in the molecular layer of the hippocampus.

CA1 and CA3; cornu ammonis subdivisions 1 and 3, DG; dentate gyrus, H&E; hematoxylin and eosin, MS; maternal separation (n = 5 rats)
Concerning the treated groups, it was found that CBT showed the highest restorative effect of the altered brain tissue followed by the combined treatment, while ESCIT treatment exhibited the least restorative effect. All of the observed lesions were scored and summarized in Table 2, a significant reduction in lesions’ frequencies was noticed in all treated groups compared to the untreated MS group.
| Groups | Control + Saline | MS + Saline | MS + ESCIT | MS + CBT | MS + ESCIT + CBT |
|---|---|---|---|---|---|
| Lesions | |||||
| Cerebral cortex | |||||
| Neuronal cells’ degeneration | 0* | 4 ± 3# | 3 ± 3*,@ | 2 ± 3* | 2 ± 3* |
| Neuronal cells’ necrosis | 0* | 4 ± 2# | 3 ± 2*,@ | 2 ± 2* | 2 ± 2* |
| Neuronophagia | 0* | 4 ± 2# | 3 ± 2*,@ | 3 ± 2* | 2 ± 2* |
| Apoptosis | 0* | 4 ± 2# | 4 ± 2# | 2 ± 2*,@ | 2 ± 2@ |
| Reactive astrocytosis | 0* | 4 ± 3# | 4 ± 3*,@ | 3 ± 3* | 2 ± 3* |
| Hippocampus | |||||
| Degeneration and loss of DG pyramidal cells | 0* | 4 ± 3# | 3 ± 3*,@ | 2 ± 3* | 2 ± 3* |
| Nuclear karyorrhexis and necrosis of CA1 and CA3 neurons | 0* | 4 ± 2# | 4 ± 2* | 3 ± 2* | 2 ± 2* |
| Neuronal degeneration, neuronophagia and apoptosis in molecular layer | 0* | 4 ± 2# | 4 ± 2# | 3 ± 2*,@ | 2 ± 2* |
CA1 and CA3; cornu ammonis subdivisions 1 and 3, DG; dentate gyrus, CBT; carbetocin, ESCIT; escitalopram, MS; maternal separation. Statistical analysis was performed using kruskal wallis H test for nonparametric analysis followed by the Mann–Whitney U test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as median ± interquartile range (IQR) (n = 5 rats).
Statistical analysis was performed using kruskal wallis test for nonparametric analysis followed by the Mann–Whitney U test. Data are presented as median ± IQR. Different signs (*, @ and #) within the same column are significantly different at p < 0.05. MS; maternal separation, CBT; carbetocin, ESCIT; escitalopram, DG; dentate gyrus; CA1 and CA3; cornu ammonis subdivisions 1 and 3.
Regarding the frontal cortex of MS rats that treated with CBT, it showed marked restoration of the neuronal cells’ density with scattered degenerated and necrotic cells (Figs. 6a, b). The hippocampus (Fig. 6c) showed increased cellular density in the CA1 and CA3 with scarce degenerated cells and decreased apoptotic cells (Figs. 6d, e) as well as marked restoration of the cell density of the DG with mild vacuolar degeneration and necrosis of few pyramidal cells (Fig. 6f). While rats treated with ESCIT showed moderate degree of neuronal degeneration, and some apoptotic cells and bodies in their frontal cortexes. Some neurons showed vacuolar degeneration with scattered neuronophagia (Figs. 6g, h). The hippocampi (Fig. 6i) of those rats showed mild restoration of the neuronal cells of the CA1 and CA3 with pyknosis of some neurons (Figs. 6j, k), however, neuronophagia of necrotic neurons was observed in the molecular layer. The DG showed moderate degree of cell-density restoration with pyknosis of some neurons, the molecular layer showed scattered neuronophagia and few apoptosis (Fig. 6l). In regards to, the combined treatment group, there was mild to moderate cortical neuronal degenerative and necrotic changes particularly in the pyramidal cells’ layers with scattered apoptotic bodies (Figs. 6m, n). The hippocampal areas (Fig. 6o) revealed moderate decrease in the CA1 and CA3 neuronal densities, scattered apoptotic cells (Figs. 6p, q) and restoration of the DG neurons with scattered apoptotic cells and few neuronophagia (Fig. 6r).

Nebulized CBT (100 µg/animal/d)-treated group showing marked cerebral restoration of the neuronal cells with scattered degenerated and necrotic cells (a, b), increased hippocampal (c) cellular density in the CA1 (d), CA3 (e), and DG (f) with scarce degenerated, necrotic and apoptotic neurons. ESCIT (20 mg/kg)-treated group showing; cerebral moderate degree of neuronal degeneration, some apoptosis and neuronophagia (g, h), mild restoration of the hippocampal (i) neuronal cells of the CA1 (j) and CA3 (k) with scattered degenerated and necrotic neurons, moderate degree of cell-density restoration of the DG (l) with few necrotic cells (m–r). The combined treatment group showing; mild cerebral neuronal degenerative and necrotic changes particularly the pyramidal cells’ layers with scattered apoptosis (m and n), moderate decrease in the hippocampal (o) CA1 (p) and CA3 (q) neuronal densities and good restoration of the DG neurons (r). CA1 and CA3; cornu ammonis subdivisions 1 and 3, CBT; carbetocin, DG; dentate gyrus, ESCIT ; escitalopram, H&E; hematoxylin and eosin (n = 5 rats).
The cell density in various hippocampal subdivisions in MS and various treated groups revealed significant reduction in the cell density in the hippocampal subdivisions’ CA1 (54.3%), CA2 (51.11%), CA3 (56.6%) and DG (58.28%) in the MS rats. CBT increased the cell density by 1.75 folds in CA1, 2, and 3, and by 1.87 folds in DG as compared to MS rats. ESCIT elevated, also, the cell density of CA1, CA2, CA3, and DG by 1.34, 1.3, 1.27, and 1.39, respectively (Fig. 7).

Statistical analysis was performed using kruskal wallis H test for non-parametric analysis followed by the Mann–Whitney U test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as median and range (n = 5 rats).
Microscopic examination of Cresyl Violet stained cerebral sections showed normal morphology of the neuronal cells (Fig. 8a) that appeared with clear vesicular nuclei in the control non-MS rats. While the MS rats showed presence of large number of shrunken darkly stained neurons in their cortexes (Figs. 8b, c). Concerning the treated groups, the use of CBT markedly decreased the number of degenerated and necrotic neurons (Fig. 8d) compared to the effect of ESCIT (Fig. 8e) and their combined treatment (Fig. 8f).

CBT; carbetocin, ESCIT; escitalopram, MS; maternal separation.
The percentage of the intact neurons and the necrotic ones is presented in Table 3; rats of the MS group showed significant (p < 0.05) diminishment in the percentage of intact cells compared to the control non-MS group. Whereas, the treated groups showed variable significant (p < 0.05) elevation of the number of intact neuronal cells particularly in CBT treated group followed by the combination and the sole ESCIT treated groups, respectively compared to the MS group.
| Parameters/Groups | Intact cell count median (Range) | Necrotic cell count median (Range) |
|---|---|---|
| Control + saline | 182.5 (177–188) | 2.5 (0–5) |
| MS + saline | 61.5 (54–69)* | 89.5 (78–101)* |
| MS + ESCIT | 99.5 (89–110)*@ | 44.5 (39–50)*@ |
| MS + CBT | 158.5 (149–168)*@# | 30.5 (27–34)*@# |
| MS + ESCIT + CBT | 107 (99–115)*@ | 41.5 (36–47)*@ |
CBT; carbetocin, ESCIT; escitalopram, MS; maternal separation. Statistical analysis was performed using kruskal wallis test for nonparametric analysis followed by the Mann–Whitney U test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as median and range (n = 5 rats).
Cerebral cortex and hippocampal areas of control rats revealed few normal and few widely apart small sized GFAP positive astrocytes with few short processes (Figs. 9a, b). While, brain sections of MS rats showed marked active astrogliosis expressed by widely spread GFAP reactive astrocytes that appeared large, hypertrophic with multiple elongated processes having spider-like morphology in both cerebral cortex (Fig. 9c) and hippocampus (Fig. 9d). ESCIT treated rats showed moderate decrease in GFAP reactive astrocytes in both cerebral cortex and hippocampus (Figs. 9e, f). While, marked decreased GFAP reactivity was observed in brain sections of CBT treated rats (Figs. 9g, h). The cerebral cortex and hippocampus of the combined treatment group showed moderate decrease in GFAP reactive astrocytes (Figs. 9i, j). The area percent of the positive brown color of GFAP expression (Fig. 9k) in 5 different microscopic fields and presented as optical density revealed significant (P ≤ 0.05) high GFAP expression in brain of MS group compared to that of control and other treated groups.

Control rat (a, b) showing scattered normal few small sized with few short processes GFAP positive astrocytes in cerebral cortex (a) and hippocampus (b). MS rat (c, d) showing strongly positive GFAP hypertrophic spider-like astrocytes with multiple elongated processes in cerebral cortex (c) and hippocampus (d). ESCIT-treated rat (e, f) showing moderate cortical (e) and hippocampal (f) decrease in GFAP reactive astrocytes. CBT-treated rat (g, h) showing marked decreased cortical (g) and hippocampal (h) GFAP immunoreactivity. Combined CBT and ESCIT-treated rats showing moderate decrease in GFAP reactive astrocytes in both cerebral cortexes (e, i) and hippocampi (f, h). The area percent of the positive brown color of GFAP expression presented as optical density (k). CBT; carbetocin, ESCIT; escitalopram, GFAP; Glial fibrillary acidic protein, MS; maternal separation. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05), where * Significantly different from normal control, @ Significantly different from MS group, and # Significantly different from ESCIT-treated group. Values are presented as X ± S.E. (n = 5 rats). The F value (4, 20) = 158.106.
Early life adversity could increase vulnerability to MDD in different age groups. Early intervention would protect against depression and substance abuse in teenagers and adolescents.30) It has been shown that early MS results in elevated stress response with associated behavioral and neuroendocrine signs of depression.31) In the current study, an experimental model of depression was set through MS of infantile rats from their mothers, as an example for early life adversity. OXT —the love hormone— has a major as an endogenous antidepressant hormone and has been reported to mitigate the anxiety of breastfeeding mothers.14,15) The present investigation is the first to document the effect of nebulized intranasal CBT on depressive-like behavior, biochemical, and histopathological hallmarks.
Our results revealed that MS rats exhibited increased immobility time at FST accompanied by decreased ambulation and rearing frequencies in OFT. Previous experimental studies in rats showed MS affected depressive behavior in FST32,33) and motor activity in OFT.34) It has been, also, shown that MS resulted in decreased orienting behavior as indicated by reduced rearing frequency.35) In the current work, both CBT and ESCIT showed reduction of immobility duration in the FST, as well as, restoration of the stress-induced deficits in locomotion and rearing in OFT. Carbetocin (CBT)11,36,37) and ESCIT38) demonstrated antidepressant activity in rodent models of stress and depression. The current results for intranasal CBT represent new finding of its potential anti-depressant effect, particularly on MS.
Results of the present study revealed that following MS, plasma OXT was decreased. OXT was found to be reduced in depressed patients,13,39) in addition to a negative association between plasma OXT levels and depression scores.40) Another study, however, showed contradictory results where an increase in OXT was reported in individuals with MDD.41) Another interesting point in this investigation is the relation between OXT and 5-HT. Our results showed that ESCIT elevated OXT concentration in MS rats. In agreement, administration of SSRI; citalopram resulted in increased plasma OXT level.17) This shed light on the possibility that SSRIs could citalopram —racemate of ESCIT— promote OXT release via stimulation of 5HT1A-R. It has been proved that OXT-stimulated release in 5HT was inhibited by 5HT2A/C-R antagonists.42) Furthermore, OXT has been found to be critically related to 5-HT, anatomically and functionally, where OXT modulate 5-HT release.16) This is supported by reduced depression scores in patients resistant to anti-depressant treatments when intranasal OXT was administered with ESCIT.43) Accordingly, in the current study, CBT caused elevation in plasma OXT level. It has been demonstrated in prenatally restraint stress rats that CBT boosted their memory to recognize a juvenile intruder. Activation of presynaptic OXT-R by CBT via enhanced glutamate release in the hippocampus, explains this social behavior.11) There has been a claim that activation of glutamate receptors stimulates OXT release from hypothalamus.44) This might clarify, in part, the indirect effect of CBT on increasing plasma OXT level.
To further examine the correlation between OXT pathway and MS-induced depression, the present work revealed that MS resulted in no significant effect on the hippocampal expression level of OXT-R. Interestingly, an experimental study showed that MS lowered OXT-R binding resulting in the associated changes in social behaviors.45) According to our findings, ESCIT increased the OXT-R expression in comparison with MS group but was kept within normal levels. There was no data found on the effect of ESCIT on OXT-R. However, it is reported that hyperserotonemia induced by 5HT1A-R agonists cause decrease in OXT centrally in the paraventricular nucleus of the hypothalamus.46) Another compelling finding in our study, is that the use of CBT, either alone or in combination with ESCIT, caused up-regulation of the OXT-R above normal level. It has been also found that CBT, unlike OXT, promotes OXT-R internalization which also negatively influences OXT-R recycling.47) Exposure to OXT leads to desensitization, internalization of the OXT-R, and receptor recycling back to the plasma membrane. This process, in turn, protects cells from overstimulation after prolonged agonist exposure.48) By contrast, after CBT, OXT-Rs remain in intracellular compartments and do not recycle to the plasma membrane. Thus, tolerance from repeated applications of CBT can occur49) which is due to a reduction in functional receptors at the plasma membrane.50) It is worthy to mention that a rebound effect can appear after the discontinuation of anti-depressants, and, in most cases, associated with an up-regulation of receptors.51)
Our work provides a new focus on the link between oxytocinergic system and stress response. The hippocampus expresses a lot of OXT-Rs and OXT secretion is regulated by the same hippocampal projections that regulate HPA activity.52) Increased HPA activity in depression is probably associated with decreased OXT levels in human.14) Consistent with an anti-stress effect, intranasal OXT lower ACTH in monkeys53) and intraperitoneal OXT increased the adult neurogenesis in rats following stress or GC administration thus protects the hippocampus from harmful effects such as neuronal growth inhibition.54)
In the current study, MS rats showed higher plasma ACTH levels than controls which was also confirmed in another study that used MS rats as well.55) The risk of developing MS-induced depressive-like behavior after subsequent chronic stress may be explained via alteration of corticosterone and neurotrophin levels in the hippocampus.33) Therefore, the stress hyper-responsiveness observed in MS rats could be attributed, at least in part, to an impaired feedback sensitivity mediated by hippocampal GC receptors.55) In the present investigation, the use of ESCIT was associated with reduction in ACTH. Citalopram also reduced ACTH precursor in control rats.56) Additionally, a previous clinical study observed that using ESCIT in higher doses reduced HPA-response in MDD.57) ESCIT is known to alter HPA axis by reducing hippocampal corticotrophin-releasing factor in stressed rats.58) Similarly, our result for CBT agrees with a previous study that showed a relieve in HPA response to stress, and decline in the expression of stress-related genes in the hippocampus and amygdala.11)
Brain monoamine 5-HT appears to be down-regulated in the hippocampus of rat pups after repeated MS,59) suggesting counterbalanced regulation between 5-HT and 5HT1A-R, which is found to be up-regulated.60) Moreover, it has been evidenced that increased regional availability of SERT, indicate reduced extracellular 5-HT levels which is found for severe form of depression.61) This comes in agreement with findings of the present work, where the hippocampi of MS group showed notable elevation of 5HT1A-R and SERT. It is well known that ESCIT has highly selective and dose-dependent inhibitory effect on SERT,62) which serve as a support to our data. An increase of 5-HT levels at the synaptic cleft induced by SSRI treatment initiates a negative feedback mechanism for the post-synaptic 5HT1A-R in the hippocampus, which as revealed in the current study, cause the density of 5HT1A-R to reduce, in comparison to the MS group. Subsequently, reduction in 5HT1A-R binding potential after ESCIT treatment may be attributed to the reduction in receptor density. This suggestion was based on the assumption that the receptor binding potential reflects receptor density divided by the distribution constant Kd (Bmax/Kd). This down-regulation could either be as a result of decreased receptor density or through a decreased receptor affinity.62) The present work revealed that 5HT1A-R hippocampal content was corrected after use of CBT, in addition to, a decline in SERT. In agreement with our results, earlier findings show that OXT modulates the serotoninergic system, specifically regulating 5-HT1A network. This suggests that OXT interferes with 5-HT neurotransmission.16) Alternately, administration of 5-HT agonist was documented to cause hyperserotonemia and exhibit a decrease of OXT in the hypothalamus and a form of autistic-like behavior, suggesting the existence of mutual interactions between the 5-HT and OXT systems.46)
To further strengthen our work, in addition to serotoninergic and oxytocinergic-related investigations, we examined the histopathological alterations in response to MS as well as ESCIT and CBT treatments. The histopathological analysis of the present work showed that MS produced shrinkage and decrease in the neuronal cell density in both cerebral cortex and hippocampal granular layer of the DG and pyramidal cells of different areas of CA. In support, reduced hippocampal volume and low cell proliferation were reported in MDD induced in experimental MS rats.63) Moreover, marked neuronal cells’ degeneration and necrosis was observed with MS in the present study which resonate with the notion that early exposure to stress causes hippocampal remodeling and/or irreversible atrophy.64) Our results revealed that ESCIT only moderately improved these histopathological alterations, as similarly observed with another study conducted on rats with vascular dementia.65) On the other hand, our results showed that CBT markedly decreased the neuronal degeneration and increased integrity of neuronal cells. Earlier investigation demonstrated that OXT induced neurogenesis especially in hippocampal CA3 neurons in mice66) and DG in rats subjected to stress.54) Intranasal OXT increased adult neurogenesis in hippocampus of rats exposed to chronic stress possibly through elevating brain-derived neurotrophic factor (BDNF) levels,67) all of which support our findings for CBT-induced neuro-protection.
GFAP, a histopathologic marker of astrogliosis,68) is known to be involved in MDD.69) Astrogliosis, as observed in the current study, accompanied with hypertrophy and spider-like morphology in MS rats confirm results in previous clinical studies.70,71) Such findings support the inflammatory and neuro-degenerative theory of depression72) and elucidates the astrocytic compensatory reaction to neuronal damage in MDD.73) Additionally, evidence from an experimental study suggests an increase in size of GFAP-positive astrocytes in hippocampus of rats exposed to chronic stress.74) In contrast, GFAP expression is shown to be reduced in hippocampus of MS rats.75) ESCIT either alone or combined with CBT only brought about moderate reduction in GFAP expression, while CBT markedly decreased the GFAP reactivity. Interestingly, OXT is known to facilitate neuronal activity by eliciting retraction of astrocyte processes.76) On the other hand, ESCIT has shown to have little influence on stimulated astrocytes in ventral hippocampus and failed to reverse changes in GFAP positive astrocytes.77)
The present work indicated the significance of oxytocinergic system as a crucial factor in the context of MS-induced depressive-like behaviors. Conclusively, the anti-depressant actions of CBT are possibly mediated via mixed oxytocinergic and serotonergic mechanisms in addition to the suppression of the hyper-activated HPA axis and improvement of the histopathological picture. Additionally, the combination of CBT and ESCIT had a synergistic effect during the despair test. The combined treatment, also, showed additive effect on reducing ACTH level and elevating OXT-R. Variations in OXT-R expression levels after CBT administration should be subject to careful future investigation when planning its use in vivo. Further studies are warranted in order to select the best dose and duration of treatment to support the use of CBT in MDD.
The authors are grateful to Dr. Ahmed El-Nabrawy for his inspiration to come up with the first raw idea of this work, and to Khaled Sayed and Tarek Abdel-Hamid, the trained technicians who offered a great assistance with animal handling and behavioral testing and sampling.
The authors declare no conflict of interest.