Abstract
The antidepressant effect of eicosapentaenoic acid-derived bioactive lipid, resolvin E1 (RvE1), was examined in a murine model of chronic pain-induced depression using a tail suspension test. Because RvE1 reportedly possesses agonistic activity on a chemerin receptor ChemR23, we also examined the antidepressant effect of chemerin. Two weeks after surgery for unilateral spared nerve injury to prepare neuropathic pain model mice, immobility time was measured in a tail suspension test. Chronic pain significantly increased immobility time, and this depression-like behavior was attenuated by intracerebroventricular injection of RvE1 (1 ng) or chemerin (500 ng). These results demonstrate that RvE1 exerts an antidepressant effect in a murine model of chronic pain-induced depression, which is likely to be via ChemR23. RvE1 and its receptor may be promising targets to develop novel antidepressants.
INTRODUCTION
Major depressive disorder (MDD) is a psychiatric disorder that imposes heavy personal and socioeconomic burdens.1) Currently, antidepressants that act on monoaminergic nervous systems are used, but these drugs have significant limitations, including delayed onset of treatment response, which increases risk of suicide,2) and relatively low efficacy (about one-third of patients are refractory to treatment).3) Therefore, there is a need to develop rapid-acting and more effective antidepressants. We previously demonstrated that intracerebroventricular (i.c.v.) injections of resolvin E1 (RvE1), a bioactive lipid mediator derived from eicosapentaenoic acid, exert rapid-acting antidepressant effects in a mouse model of lipopolysaccharide (LPS)-induced depression, an inflammation-based mouse model of depression.4) To determine whether RvE1 and its receptors are useful targets to develop novel antidepressants, further studies are needed to examine whether RvE1 exerts antidepressant effects in rodent models of depression that more closely relate to clinical situations. Comorbidity of chronic pain and depression has been well known in the clinic,5) and depression-like behaviors have been observed in animal models of chronic pain.6) Therefore, in this study, we examined the antidepressant effect of RvE1 on depression-like behavior induced by chronic pain.
MATERIALS AND METHODS
AnimalsMale BALB/c mice (Japan SLC, Hamamatsu, Japan) were group-housed (four per cage) under a constant ambient temperature (23 ± 1 °C) and a 12 h light/dark cycle (lights on 07 : 00) with free access to food and water. All experiments were approved by the Institutional Animal Care and Use Committee at Hokkaido University.
DrugsRvE1 was synthesized using organic synthetic methods,7) dissolved in 100% ethanol, and stored at −80 °C. The stock solution of RvE1 was diluted with sterile phosphate-buffered saline (PBS) to a final ethanol concentration of 2% immediately before use while minimizing exposure to light. Recombinant mouse chemerin (R&D Systems, Minneapolis, MN, U.S.A.) was dissolved in PBS containing 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, U.S.A.).
Surgery and von Frey TestMice were subjected to surgery for unilateral spared nerve injury (SNI) to prepare a neuropathic pain model.8) Mice (6 weeks-old) were anesthetized using isoflurane (induction: 3.0%, maintenance: 1.5–2.0%) or chloral hydrate (400 mg/kg, intraperitoneal). The SNI model was prepared by unilateral ligations (left side) of the common peroneal nerve and tibial nerve with 5-0 silk followed by cutting their peripheral sides. In sham-operated control group, the sciatic nerve was exposed, but the common peroneal and tibial nerves were not injured. Thresholds to mechanical stimuli was measured using a von Frey test. Mice were acclimated to the experimental environment by placing on the elevated wire grid for at least 20 min prior to the start of testing. Pressure was applied to the hind paw plantar surface using von Frey filaments of different thicknesses (0.04, 0.07, 0.16, 0.40, 0.60, 1.00, and 2.00 g strength: starting with the 0.16 g filament). The up-down method9) was used to determine the 50% paw withdrawal threshold. When mice showed motor impairment or no tactile allodynia, such mice were not used for the following experiments.
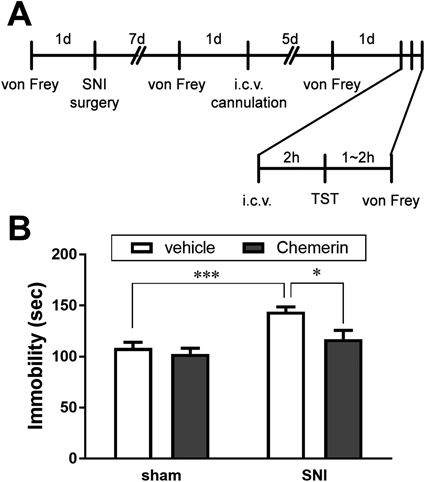
Drug Treatments and Behavioral TestsThe schedule of the experiments is shown in Figs. 1A and 2A. I.c.v. injection was performed as described previously.4) Briefly, RvE1 (1 ng/5 µL), chemerin (500 ng/5 µL), or vehicle (5 µL) unilaterally injected to the lateral ventricle at a rate of 2.5 µL/min using an injection cannula. Two hours after the i.c.v. infusion, we conducted a tail suspension test (TST) to examine the antidepressant effects of RvE1 and chemerin. Immobility time was measured automatically for 6 min using an IR senser-equipped activity monitoring apparatus (SUPERMEX, CompACT FSS software; Muromachi Kikai, Tokyo, Japan). Data from mice that climbed on their tails during the test period were not included in the analyses. Locomotor activity was examined 2 h after i.c.v. injection of RvE1 using an open field test (OFT) as described previously.4) To measure the distance traveled, the movements of mice were recorded on video for 10 min and analyzed using the EthoVision video-tracking system (Noldus Information Technology, Wageningen, The Netherlands). We used different cohorts of mice for the TST and OFT. After the behavioral tests, histological analyses were conducted as described previously.4) The data of mice with incorrect i.c.v. injection were excluded from analyses.
Statistical AnalysesData are presented as means ± standard error of the mean (S.E.M.). The data of the von Frey test were analyzed by Kruskal–Wallis test followed by Dunn's multiple comparisons test. The data of the TST and OFT were analyzed by two-way ANOVA followed by the Holm–Sidak’s post hoc test. GraphPad Prism 7 software (GraphPad Software, La Jolla, CA, U.S.A.) was used for statistical analyses. p < 0.05 was considered indicative of statistical significance.
RESULTS
Antidepressant Effect of RvE1 in a Murine Model of Chronic Pain-Induced DepressionBefore RvE1 injection (2 weeks after the SNI or sham surgery), the pain threshold was significantly lower in the SNI group than the sham group. Mechanical thresholds were 1.690, 1.876, 0.143, and 0.096 g in the sham-vehicle, sham-RvE1, SNI-vehicle and SNI-RvE1 groups, respectively. There was no significant difference between the sham-vehicle and sham-RvE1 groups (1.819 and 1.889 g, respectively) or between the SNI-vehicle and SNI-RvE1 groups (0.084 and 0.083 g, respectively) at 3–4 h after RvE1 injection. In the TST (Fig. 1B), chronic pain significantly increased immobility time (92.8 ± 4.6 s and 133.7 ± 5.3 s in sham-vehicle and SNI-vehicle groups, respectively, p < 0.0001, Holm–Sidak’s post hoc test). The increased immobility time was significantly lowered by i.c.v. injection of RvE1 (133.7 ± 5.3 and 107.1 ± 6.0 s in SNI-vehicle and SNI-RvE1 groups, respectively, p = 0.0027, Holm–Sidak’s post hoc test), whereas RvE1 had no effect on immobility time in sham-operated control mice (92.8 ± 4.6 and 92.6 ± 4.7 s in sham-vehicle and sham-RvE1 groups, respectively, p = 0.9803, Holm–Sidak’s post hoc test). Neither the SNI surgery nor i.c.v. injections of RvE1 affected locomotor activity (Fig. 1C; surgery: F1, 24 = 0.9619, p = 0.3365; treatment: F1, 24 = 1.809, p = 0.1912; interaction: F1, 24 = 2.573e-005, p = 0.9960, n = 6–8), indicating that the differences observed in the TST were not due to locomotor activity alterations.
Antidepressant Effect of Chemerin in a Murine Model of Chronic Pain-Induced DepressionBefore chemerin injection (2 weeks after the SNI or sham surgery), the pain threshold was significantly lower in the SNI group than the sham group. There was no significant difference between the sham-vehicle and sham-chemerin groups (1.874 and 2.000 g, respectively) or between the SNI-vehicle and SNI-chemerin groups (0.040 and 0.051 g, respectively) at 3–4 h after chemerin injection. In the TST (Fig. 2B), chronic pain significantly increased immobility time (108.6 ± 5.5 and 144.1 ± 4.6 s in sham-vehicle and SNI-vehicle groups, respectively, p = 0.0009, Holm–Sidak’s post hoc test). The increased immobility time was significantly lowered by i.c.v. injection of chemerin (144.1 ± 4.6 and 117.2 ± 8.5 s in SNI-vehicle and SNI-chemerin groups, respectively, p = 0.016, Holm–Sidak’s post hoc test), whereas chemerin had no effect on immobility time in sham-operated control mice (108.6 ± 5.5 and 102.8 ± 5.4 s in sham-vehicle and sham-chemerin groups, respectively, p = 0.5419, Holm–Sidak’s post hoc test).
DISCUSSION
We previously demonstrated that i.c.v. injections of RvE1 attenuate depression-like behavior in an LPS-induced mouse model of depression.4) This study using neuropathic pain model mice extends those findings by showing that i.c.v. injections of RvE1 ameliorated the depression-like behavior induced by chronic pain.
RvE1 reportedly possesses agonistic activity on chemerin receptor ChemR23 and antagonistic activity on leukotriene B4 (LTB4) receptor BLT1.10,11) We peviously demonstrated that the ChemR23 agonist chemerin, but not the BLT1 antagonist U75302, attenuated LPS-induced depression-like behaviors.4) Therefore, in the present study, we examined the effect of chemerin in a mouse model of chronic pain-induced depression. The results show the antidepressant-like effect of i.c.v. injection of chemerin, suggesting the possible involvement of ChemR23 in the antidepressant effect of RvE1. Since there are no commercially available selective antagonists for ChemR23, further experiments to examine whether gene knockout or viral-mediated knockdown of ChemR23 would block the antidepressant action of RvE1 are required to elucidate the role of ChemR23 in the antidepressant effects of RvE1.
Xu et al. reported that intrathecal (i.t.) injection of RvE1 reduced inflammatory pain behaviors: preemptive injection (i.t.) of RvE1 at a dose of 1 ng decreased second-phase but not first-phase pain behavior induced by intraplantar formalin injection and i.t. injection of RvE1, given 3 d after intraplantar injection of complete Freund’s adjuvant (CFA), attenuated CFA-induced hyperalgesia in a dose-dependent manner (1–10 ng).12) They also reported that i.t. injection of RvE1 at a dose of 100 ng, given 3 weeks after spinal nerve ligation (SNL), reduced SNL-induced mechanical allodynia and heat hyperalgesia.13) To our knowledge, there are no reports on the anti-allodynic effect of i.c.v. administration of RvE1. In this study, the anti-allodynic effect of i.c.v. administered RvE1 was not observed. However, this study has limitations that most of the animals in the SNI group showed withdrawal responses to the thinnest von Frey filament used in this study (0.04 g) and that the von Frey test was conducted only at one time point after the injection of RvE1. Further studies with von Frey test using thinner filaments (0.02 or 0.008 g) at multiple time points after RvE1 injection are needed for detailed analysis of the anti-allodynic effect of i.c.v. administered RvE1.
Our results demonstrated that RvE1 and chemerin ameliorated chronic-pain-induced depression-like behavior, suggesting that these chemical mediators and their receptors are promising targets to develop novel antidepressants. Further studies are needed to elucidate the cellular and molecular mechanisms underlying the antidepressant effects of these chemical mediators.
Acknowledgments
This work was supported by Japan Agency for Medical Research and Development (AMED) (20gm0910012s0104, 21gm0910012h0004, and JP21zf0127004 to M.M.).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry, 76, 155–162 (2015).
- 2) Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA, 292, 338–343 (2004).
- 3) Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry, 163, 28–40 (2006).
- 4) Deyama S, Shimoda K, Suzuki H, Ishikawa Y, Ishimura K, Fukuda H, Hitora-Imamura N, Ide S, Satoh M, Kaneda K, Shuto S, Minami M. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology (Berl.), 235, 329–336 (2018).
- 5) Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch. Intern. Med., 163, 2433–2445 (2003).
- 6) Yalcin I, Barthas F, Barrot M. Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci. Biobehav. Rev., 47, 154–164 (2014).
- 7) Ishimura K, Fukuda H, Fujiwara K, Muromoto R, Hirashima K, Murakami Y, Watanabe M, Ishihara J, Matsuda T, Shuto S. Synthesis of resolvin E1 and its conformationally restricted cyclopropane congeners with potent anti-inflammatory effect. ACS Med. Chem. Lett., 12, 256–261 (2021).
- 8) Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain, 87, 149–158 (2000).
- 9) Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods, 53, 55–63 (1994).
- 10) Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol., 178, 3912–3917 (2007).
- 11) Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol., 13, 632–640 (2013).
- 12) Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med., 16, 592–597 (2010).
- 13) Xu ZZ, Berta T, Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol., 8, 37–41 (2013).