2021 Volume 44 Issue 10 Pages 1536-1547
2021 Volume 44 Issue 10 Pages 1536-1547
This study aimed to investigate the effect of norisopoldine (NOR) on acute lung injury in septic mice. Lipopolysaccharide (LPS) was used to establish sepsis induced acute lung injury (ALI) in mice. The dry and wet weight of mice lung was detected, and the pathological changes of lung were observed by hematoxylin and eosin (H&E) staining. Bronchoalveolar lavage fluid (BALF) was detected. Inflammatory factors in BALF were detected by enzyme-linked immunosorbent assay (ELISA). The polarization of macrophages in lung tissue was detected by flow cytometry. The markers of M1 and M2 macrophages were detected by RT-PCR. LPS induced RAW264.7 cells were treated with NOR. Inflammatory response, macrophage polarization, glycolysis, and M2 pyruvate kinase (PKM2)/hypoxia inducible factor-1α (HIF-1α)/peroxisome proliferator activated receptor-γ co-activator 1-α (PGC-1α) signaling pathway were detected. NOR could effectively alleviate sepsis induced ALI, and reduce the number of total cells, total protein concentration, neutrophils, macrophages in BALF. NOR decreased the level of inflammatory factors and promoted macrophages from M1 to M2 type in vivo and vitro. Moreover, NOR could activated PKM2, and inhibited PKM2 from cytoplasm to nuclear, attenuated HIF-1α expression, and increased PGC-1α and peroxisome proliferator-activated receptor (PPAR)-γ expression. In addition, NOR inhibited glycolysis and promoted oxidative phosphorylation in RAW264.7 cells. Furthermore, PKM2 inhibitors could reverse the effect of NOR on PKM2/HIF-1α/PGC-1α signaling pathway in RAW264.7 cells. NOR alleviated sepsis induced AIL in mice, inhibited the inflammatory response, promote M2 polarization of macrophages through regulating PKM2/HIF-1α/PGC-1α signaling pathway.
Sepsis is a systemic inflammatory reaction of damaging autologous tissues and organs during defense against bacterial, viral and parasitic infection in organism.1) Sepsis is the chief cause of death in intensive care unit (ICU). Severe sepsis frequently leads to multiple organ dysfunction syndrome (MODS), of which acute lung injury (ALI) is the most common.2) At present, it is believed that ALI is closely related to the uncontrolled inflammatory reaction process.3,4) The infiltration of inflammatory cells and the release of pro-inflammatory mediators play a major role in the development of ALI.5,6) Macrophages, as the first line of defense of lung immune defense, are widely distributed on the surface of large and small airways and alveoli, which are also the most important immune cells in lung tissue.7,8) In sepsis, endotoxic shock and severe trauma, alveolar macrophages are activated, which release abundant inflammatory factors, recruit neutrophil to alveoli, thus continuously expand the inflammatory effect, eventually lead to acute lung injury.9,10) Therefore, based on the pathological mechanism of excessive inflammatory response in sepsis induced ALI, screening drugs that can effectively treat ALI is the current research hotspot.
Macrophages were divided into pro-inflammatory M1 type and anti-inflammatory M2 type. In different tissue microenvironments, macrophages can conduct inflammatory signals through polarization in two directions.11,12) With the stimulation of lipopolysaccharide (LPS), interferon-γ or tumor necrosis factor (TNF)-α, the classical macrophage M1 type can be activated, which secrete a large number of inflammatory factors (TNF-α, interleukin-1β (IL-1β), and IL-6) and cytokines, thus promote the cascade amplification effect of inflammation, ultimately lead to tissue injury. On the other hand, when stimulated with IL-4 and IL-13, macrophages are induced to M2 type, which can produce anti-inflammatory reactions, and promote tissue healing and repair.12) Therefore, we could regulate the inflammatory response in the progression of ALI through modulating the balance of M1 and M2 polarization of alveolar macrophages. Previous studies have reported that in the research of sepsis induced lung injury animal model, multiple drugs or preparations could alleviate and recover the inflammation of organism. Moreover, at the same time, the treatments were often accompanied with the increase of M2 macrophages in tissues.13–16)
Inflammatory cells generally undergo a series of metabolic transitions, such as enhanced glycolysis and inhibited oxidative phosphorylation. Hence, regulating energy metabolism may become a new way to limit inflammatory injury.17,18) M2 pyruvate kinase (PKM2) is one of the rate-limiting enzyme in glycolysis pathway, which can rapidly regulate its metabolic activity by changing its distribution in cytoplasm and nucleus.19,20) In the normal state, PKM2 is mainly distributed in the cytoplasm in the form of tetramer, which plays the role of metabolic kinase activity. In the inflammatory state, PKM2 can be converted into dimer and translocated to the nucleus, which reduces the metabolic kinase activity of PKM2 in the cytoplasm, and enhances glycolysis by enhancing the activity of hypoxia inducible factor-1 (HIF-1).21,22) In monocytes and macrophages of patients with coronary artery disease (CAD), PKM2 dimers were transferred into the nucleus, which activated signal transducer and activator of transcription 3 (STAT3) signaling pathway, promoted glycolysis, oxidative stress, and inflammation, as well as increased M1 polarization of macrophages.23) In LPS induced RAW264.7 macrophages, up-regulation of PKM2 expression can convert metabolism to aerobic glycolysis, resulting in excessive production of lactic acid. Lactate in turn increased the release of early (e.g., TNF-α and IL-1β) and late (e.g., HMGB1) proinflammatory cytokines 2.24) Therefore, PKM2 is a key switch molecule in the regulation of metabolic transition in inflammatory response.
Norisoboldine (NOR) is a kind of isoquinoline alkaloid in Radix Linderae, which shows anti-inflammatory, immunoregulation and other biological activities.25–27) It was reported that NOR promoted Treg differentiation by regulating the AhR/glycolysis axis and the subsequent oxidized form of nicotinamide adenine dinucleotide (NAD+)/SIRT1/SUV39H1/H3K9me3 signaling pathway, thereby reducing the development of colitis.26) It was also reported that NOR could improve collagen-induced arthritis (CIA) in mice by inhibiting mitogen-activated protein kinase (MAPK) activity and activating RAW264.7 cells.28) However, the role of NOR in sepsis ALI has not been studied. Therefore, this study focused on whether NOR can alleviate LPS induced sepsis ALI in mice. Through the experiments, it was found that NOR could improve lung pathological injury, decrease M1 polarization of macrophages, inhibit inflammatory reaction in LPS induced mice and RAW264.7 cells. Interestingly, NOR regulate polarization of macrophages by PKM2/HIF-1α/peroxisome proliferator activated receptor-γ co-activator 1-α (PGC-1α) signaling pathway. This study provides a potential drug candidate for the treatment of sepsis ALI.
Male C57BL6/N mice (6–8 weeks old, 16–20 g) were purchased from Shanghai Lab. Animal Research Center (Shanghai, China) and raised in specific-pathogen-free (SPF) environment. The animals were housed in 23 + 1 °C, humidity of 50%, kept at natural light cycle lighting system (7:00–19:00 light and 19:00–7:00 dark). Food, drinking water and cages were sterilized. The model was established one week after adaptation. In this study, all animal experiments involving mice were carried out according to the standards of Animal Care, and approved by the Animal Research Committee of the First People’s Hospital of Fuyang District.
Sepsis Induced ALI Model and TreatmentNOR (98%) was purchased from AbMole (U.S.A.). Its structure was identified by 1H- and 13C-NMR, and its purity was tested by HPLC. Forty mice were randomly divided into five groups: normal control group, model control group, model group + NOR (10 mg/kg) group, model group + NOR (20 mg/kg) group, model group + NOR (40 mg/kg) group. Sepsis induced ALI model was obtained by intraperitoneal injection of LPS (10 mg/kg) as previously described. The normal control group was treated with the same amount of normal saline. NOR (10, 20, or 40 mg/kg) was administered by intraperitoneal injection, and LPS (10 mg/kg) was injected intraperitoneally 2 h after the administration of NOR. After 24 h of LPS challenge, the mice were euthanized with pentobarbital sodium (120 mg/kg) by intraperitoneal injection. After that, the serum was extracted, bronchoalveolar lavage fluid (BALF) was performed, and lung tissues were carefully extracted.
Determination of Pulmonary EdemaThe moisture on the surface of lung tissues is removed using filter paper. Then, lung tissues were weighed to obtain wet weight. Following that, the lung tissue was put in a 60 °C oven for 72 h, and immediately weighed to get the dry weight. The ratio of wet weight to dry weight was calculated.
Histologic ExaminationThe lung tissue of mice was fixed in 4% polyformaldehyde solution for 24 h. After the conventional paraffin was embedded, 4 µm sections were made. After dewaxing using xylene, the slices were treated with different alcohol concentrations (95, 90, and 85%), and then stained with hematoxylin for 10 min after washed with water. The slices were differentiated with hydrochloric acid alcohol for 5s, and then different concentrations of alcohol (85, 90, 95, 100%) was used for dehydration after washed with water. Following that, the slices were treated with xylene for transparent. Finally, neutral resin is used for seal. The results were observed under an optical microscope (×200, Olympus, Tokyo, Japan).
Bronchoalveolar Lavage Fluid (BALF) DetectionAfter euthanasia, the skin of mice in front of the neck was disinfected with 75% alcohol. An incision was made in front of the neck of mice with sterilized surgical scissors to expose the hyoid muscle group and sternal thyroid muscle on both sides. Then, the trachea was exposed by separating surrounding tissue. The 24Y venous indwelling needle was inserted into the trachea, and then fixed with surgical line. The sterile syringe with 0.5 mL phosphate buffered saline (PBS) (containing 1 mM ethylenediaminetetraacetic acid (EDTA)) was connected with indwelling needle, and slowly aspirated for 5 times. The above operations were repeated for 3 times to obtain 1.4–1.5 mL of BALF. BALF was centrifuged at room temperature (500 × g, 5 min) to collect the precipitated cells, and then cells were resuspended in 100 mL of PBS. The total number of cells was counted by hemocytometer.
Immune cells were observed and counted by Diff-Quik staining (D030-1-1, Nanjing Jiancheng, China). Simply, the cell smears were dried naturally and was fixed at room temperature with R1 reagent. After RI was removed, the sections were infiltrated in R2 reagent for 8 s. After R2 was removed, the sections were stained for 8s with R3 reagent. the staining sections were washed with small flow tap water, dehydrated twice with anhydrous alcohol, and then sealed with neutral resin. Finally, cell types were identified and counted using an optical microscope (Olympus). The concentration of BALF protein was measured with Bicinchoninic Acid (BCA) Protein Assay Kit (Beyotime Biotechnology, Shanghai, China).
Enzyme-Linked Immunosorbent Assay (ELISA) AssayRAW264.7 cells (1 × 106 cells/mL) were seeded in 6 well plate and cultured for 12 h at 37 °C in 5% CO2. Following that, the cells were pretreated with NOR (10, 20, 40 µM) for 2 h, and then stimulated with 10 µg/mL LPS for 24 h. The supernatant was collected. Then the levels of TNF-α, IL-1β, IL-6, and macrophage inflammatory protein (MIP)-2 in the supernatant and BALF were detected by the corresponding ELISA kit (R&D Systems Inc., U.S.A.) following the application manual.
Cell Culture and Cytotoxic AssayMice RAW264.7 cells were obtained from American Type Culture Collection (ATC C, Rockville, MD, U.S.A.). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium (Gibco, U.S.A.) containing 10% fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin, and the culture conditions were as follows: 37 °C and 5% CO2.
Cell cytotoxic was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. RAW264.7 cells with a concentration of 1 × 105 cell/well were seeded in 96 well plate for 12 h at 37 °C in 5% CO2 incubator. After treated with NOR (0, 5, 10, 20, 40, 80, 100 µM), the cells were cultured for 24 h. Following that, the cells were treated with MTT (5 mg/mL, 20 µL/well) for 4 h, and then the supernatant was discarded, and 150 µL dimethyl sulfoxide (DMSO) was added to each well. Finally, the absorbance at 570 nm was measured on a microplate reader (BioTek, VT, U.S.A.).
Cell TreatmentRAW264.7 cells (1 × 106 cells/mL) were seeded in 6 well plate and cultured for 12 h at 37 °C in 5% CO2. Following that, the cells were pretreated with NOR (10, 20, 40 µM) for 2 h, and then stimulated with 1 µg/mL LPS for 24 h. Then, cell function experiments were performed.
Reverse Transcription and Real-Time PCR (RT-PCR)Total RNA was isolated from cultured cells with Trizol Reagent (Life Technology), and reversely transcribed into cDNA using the TaqMan microRNA Reverse Transcription Kit (Life Technology). RT-PCR was performed with the TaqMan Universal PCR Master Mix II (Life Technology) using the 7500 FAST real-time PCR System (Applied Biosystems, Carlsbad, CA, U.S.A.). The PCR primers used in this case were: iNOS (forward 5′-CCG AAG CAA ACA TCA CAT TCA-3′ and reverse 5′-GGT CTA AAG GCT CCG GGC T-3′), IL-12 (forward 5′-GAT GAG CTG ATG CAG GCC-3′ and reverse 5′-AGT CCT CCA CCT CGT TGT CCG TGA-3′), IL-10 (forward 5′-AAC AAG AGC AAG GCC GTG G-3′ and reverse 5′-GAA GAT GTC AAA CTC ACT CAT GGC-3′) and IRF4 (forward 5′-AGT CCC TTA TTC TTT CAC TTC ATT TCC TTC C-3′ and reverse 5′-GGA AGG AAA TGA AGT GAA AGA ATA AGG GAC T-3′). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The primers for GAPDH were forward 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ and reverse 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′.
Western BlottingImmunoblotting was conducted in cultured cells according to the standard procedures as previously described.26) Total proteins were obtained from cells with RIPA Reagent (Sigma). Equal amounts of protein (20 µg) were separated with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which were moved to polyvinylidene difluoride (PVDF) membranes (Millipore). Following that, the membranes were incubated in 5% non-fat milk for 1 h at room temperature, and then treated with the primary antibodies overnight at 4 °C. After washed for 3 times, the membranes were treated with second antibody (1 : 5000, ab150077 or ab150113, Abcam) for 1 h at room temperature. Finally, the protein bands were detected using an ECL detection system (Thermo). The data were analyzed using Image J software (NIH). The antibodies used in this analysis included PKM2 (1 : 1000, ab85555), HIF-1α (1 : 1000, ab15212), PGC-1α (1 : 1000, ab15580), PPARγ (1 : 1000, ab53154), GAPDH (1 : 1000, ab8805), and Lamin B1 (1 : 1000, ab38449), which were purchased from Abcam (Cambridge, U.K.). In addition, GAPDH or Lamin B1 was used as a loading control.
Glucose Uptake AssayRAW264.7 cells were counted at 2 × 106/mL, which were treated with 0.9 mol/L of perchloric acid solution, and then the supernatant was collected by centrifugation (12000 × g, 4 °C, 5 min). After neutralized with KHCO3, the supernatant was again collected by centrifugation (12000 × g, 4 °C, 5 min). According to the applied instruction of glucose assay kit (Sigma), glucose uptake assay was performed. The microplate reader was used for detection at 340 nm.
Lactate Content AssayLactic acid content was detected by L-lactic acid colorimetric test kit (Biovision) referring to the instruction. The supernatant of RAW264.7 cells was taken and centrifuged at 4 °C 12000 × g for 10 min. Above supernatant (50 µL) was added to 96 well plate, and then which was mixed with 50 µL reaction solution and incubated at room temperature for 0.5 h. the microplate reader was used for detection at 450 nm. L-Lactic acid = L-lactic acid content (nmol)/sample volume (µL).
Mitochondrial Membrane Potential AssayThe collected RAW264.7 cells were resuspended with 0.5 mL JC-1 staining solution and incubated for 20 min at 37 °C in a cell incubator. Following that, the cells were washed twice with JC-1 staining buffer (1×), and then the cells were analyzed by flow cytometry (FACScan, BD Biosciences).
Polarization Analysis of MacrophagesThe collected BLAF was plated in 6-well plates. After repeated medium changes, the adherent cells were alveolar macrophages. RAW264.7 cells and alveolar macrophages were collected through centrifuging at 1500 rpm for 5 min at 4 °C, and then resuspended with staining buffer. RAW264.7 cells and alveolar macrophages were stained with anti-CD86-PE and anti-CD163-AF647 for 30 min in the dark at 4 °C. Following that, the cells were permeabilized with fixation/per-meabilization buffer (FcMACS, Nanjing, Jiangsu, China) for 20 min at 4 °C, and then stained with fluorescein isothiocyanate labeled anti-F4/80 (BD Biosciences). The data were acquired on an Attune NxT Acoustic Focusing Cytometer (ThermoFisher, Waltham, MA, U.S.A.) and analyzed with FlowJo software (Tree Star, Ashland, OR, U.S.A.).
Statistical AnalysisOne-way ANOVA was used to analyze difference among groups using Graphpad prism version 7.0 (GraphPad Software, San Diego, CA, U.S.A.), and post hoc test (Tukey) was performed after ANOVA. All results were expressed as mean ± standard deviation (S.D.) and values of p < 0.05 were considered statistically significant. In our experiment, the sample size of each group was set to n = 5. All experiment was repeated for three times
Effect of NOR on lung pathological changes LPS induced acute lung injury in mice. As Fig. 1A, the chemical structure formula of NOR was analyzed by PubChem database (https://pubchem.ncbi.nlm.nih.gov/compound/12313549). The effect of NOR on LPS induced acute lung injury was evaluated. Pulmonary edema was detected by calculating the ratio of wet weight to dry weight of mice. The results showed that the ratio of wet weight to dry weight of mice in the model group was significantly increased compared with the control group, but NOR could significantly reduce the ratio of wet weight to dry weight of mice induced by LPS (Fig. 1B). Pathological changes were observed by HE staining. The results showed that the alveolar structure was intact, and there was no inflammatory cell infiltration in the alveolar septum and alveolar cavity in control group. In the model group, the alveolar septum was broken and a large number of inflammatory cells infiltrated into the alveolar septum and cavity. However, compared with the model group, NOR treatment group showed that alveolar septum was repaired and inflammatory cell infiltration in alveolar septum and alveolar cavity was reduced in a dose-dependent manner (Fig. 1C). These results suggested that NOR administration could significantly improve LPS induced acute lung injury in mice
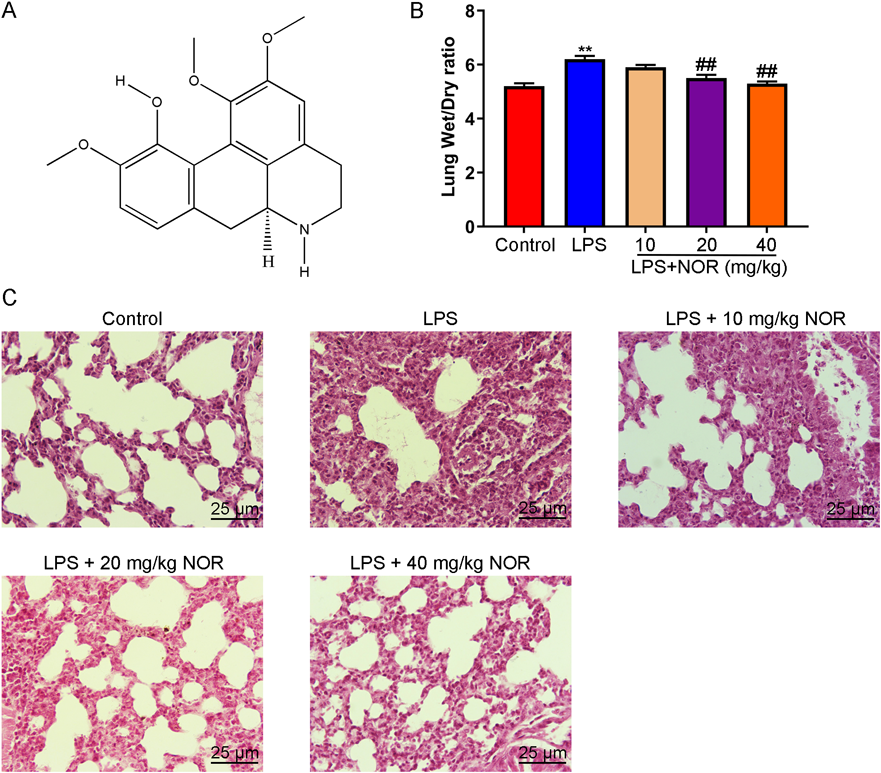
After treated with NOR (10, 20, 40 mg/mL) for 2 h, mice were challenged with LPS for 24 h. (A) The chemical structure formula of NOR was analyzed by PubChem database (https://pubchem.ncbi.nlm.nih.gov/compound/12313549). (B) The wet weight and dry weight ratio of lung tissue were measured. (C) Pathological changes were evaluated by H&E staining (× 200). ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group. (Color figure can be accessed in the online version.)
Next, we studied the effect of NOR on inflammation induced by LPS in mice. The number of total cells, total protein concentration, neutrophils and macrophages in BALF were significantly increased in the model group, but the enhanced effects were inhibited in the NOR treatment group (Figs. 2A–D). Furthermore, the levels of inflammatory factors in BALF were measured. The results showed that the levels of IL-1β, TNF-α, IL-6, and MIP-2 were significantly increased in the model group, which were significantly weakened in the NOR treatment group (Figs. 2E–H). Our results suggested that NOR could inhibit LPS induced inflammation in mice.
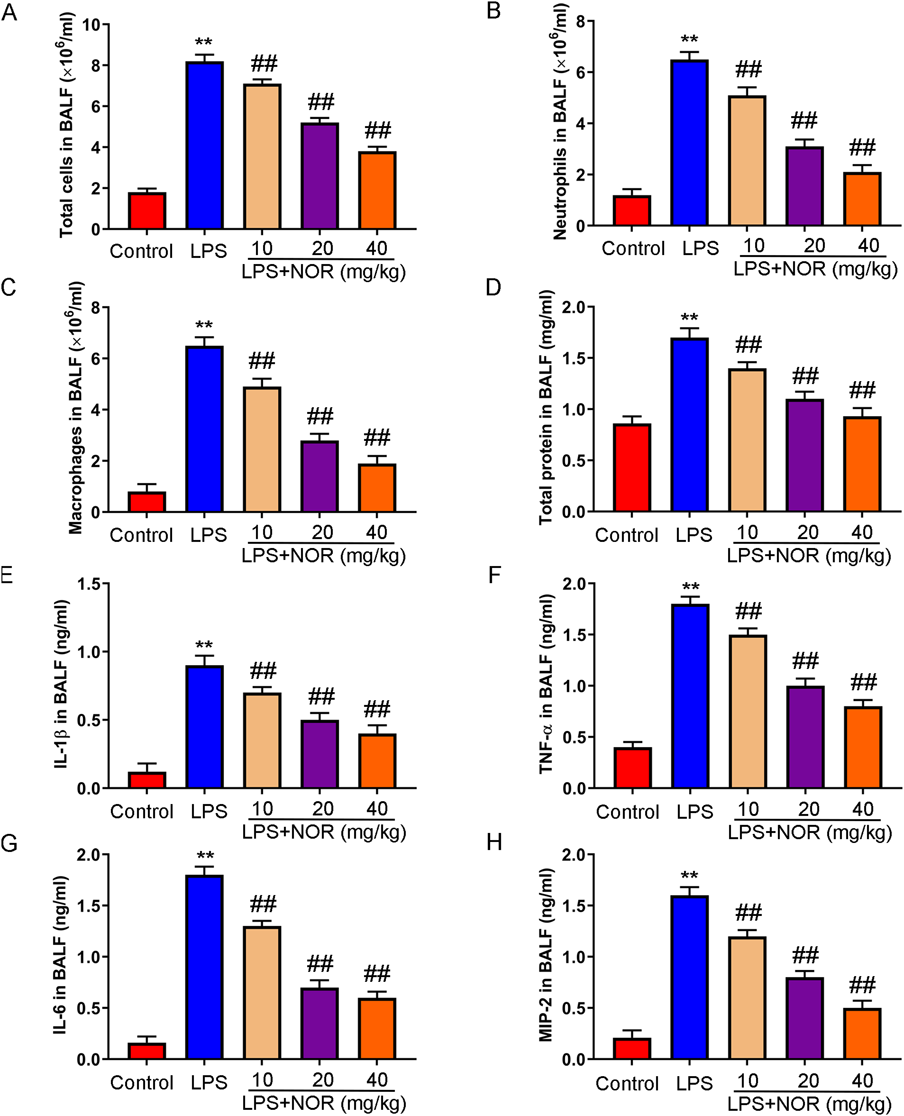
(A–D) The number of total cells, neutrophils, macrophages, and total protein concentration in BALF were detected. (E–H) Inflammatory factors (IL-1β, TNF-α, IL-6, and MIP-2) were assessed by ELISA. ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group. (Color figure can be accessed in the online version.)
Whether NOR affected macrophage polarization was explored. The results showed that CD86, a marker of M1 polarized macrophages, was increased and CD163, a marker of M2 polarized macrophages, was decreased in BLAF in the model group. After NOR treatment, CD86 was inhibited and CD163 was increased compared with model group (Figs. 3A–C). In addition, RT-PCR results showed that NOR could remarkably inhibit the expression of M1 polarization marker gene (inducible nitric oxide synthase (iNOS) and IL-12), and promote the expression of M2 marker gene (IL-10 and IRF4) (Figs. 3D–G). The results showed that NOR promoted M2 polarized macrophages, and inhibited M1 polarized macrophages in LPS induced mice.

(A–C) Macrophage polarization (F4/80+ CD86+ for M1-type macrophages and F4/80+ CD163+ for M2-type macrophages) was detected by flow cytometry. (D–G) Markers of M1-type macrophages (INOS and IL-12) and Markers of M2-type macrophages (IL-10 and IRF4) were measured by RT-PCR. ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group. (Color figure can be accessed in the online version.)
NOR showed no toxicity to macrophages at concentrations up to 40 µM (Fig. 4A). NOR decreased level of IL-1β, TNF-α, IL-6, and MIP-2 in LPS induced RAW264.7 cells (Figs. 4B–E). Moreover, NOR prevented the increase of LPS induced M1 polarized macrophages (CD86). On the contrary, NOR reversed the decrease of M2 polarized macrophages (CD163) induced by LPS (Figs. 5A–C). After LPS challenge, NOR significantly inhibited the expression of iNOS and IL-12, and increased the expression of IL10 and IRF4 (Figs. 5D–G). The results demonstrated that NOR inhibited Macrophage-mediated inflammation in LPS induced mice.
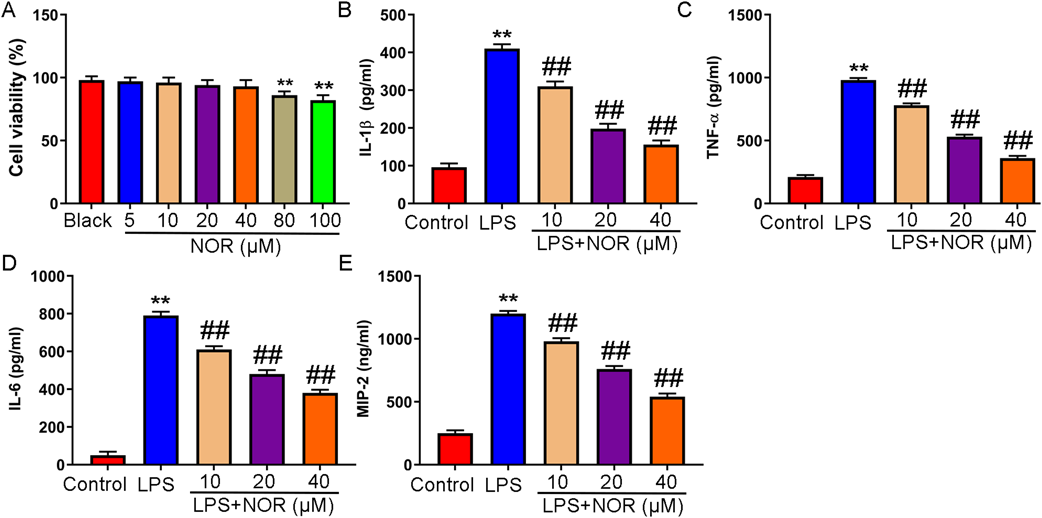
To explore the effect of NOR on macrophages in vitro. The cells were pretreated with NOR (10, 20, 40 µM) for 2 h, and then stimulated with 10 µg/mL LPS for 24 h. (A) The cytotoxicity of NOR was assessed by MTT assay. ** p < 0.01 vs. black group. (B–E) Level of IL-1β, TNF-α, IL-6, and MIP-2 was detected by ELISA. ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group. (Color figure can be accessed in the online version.)
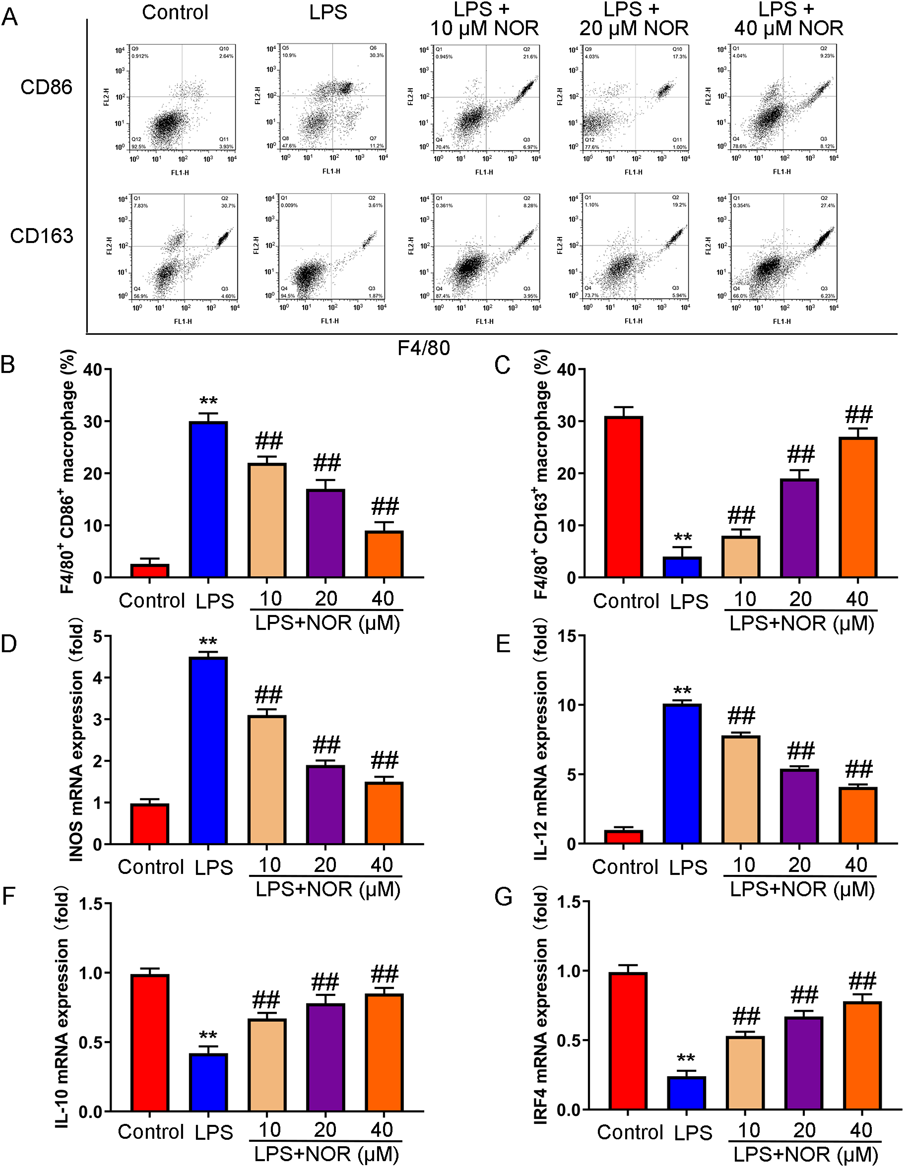
(A–C) Macrophage polarization (F4/80+ CD86+ for M1-type macrophages and F4/80+ CD163+ for M2-type macrophages) was detected by flow cytometry. (D–G) Markers of M1-type macrophages (INOS and IL-12) and Markers of M2-type macrophages (IL-10 and IRF4) were measured by RT-PCR. ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group. (Color figure can be accessed in the online version.)
Because glycolysis is mainly energy metabolism in M1 polarized macrophages, and oxidative phosphorylation is mainly energy metabolism in M1 polarized macrophages, glycolysis (glucose uptake and lactate production) and oxidative phosphorylation (mitochondrial membrane potential) related indicators were assessed. The results showed that glucose uptake, lactate production and mitochondrial membrane potential were significantly increased in LPS induced macrophages. Macrophages were treated with NOR after LPS challenge, glucose uptake lactate production and mitochondrial membrane potential were significantly reduced in a dose-dependent manner (Figs. 6A–C). Besides, NOR dose-dependently decreased the expression of PKM2 in macrophage nucleus and increased the expression of PKM2 in macrophage cytoplasm. Moreover, NOR promoted the expression of HIF-1α and inhibited the expression of PGC-1α and PPAR-γ in a dose-dependent manner (Figs. 6D–K). The results indicated that NOR promoted oxidative phosphorylation, inhibited glycolysis, and activated PKM2/HIF-1α/PGC-1α signaling pathway in LPS induced RAW264.7 cells.
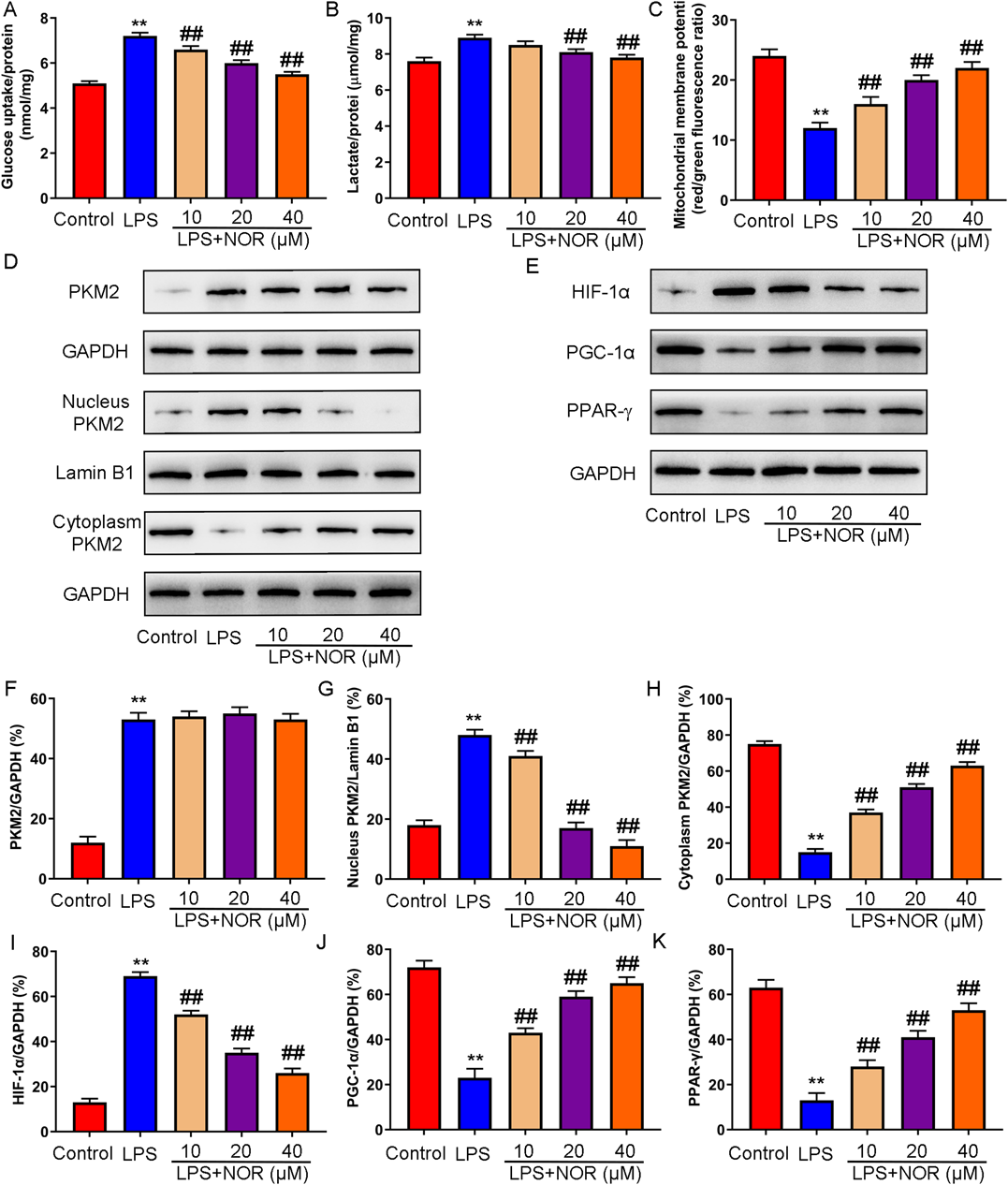
(A–C) Glycolysis (glucose uptake and lactate production) and oxidative phosphorylation (mitochondrial membrane potential) were detected. (D–K) The expression of PKM2 (total, nucleus, and cytoplasm), HIF-1α, PGC-1α, PPAR-γ was measured by Western blot. ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group. (Color figure can be accessed in the online version.)
PKM2 inhibitor compound 3K also prevented the inhibitory effect of NOR on glucose uptake, lactate production and mitochondrial membrane potential in LPS induced macrophages (Figs. 7A–C). Besides, NOR-inhibited PKM2 nuclear transcription was blocked by PKM2 inhibitor compound 3K in LPS induced RAW264.7 cells. Moreover, PKM2 inhibitor compound 3K attenuated the enhancement of NOR on HIF-1α expression and the inhibition of NOR on PGC-1α and PPAR-γ expression (Figs. 7D–L). The data demonstrated that NOR could regulated metabolism of macrophages by activating PKM2/HIF-1α/PGC-1α signaling pathway in RAW264.7 cells.
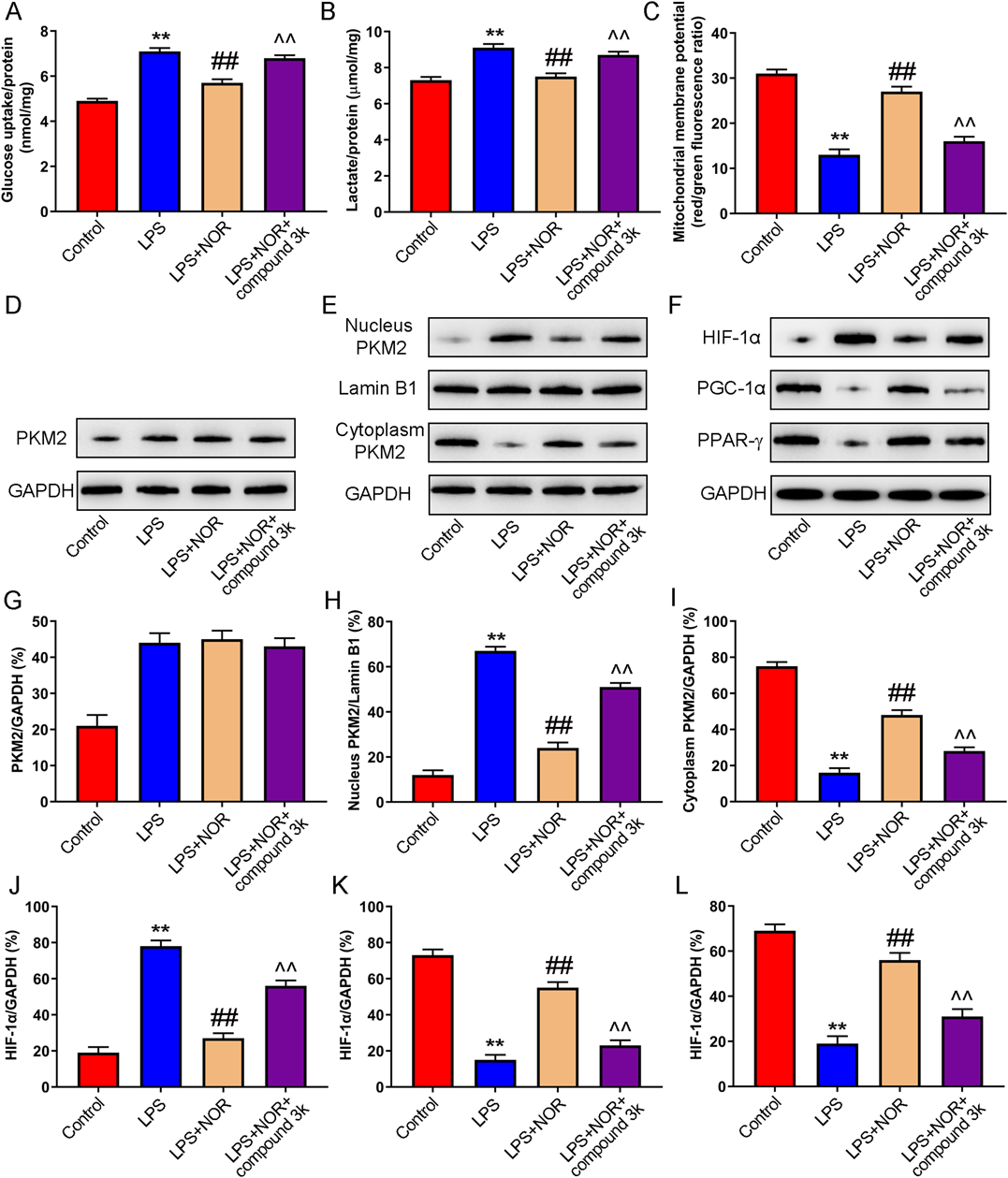
(A–C) Glycolysis (glucose uptake and lactate production) and oxidative phosphorylation (mitochondrial membrane potential) were detected. (D–L) The expression of PKM2 (total, nucleus, and cytoplasm), HIF-1α, PGC-1α, PPAR-γ was measured by Western blot. ** p < 0.01 vs. control group, ## p < 0.01 vs. LPS group, ^^ p < 0.01 vs. LPS + NOR group. (Color figure can be accessed in the online version.)
The purpose of this study was to evaluate whether NOR could alleviate LPS induced ALI by regulating macrophage polarization, and further investigate the underlying mechanism. Our results demonstrated that NOR reduced pulmonary edema and alveolar inflammatory cell infiltration and improved LPS induced ALI. Moreover, NOR decreased glycolysis and M1 polarized macrophages, increased oxidative phosphorylation and M2 polarized macrophages, and inhibit inflammatory response in LPS induced mice and RAW264.7 cells. Furthermore, our findings suggested that the involvement of NOR in macrophage M2 polarization and anti-inflammatory response was related with PKM2/HIF-1α/PGC-1α pathway.
ALI possessed the characteristics of high incidence rate, limited therapeutic effect and high mortality in intensive care unit (ICU). The main mechanism of ALI includes interstitial edema and alveolar collapse, which are caused by imbalance of inflammatory response.29,30) Alveolar macrophages in the alveolar cavity are derived from monocytes with the function of phagocytosis and secretion, forming the body’s first line of defense against foreign pathogens. Alveolar macrophages participate in adjusting the balance of pro-inflammatory and anti-inflammatory and tissue repair.31,32) Macrophages can be divided into pro-inflammatory/cytotoxic M1 type and anti-inflammatory/tissue repair M2 type. In ALI, the number of M1 macrophages in alveoli are increased, which promotes the secretion of a variety of inflammatory factors, such as TNF-a, IL-6, IL-18, IL-12, etc. The secretion of these pro-inflammatory cytokines further aggravates lung injury.33,34) As the main component of the cell wall of Gram-negative bacteria, LPS is one of the most representative inflammatory factors, which can induce M1 polarization of alveolar macrophages, promote the expression of inflammatory related cytokines, and lead to lung injury. It is often used as an experimental model for anti-inflammatory drug screening.35,36)
Accelerated evidences have shown that traditional Chinese medicine and its extracts play an important role in the treatment of inflammatory diseases.37,38) It was found that Tyrosol could improve survival rate, attenuated lung permeability, ameliorated histopathological alterations, reduced expression of the inflammatory mediators and improved expression of the antioxidant enzyme in LPS induced ALI mice.39) Some studies have found that paeoniflorin possessed function of anti-inflammatory, and could inhibit the production of iNOS and IL-6 in RAW264.7 cells induced by LPS.40) Andrographolide could improve ovalbumin induced lung injury in mice by inhibiting the activation of nuclear factor-kappaB (NF-κB) signaling pathway and NLRP3 inflammasome.41) NOR is the main alkaloid in Lindera. Studies have shown that NOR could activate AHR/nuclear factor-E2-related factor 2 (Nrf2) signaling pathway to alleviate trinitrobenzenesulfonate (TNBS) induced colitis in mice.26) It was reported that NOR inhibited the production of pro-inflammatory factors by activating MAPKs signaling pathway in LPS induced RAW264.7 cells.42) However, there is no relevant research on the use of NOR in the treatment of sepsis induced lung injury. In this study, NOR improved lung injury, and reduced the release of LPS induced inflammatory factors in lung tissue, serum and macrophages, and reduced the number of leukocytes, total protein concentration, neutrophils and macrophages in BALF. In addition, NOR could increase of M2 polarized macrophages, reduce M1 polarized macrophages, promote oxidative phosphorylation, and inhibit glycolysis in LPS induced mice and RAW264.7 cells. These results suggested that NOR prevented LPS induced ALI by inhibiting macrophage-mediated inflammatory response.
Type M2 pyruvate kinase (PKM2), a subtype of PK, plays a key role in glycolysis. Some studies have found that PKM2 is highly expressed in M1 macrophages and participates in a variety of inflammatory reactions.43,44) It has been reported that PKM2 interacted with hypoxia inducible factor 1α (HIF-1α) and activated the glycolysis of macrophages, further promoted the metabolism of HMGB1, which regulated the release of inflammatory factors, and then aggravated the mortality of septic mice.45) LPS could induce PKM2 to enter the nucleus, and then form a complex with HIF-1α, which directly promoted the expression of IL-1β and accelerate the inflammatory response.44) PKM2 activators can promote the formation of its tetramer, reduce nuclear transcription, inhibit the production of pro-inflammatory M1-type macrophages induced by LPS, and promote the production of anti-inflammatory M2-type macrophages.46,47) Our data indicated that NOR could inhibit the increase of PKM2 nuclear transcription and HIF-1α expression induced by LPS, which may be the underlying mechanism of activating M2-type macrophages. PGC-1α plays an important role in oxidative metabolism, promoting oxidative phosphorylation and mitochondrial proliferation. PGC-1α plays a variety of physiological functions by combining with PPAR-γ, such as energy metabolism, glucose metabolism, inflammatory response and so on.48–50) Studies have shown that up-regulation of PGC-1α and β could attenuate the energy metabolism disorder and inflammatory response in LPS induced septic heart failure mice.51) Our study showed that NOR could up-regulate the expression of PGC-1α and PPAR-γ in LPS induced mice and RAW264.7 cells. Furthermore, PKM2 inhibitor compound 3K could reverse the effect of NOR on HIF-1α, PGC-1α and PPAR-γ expression, which also attenuated the enhanced effect of NOR on M2 macrophages and oxidative phosphorylation metabolism. The results suggested that NOR inhibited macrophage-mediated inflammatory response in LPS induced lung injury by regulating PKM2/HIF-1α/PGC-1α signaling pathway.
In conclusion, our study demonstrated for the first time that NOR reduced M1 polarized macrophages and increase M2 polarized macrophages through regulating PKM2/HIF-1α/PGC-1α signaling pathway, thereby ameliorated LPS induced ALI. Our data suggested that NOR might be a candidate anti-inflammatory drug for the treatment of inflammatory diseases, such as sepsis induced ALI.
Qi Chen designed and concepted experiments, and prepared, edited, and reviewed the manuscript. Xuebo Shao, Yanyan He, Enkui Lu, Lijun Zhu, and Weidong Tang performed experiments and collected data. Qi Chen and Xuebo Shao analyzed data and drafted manuscript.
The authors declare no conflict of interest.