2021 Volume 44 Issue 4 Pages 515-521
2021 Volume 44 Issue 4 Pages 515-521
In 2017, Leoni et al. reported myticalins as novel cationic linear antimicrobial peptides obtained from marine mussels. The authors focused on myticalin A6 (29 amino acids), which has a relatively short chain length among myticalins and contains a repeating X-proline(Pro)-arginine (Arg) sequence in its structure. We investigated the antimicrobial activity of myticalin A6 against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus (S. aureus). Fragment derivatives of myticalin A6 were synthesized, and the site required for expression of antimicrobial activity was examined. To investigate the structure–antimicrobial activity relationship of myticalin A6, short-chain derivatives and partially substituted derivatives were synthesized, and the antimicrobial activity was measured. Furthermore, some cyclized derivatives were synthesized and examined for antimicrobial activity. Circular dichroism (CD) spectroscopy of myticalin A6 and its derivatives was carried out to evaluate the secondary structure. Myticalin A6 exhibited an antimicrobial activity of 1.9 µM against S. aureus. Myticalin A6 (3–23)-OH (21 amino acids) exhibited an antimicrobial activity of 2.4 µM against S. aureus, suggesting that the X-Pro-Arg repeat sequence is important for antimicrobial activity. Derivatives with different CD measurement results from myticalin A6 (3–23)-OH exhibited decreased activity. The myticalin A6 (3–23)-OH derivative in which all Arg residues were replaced with lysine (Lys) residues exhibited reduced antimicrobial activity against S. aureus. We succeeded in synthesizing cyclic derivatives using 9-fluorenylmethoxycarbonyl (Fmoc)-aspartic acid (Asp)(Wang resin)-[2-phenylisopropyl ester (OPis)], but the yield of derivatives with 21 amino acids was decreased. The myticalin derivatives synthesized in this study did not exhibit any enhancement in antimicrobial activity due to cyclization.
A variety of pathogenic bacteria pose a public health threat in Japan. Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and Enterococci and Gram-negative bacteria such as Pseudomonas aeruginosa (P. aeruginosa) and pathogenic Escherichia coli (E. coli) are well-known pathogens of nosocomial (opportunistic) infections. The control of diseases caused by these bacteria is difficult, and multidrug-resistant bacteria have become a problem worldwide. These infectious diseases are a threat to patients and elderly individuals whose physical strength and immunity are weakened due to disease. As a countermeasure against the outbreak of multidrug-resistant bacteria, the use of many antimicrobial agents is being curtailed, but the need for new antimicrobial agents as a countermeasure against actual diseases is increasing, and the development of new antimicrobial agents is required. Antimicrobial peptides exhibit a broad antimicrobial spectrum, have high selective toxicity to bacteria, and are active against drug-resistant bacteria.1) Due to such advantages, antimicrobial peptides are attracting attention as candidates for the development of new antimicrobial agents against drug-resistant bacteria.
Colistin is a peptide antimicrobial drug developed in Japan, and it is listed in the Japanese Pharmacopoeia. Polymyxin B, an analogue of colistin, is also listed in the Japanese Pharmacopoeia and currently being used medically as an antimicrobial drug. Colistin is commonly used to treat multidrug-resistant bacterial infections for which other antimicrobial agents are not expected to be clinically effective.
The structure of many antimicrobial peptides (magainin 2,2–4) melittin,5) alamethicin,6) etc.) carries a positive charge due to the side chains of basic amino acids and also has a lipophilic site or lipophilic amino acid. There are also reports of antimicrobial peptides containing a large number of arginine (Arg) or proline (Pro) residues in the structure (such as Tur1A7)). Colistin, by comparison, is positively charged and hydrophobic. Colistin binds strongly to the outer membrane of bacteria and exerts antimicrobial activity by displacing calcium and magnesium ions present in the membrane.8–10) In addition, our group has conducted structure–activity relationship studies on the antimicrobial peptides polymyxin B and colistin.11–14)
In 2016, an E. coli strain was isolated that carries the resistance gene mcr-1, which could transmit colistin resistance via a plasmid.15) In 2018, two additional colistin resistance genes, mcr-2 and mcr-3, were reported.16) It is expected that it will soon be difficult to treat drug-resistant bacteria only with conventional antimicrobial drugs, and thus, there is an urgent need to develop new antimicrobial agents that are effective against drug-resistant bacteria.
In 2017, Leoni et al. reported myticalins as novel cationic linear antimicrobial peptides obtained from marine mussels (Mytilus spp.).17) They reported 27 precursor sequences of myticalin: A1–9, B1, C1–10, and D1–7. Among these, seven peptides (myticalin A5, A8, B1, C6, C9, D2, and D5) were chemically synthesized, and their antimicrobial activity was evaluated against six types of bacteria, including E. coli, P. aeruginosa, and Staphylococcus aureus (S. aureus). The authors focused on myticalin A6,17) which has 29 amino acids, a relatively short chain length among myticalins, and also contains a repeating X-Pro-Arg (X = tyrosine (Tyr), tryptophan (Trp), leucine (Leu), isoleucine (Ile)) sequence in its structure. Myticalin A6 was presumed to exert antimicrobial activity due to the positively charged side chains and hydrophobic sites in its structure. Therefore, the authors of the present study selected it as the base antimicrobial peptide for further examination.
In this study, we investigated the antimicrobial activity of myticalin A6 against E. coli, P. aeruginosa, and S. aureus. Fragment derivatives of myticalin A6 were synthesized, and the site required for expression of antimicrobial activity was examined. We synthesized Lys-substituted derivatives of Arg and Tyr-substituted or Trp-substituted derivatives of X of myticalin A6 (3–23)-OH to examine the importance of peptide side chains and their usefulness as model peptides for studies examining repetitive sequences. Furthermore, some cyclized derivatives were synthesized and examined for antimicrobial activity. Circular dichroism (CD) spectroscopy of myticalin A6 and its derivatives was carried out to evaluate the secondary structure.
9-Fluorenylmethoxycarbonyl (Fmoc)-Tyr[t-Butyl (But)]-OH, Fmoc-Ser(But)-OH, Fmoc-Trp[t-Butoxycarbonyl (Boc)]-OH, Fmoc-Pro-OH (Nonhydrate), Fmoc-Arg[2,2,4,6,7-Pentamethyldihydrobenzofuran-5-sulfonyl (Pbf)]-OH, Fmoc-Leu-OH, Fmoc-Ile-OH, Fmoc-histidine (His)(Boc)-OH {Fmoc-His[trityl (Trt)]-OH for cyclic peptide syntheses}, Fmoc-Thr(But)-OH, Fmoc-alanine (Ala)-OH (Nonhydrate), Fmoc-Lys(Boc)-OH, Fmoc-Thr(But)-Wang resin, Fmoc-Arg(Pbf)-Wang resin, Fmoc-Lys(Boc)-Wang resin, N,N-dimethylformamide (DMF) (SP Grade), N,N′-diisopropylcarbodiimide (DIPCI), ethyl cyanoglyoxylate-2-oxime (OxymaPure), piperidine, 2,2,2-trifluoroacetic acid (TFA), triisopropylsilane (TIPS), 2,2,2-trifluoroethanol (TFE), and Fmoc-Asp(Wang resin)-[2-phenylisopropyl ester (OPis)] were obtained from Watanabe Chemical Industries, Ltd. (Hiroshima, Japan). Rink amide ProTide Resin was obtained from CEM Corp. (Matthews, NC, U.S.A.). Acetic acid (AcOH, Guaranteed Reagent), 1-butanol (BuOH, Guaranteed Reagent), ethyl acetate (AcOEt, Guaranteed Reagent), and pyridine (Guaranteed Reagent) were obtained from FUJIFILM Wako (Osaka, Japan). Diethyl ether (Guaranteed Reagent) was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Acetonitrile (special grade), used for HPLC after filtration, was obtained from Kanto Chemical Co. Ltd. (Tokyo, Japan). 1,2-Ethanedithiol was obtained from Alfa Aesar (Lancashire, U.K.). TOYOPEARL HW-40F was obtained from Tosoh Corp. (Tokyo, Japan).
Peptide SynthesisPeptides were synthesized with an automated microwave peptide synthesizer (Liberty Blue, CEM) using Fmoc chemistry. Rink amide resin or Fmoc-amino acid-Wang resins were used at the start of solid-phase synthesis. Piperidine (20%) in DMF was used for Fmoc removal. Fmoc-amino acids were coupled to resin using OxymaPure and DIPCI in DMF to extend the peptide chain. As Fmoc-His(Boc)-OH was used, the reaction temperature was set to 90 °C during His binding. Peptides were cleaved from the resin using TFA : TIPS : dist. water (95 : 2.5 : 2.5). After removing TFA under reduced pressure, diethyl ether precipitates were collected by filtration, dissolved in 50% acetic acid (2% 1,2-ethanedithiol was added for Trp peptide), then purified by C18 reversed phase HPLC. Peptides were obtained after gel filtration (Toyopearl HW-40F), evaporation, and lyophilization, and their molecular weights were determined by matrix-assisted laser desorption/ionization-time-of-flight MS (MALDI-TOF-MS). Peptide purity was assessed by analytical C18 HPLC and TLC.
Cyclic Peptide SynthesisCyclic peptides were synthesized on a peptide synthesizer using Fmoc-Asp(Wang resin)-OPis as the resin. Leu-Pro-Arg(Pbf)-Ile-Pro-Arg(Pbf)-Leu-Pro-Arg(Pbf)-Tyr(But)-Pro-Arg(Pbf)-Trp(Boc)-Pro- Arg(Pbf)-His(Trt)-Pro-Asp(Wang resin)-OPis was deprotected in 2% TFA/DCM at room temperature (r.t.) for 2 h to obtain Leu-Pro-Arg(Pbf)-Ile-Pro-Arg(Pbf)-Leu-Pro-Arg(Pbf)-Tyr(But)-Pro-Arg(Pbf)-Trp(Boc)-Pro-Arg(Pbf)-His(Trt)-Pro-Asp(Wang resin)-OH. After washing with 10% triethylamine/DCM (once), cyclization reactions were carried out using [O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU), (5 equivalent (eq.)), 1-hydroxy-7-azabenzotriazole (HOAt) (5.2 eq.), and N,N-diisopropylethylamine (DIEA) (9.36 eq.), r.t. overnight]. After deprotection by removal of the side chain protecting group {Reagent K [TFA/water/phenol/thioanisole/1,2-ethanedithiol, (82.5/5/5/5/2.5)]}, HPLC purification and lyophilization were performed to obtain cyclic peptides. Short-chain cyclic peptides were synthesized and purified according to the above method.
CD SpectroscopyThe secondary structures of myticalin A6 (a), myticalin A6 (3–23)-OH (h), myticalin A6 (6–26)-OH (j), and myticalin A6 (16–26)-OH (m) were examined by measuring the CD spectra on a J-720 Circular Dichroism Spectrometer (JASCO Corporation, Tokyo, Japan). Solvent: distilled water, 20% TFE, and 40% TFE; sample concentration: 20 µM; standard solution: 0.06 w/v (%) (1S)-(+)-10-ammonium camphorsulfonate.
TLCThe Rf value was calculated using high performance thin-layer chromatography (HPTLC) Silica gel 60 (Merck, Germany). Rf1: BuOH : pyridine : AcOH : H2O (30 : 20 : 6 : 24), Rf2: BuOH : AcOH : AcOEt : H2O (1 : 1 : 1 : 1).
Analysis of Antimicrobial ActivityThe antimicrobial activity of the synthetic peptides was evaluated by comparison with commercially available piperacillin sodium. Piperacillin is effective against S. aureus, E. coli, and P. aeruginosa and used as a control for antimicrobial activity measurements. Myticalin A6 and its derivatives were examined for antimicrobial activity against S. aureus NBRC12732, E. coli NBRC12734, and P. aeruginosa NBRC3080. Antimicrobial activity was determined by measuring the minimum inhibitory concentration (MIC). The MIC of each myticalin A6 derivative was calculated using the broth microdilution method.18)
Myticalin A6 (a), its short fragment peptides (b–m), cyclic fragment peptides (n–o), and replaced fragment peptides (p–r) were synthesized (Table 1). Myticalin A6 (6–26)-OH (j) and myticalin A6 (16–26)-OH (m) were fragment peptides of chain length corresponding to the cyclic peptides cyclo {[Asp26]-myticalin A6 (6–26)} (n) and cyclo {[Asp26]-myticalin A6 (16–26)} (o). [Lys5,8,11,14,17,20,23]-myticalin A6 (3–23)-OH [p, (X-Pro-Lys)7], [Trp6,9,12,15,18]-myticalin A6 (3–23)-OH [q, (Trp-Pro-Arg)7], and [Tyr3,6,9,12,21]-myticalin A6 (3–23)-OH [r, (Tyr-Pro-Arg)7] were synthesized as substituted derivatives to examine the importance of the side chains of Arg, Trp, and Tyr in the repeat sequence. The usefulness of these derivatives as model peptides having repetitive sequences was also evaluated.
| Peptide | Sequence | |
|---|---|---|
| a | myticalin A6 | H-YSWPR LPRIP RLPRY PRYPR WPRHP TIYA-NH2 |
| b | myticalin A6 (6–29)-NH2 | H-LPRIP RLPRY PRYPR WPRHP TIYA-NH2 |
| c | myticalin A6 (11–29)-NH2 | H-RLPRY PRYPR WPRHP TIYA-NH2 |
| d | myticalin A6 (16–29)-NH2 | H-PRYPR WPRHP TIYA-NH2 |
| e | myticalin A6 (21–29)-NH2 | H-WPRHP TIYA-NH2 |
| f | myticalin A6 (4–29)-NH2 | H-PR LPRIP RLPRY PRYPR WPRHP TIYA-NH2 |
| g | myticalin A6 (13–29)-NH2 | H-PRY PRYPR WPRHP TIYA-NH2 |
| h | myticalin A6 (3–23)-OH | H-WPR LPRIP RLPRY PRYPR WPR-OH |
| i | myticalin A6 (4–26)-OH | H-PR LPRIP RLPRY PRYPR WPRHP T-OH |
| j | myticalin A6 (6–26)-OH | H-LPRIP RLPRY PRYPR WPRHP T-OH |
| k | myticalin A6 (6–20)-OH | H-LPRIP RLPRY PRYPR-OH |
| l | myticalin A6 (11–26)-OH | H-RLPRY PRYPR WPRHP T-OH |
| m | myticalin A6 (16–26)-OH | H-PRYPR WPRHP T-OH |
| n | cyclo {[Asp26]-myticalin A6 (6–26)} | cyclo (-LPRIP RLPRY PRYPR WPRHP D-) |
| o | cyclo {[Asp26]-myticalin A6 (16–26)} | cyclo (-PRYPR WPRHP D-) |
| p | [Lys5,8,11,14,17,20,23]-myticalin A6 (3–23)-OH | H-WPK LPKIP KLPKY PKYPK WPK-OH |
| q | [Trp6,9,12,15,18]-myticalin A6 (3–23)-OH | H-WPR WPRWP RWPRW PRWPR WPR-OH |
| r | [Tyr3,6,9,12,21]-myticalin A6 (3–23)-OH | H-YPR YPRYP RYPRY PRYPR YPR-OH |
It is generally well known that a cyclic structure stabilizes the conformation of a peptide, making it less susceptible to hydrolysis. A number of structure–activity relationship studies have been conducted to date on cyclic antimicrobial peptides obtained from nature, such as gramicidin S,19,20) and BPC194,21,22) which was designed and identified by screening. In this study, we synthesized two derivatives of myticalin A6 with a cyclic structure and compared their antimicrobial activity with linear derivatives of corresponding chain length.
Table 2 shows the yield, purity, retention time, Rf value, and molecular weight of the synthetic peptides. In the cyclic peptide syntheses in this study, the synthetic yield decreased for long-chain peptides. Cyclized peptides were synthesized using Fmoc-Asp(Wang resin)-OPis as resin by substituting Asp for Thr at the 26-position of myticalin A6 (6–26)-OH (j) and myticalin A6 (16–26)-OH (m). The yield of the cyclic derivatives was 2.9% for cyclo {[Asp26]-myticalin A6 (16–26)} (o, 11 amino acids) and 0.1% for cyclo {[Asp26]-myticalin A6 (6–26)} (n, 21 amino acids). After cyclization on the resin, the side chain-protected cyclic peptide was cleaved from the resin, followed by deprotection of the side chain and subsequent purification. It is thought that a long chain length causes congestion on the resin at the point of cyclization, resulting in a decrease in the synthetic yield of the target cyclic peptide.
| Peptide | Yielda) (mg) | Yielda) (%) | tRb) (min) | Purityb) (HPLC) | Rf1 | Rf2 | Molecular weight of salt | Salt | |
|---|---|---|---|---|---|---|---|---|---|
| a | myticalin A6 | 22.9 | 5.4 | 15.8 | >95.0% | 0.48 | 0.04 | 4241.5 | acetate |
| b | myticalin A6 (6–29)-NH2 | 36.9 | 11.1 | 15.3 | >95.0% | 0.41 | 0.03 | 3326.6 | hydrochloride |
| c | myticalin A6 (11–29)-NH2 | 31.8 | 11.1 | 14.5 | >95.0% | 0.38 | 0.05 | 2855.0 | acetate |
| d | myticalin A6 (16–29)-NH2 | 49.4 | 25.3 | 14.3 | >95.0% | 0.38 | 0.01 | 1954.8 | hydrochloride |
| e | myticalin A6 (21–29)-NH2 | 14.7 | 11.7 | 14.4 | 88.8% | 0.47 | 0.44 | 1259.3 | acetate |
| f | myticalin A6 (4–29)-NH2 | 27.2 | 7.2 | 15.5 | >95.0% | 0.40 | 0.04 | 3805.1 | acetate |
| g | myticalin A6 (13–29)-NH2 | 29.8 | 11.8 | 14.6 | >95.0% | 0.40 | 0.08 | 2525.7 | acetate |
| h | myticalin A6 (3–23)-OH | 25.2 | 7.6 | 16.0 | >95.0% | 0.40 | 0.03 | 3309.6 | acetate |
| i | myticalin A6 (4–26)-OH | 42.2 | 12.2 | 15.0 | >95.0% | 0.36 | 0.04 | 3458.7 | acetate |
| j | myticalin A6 (6–26)-OH | 41.1 | 13.1 | 15.1 | 93.6% | 0.38 | 0.04 | 3145.4 | acetate |
| k | myticalin A6 (6–20)-OH | 38.7 | 16.7 | 14.8 | 94.7% | 0.39 | 0.04 | 2310.5 | acetate |
| l | myticalin A6 (11–26)-OH | 40.7 | 16.2 | 14.0 | >95.0% | 0.34 | 0.04 | 2508.6 | acetate |
| m | myticalin A6 (16–26)-OH | 35.5 | 20.8 | 13.4 | 93.0% | 0.25 | 0.04 | 1702.8 | acetate |
| n | cyclo {[Asp26]-myticalin A6 (6–26)} | 0.4 | 0.1 | NT | >95.0% | NT | NT | 3405.1 | trifluoroacetate |
| o | cyclo {[Asp26]-myticalin A6 (16–26)} | 5.2 | 2.9 | 13.5 | >95.0% | 0.40 | 0.09 | 1800.6 | trifluoroacetate |
| p | [Lys5,8,11,14,17,20,23]-myticalin A6 (3–23)-OH | 60.0 | 19.3 | 15.8 | >95.0% | 0.39 | 0.01 | 3113.5 | acetate |
| q | [Trp6,9,12,15,18]-myticalin A6 (3–23)-OH | 25.3 | 7.1 | 17.3 | >95.0% | 0.44 | 0.04 | 3574.8 | acetate |
| r | [Tyr3,6,9,12,21]-myticalin A6 (3–23)-OH | 15.2 | 4.5 | 13.9 | >95.0% | 0.42 | 0.03 | 3413.5 | acetate |
a) Peptides were synthesized at 0.1 mmol. b) Column: Waters SunFire Prep C18, 10 × 250 mm, 5 µm, B = 5% (0 min)→55% (15 min) (A = 0.1% TFA, B = 80% MeCN in 0.1% TFA), detection 214 nm, flow rate 2.5 mL/min. Rf1: BuOH : pyridine : AcOH : H2O (30 : 20 : 6 : 24), Rf2: BuOH : AcOH : AcOEt : H2O (1 : 1 : 1 : 1). NT: not tested.
MALDI-TOF-MS data for the synthetic peptides in this study are shown in Table 3. The results are in good agreement with the theoretical values.
| Peptide | Molecular formula | Monoisotopic molecular weight | MALDI-TOF-MSa) | |
|---|---|---|---|---|
| a | myticalin A6 | C180H267N55O35 | 3759.080 | 3759.830 |
| b | myticalin A6 (6–29)-NH2 | C146H224N46O28 | 3069.752 | 3070.579 |
| c | myticalin A6 (11–29)-NH2 | C118H176N38O23 | 2493.377 | 2494.460 |
| d | myticalin A6 (16–29)-NH2 | C86H125N27O17 | 1807.975 | 1808.831 |
| e | myticalin A6 (21–29)-NH2 | C55H78N16O11 | 1138.604 | 1139.935 |
| f | myticalin A6 (4–29)-NH2 | C157H243N51O30 | 3322.906 | 3322.701 |
| g | myticalin A6 (13–29)-NH2 | C106H153N33O21 | 2224.192 | 2224.560 |
| h | myticalin A6 (3–23)-OH | C135H206N44O24 | 2827.625 | 2827.806 |
| i | myticalin A6 (4–26)-OH | C139H217N47O27 | 2976.705 | 2976.358 |
| j | myticalin A6 (6–26)-OH | C128H198N42O25 | 2723.551 | 2722.832 |
| k | myticalin A6 (6–20)-OH | C91H148N30O18 | 1949.159 | 1949.569 |
| l | myticalin A6 (11–26)-OH | C100H150N34O20 | 2147.177 | 2147.408 |
| m | myticalin A6 (16–26)-OH | C68H99N23O14 | 1461.774 | 1461.009 |
| n | cyclo {[Asp26]-myticalin A6 (6–26)} | C128H194N42O25 | 2720.996b) | 2721.091c) |
| o | cyclo {[Asp26]-myticalin A6 (16–26)} | C68H95N23O14 | 1458.532b) | 1458.516c) |
| p | [Lys5,8,11,14,17,20,23]-myticalin A6 (3–23)-OH | C135H206N30O24 | 2631.582 | 2632.543 |
| q | [Trp6,9,12,15,18]-myticalin A6 (3–23)-OH | C154H205N49O22 | 3092.643 | 3093.420 |
| r | [Tyr3,6,9,12,21]-myticalin A6 (3–23)-OH | C140H198N42O29 | 2931.531 | 2931.007 |
a) Reflector-mode measurements. b) Natural abundance molecular weight. c) Linear-mode measurements.
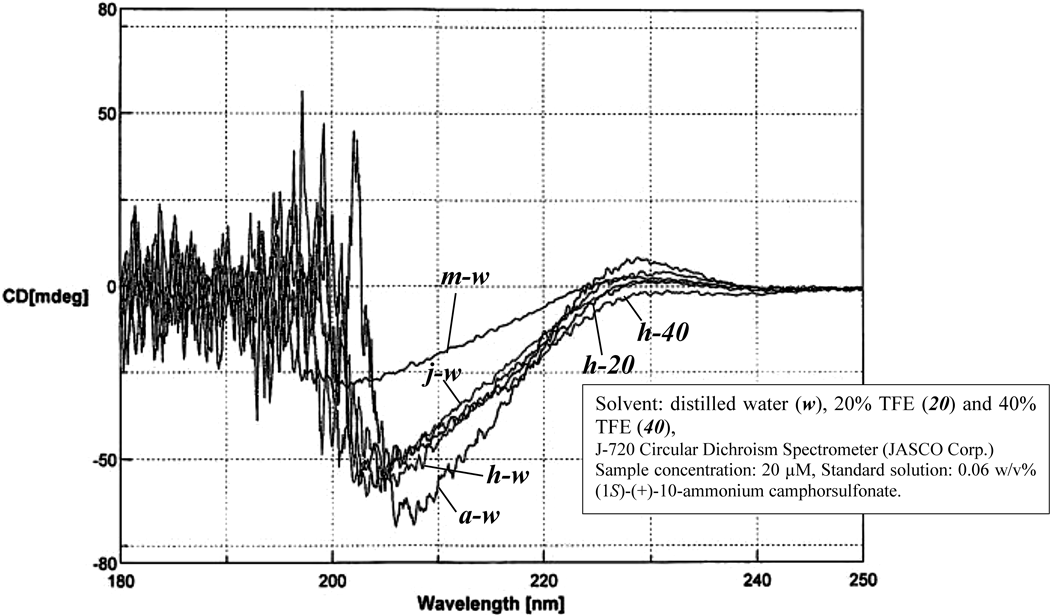
CD measurements were performed to investigate the secondary structure of myticalin A6 (a) and its derivatives. The CD spectrum of myticalin A6 (3–23)-OH (h) did not clearly indicate an α-helix or a β-sheet structure (Fig. 1), similar to the results of CD measurement of myticalin A6 (a).23) The CD spectra of myticalin A6 (6–26)-OH (j) and myticalin A6 (16–26)-OH (m) in water deviated from those of myticalin A6 (a) as the chain length of the peptides decreased (Fig. 1). The results of CD spectroscopy and antimicrobial activity analyses showed that the antimicrobial activity against S. aureus decreased as the peptide chain length decreased (Fig. 1, Table 4). Myticalin A6 (3–23)-OH (h) with a 7-fold repeat sequence and myticalin A6 (6–26)-OH (j) with a 6-fold repeat sequence retained antimicrobial activity against S. aureus, but myticalin A6 (16–26)-OH (m) with a short repeat sequence did not exhibit antimicrobial activity against S. aureus (Table 4). These results suggest the importance of the repeat sequence portion of the peptide in determining antimicrobial activity against S. aureus.
| Peptide | Gram (+) | Gram (−) | n | |||||
|---|---|---|---|---|---|---|---|---|
| S. aureus NBRC12732 | E. coli NBRC12734 | P. aeruginosa NBRC3080 | ||||||
| MIC (µg/mL) | Antimicrobial activity (µM) | MIC (µg/mL) | Antimicrobial activity (µM) | MIC (µg/mL) | Antimicrobial activity (µM) | |||
| a | myticalin A6 | 8 | 1.9 | 64 | 15.1 | >128 | >30.2 | 8 |
| b | myticalin A6 (6–29)-NH2 | 16 | 4.8 | >128 | >38.5 | 128 | >38.5 | 1 |
| c | myticalin A6 (11–29)-NH2 | 64 | 22.4 | 128 | 44.8 | >128 | >44.8 | 1 |
| d | myticalin A6 (16–29)-NH2 | 32 | 16.4 | >128 | >65.5 | >128 | >65.5 | 1 |
| e | myticalin A6 (21–29)-NH2 | >128 | >101.6 | >128 | >101.6 | >128 | >101.6 | 1 |
| f | myticalin A6 (4–29)-NH2 | 8 | 2.1 | 128 | 33.6 | >128 | >33.6 | 3 |
| g | myticalin A6 (13–29)-NH2 | 64 | 25.3 | >128 | >50.7 | >128 | >50.7 | 3 |
| h | myticalin A6 (3–23)-OH | 8 | 2.4 | 64 | 19.3 | >128 | >38.7 | 3 |
| i | myticalin A6 (4–26)-OH | 32 | 9.3 | >128 | >37.0 | >128 | >37.0 | 3 |
| j | myticalin A6 (6–26)-OH | 64 | 20.3 | >128 | >40.7 | >128 | >40.7 | 3 |
| k | myticalin A6 (6–20)-OH | >128 | >55.4 | >128 | >55.4 | >128 | >55.4 | 3 |
| l | myticalin A6 (11–26)-OH | >128 | >51.0 | >128 | >51.0 | >128 | >51.0 | 3 |
| m | myticalin A6 (16–26)-OH | >128 | >75.2 | >128 | >75.2 | >128 | >75.2 | 3 |
| n | cyclo {[Asp26]-myticalin A6 (6–26)} | >128 | >37.6 | >128 | >37.6 | >128 | >37.6 | 1 |
| o | cyclo {[Asp26]-myticalin A6 (16–26)} | >128 | >71.1 | >128 | >71.1 | >128 | >71.1 | 3 |
| p | [Lys5,8,11,14,17,20,23]-myticalin A6 (3–23)-OH | 128 | 41.1 | >128 | >41.1 | >128 | >41.1 | 4 |
| q | [Trp6,9,12,15,18]-myticalin A6 (3–23)-OH | 16 | 4.5 | 64 | 17.9 | 128 | 35.8 | 4 |
| r | [Tyr3,6,9,12,21]-myticalin A6 (3–23)-OH | 32 | 9.4 | >128 | >37.5 | >128 | >37.5 | 4 |
| Piperacillin sodium | 1 | 1.9 | 4 | 7.4 | 2 | 3.7 | 16 | |
MIC: minimum inhibitory concentration.
Myticalin A6 (a) exhibited an MIC of 8 µg/mL (1.9 µM) against Gram-positive S. aureus NBRC12732. In contrast, myticalin A6 (a) exhibited low or no antimicrobial activity against Gram-negative E. coli NBRC12734 and P. aeruginosa NBRC3080. Myticalin A6 (a) exhibited antimicrobial activity (µM) approximately equivalent to that of piperacillin against S. aureus (Table 4).
We first synthesized peptides (b–e) in which for each 5 residues were removed from the N-terminal part of myticalin A6 (a), and the antimicrobial activity of the derivatives was then measured. Myticalin A6 (6–29)-NH2 (b), in which the 5 N-terminal residues from myticalin A6 (a) were removed, exhibited a slight decrease in antimicrobial activity against S. aureus and a greater decrease in antimicrobial activity against E. coli. Myticalin A6 (21–29)-NH2 (e), corresponding to the 9 C-terminal residues of myticalin A6 (a), exhibited no antimicrobial activity. Myticalin A6 (4–29)-NH2 (f) lacks 3 N-terminal amino acids compared with myticalin A6 (a), but its antimicrobial activity against S. aureus was not diminished. In addition, myticalin A6 (13–29)-NH2 (g) exhibited antimicrobial activity against S. aureus. These results suggest that a deficiency of 3 N-terminal amino acids does not diminish the antimicrobial activity, but a deficiency of 5 or more amino acids tends to reduce antimicrobial activity against S. aureus (Table 4).
Next, we synthesized derivatives (h–m) containing the X-Pro-Arg repeat portion of myticalin A6 (a) and examined their antimicrobial activity. Myticalin A6 (3–23)-OH (h) exhibited antimicrobial activity comparable to that of myticalin A6 (a) against S. aureus (Table 4). Myticalin A6 (3–23)-OH (h, composed of 21 amino acids) carried the X-Pro-Arg 7-fold repeat sequence in the structure of myticalin A6 (a) (Table 1). Myticalin A6 (3–23)-OH (h) exhibited higher antimicrobial activity against S. aureus than myticalin A6 (6–26)-OH (j) having the same number of amino acids, suggesting the importance of the X-Pro-Arg repeat sequence to antimicrobial activity (Table 4). Among the derivatives lacking both the N- and C-terminus synthesized in this study, no antimicrobial activity was observed for the short fragments (Table 4, k, l, m).
Myticalin A6 (6–26)-OH (j) exhibited an MIC of 64 µg/mL against S. aureus, but the corresponding cyclic peptide cyclo {[Asp26]-myticalin A6 (6–26)} (n) had no antimicrobial activity (Table 4). As the yield of cyclo {[Asp26]-myticalin A6 (6–26)} (n) was very low, the antimicrobial activity could be assayed only once. Neither myticalin A6 (16–26)-OH (m) nor the corresponding cyclic peptide cyclo {[Asp26]-myticalin A6 (16–26)} (o) exhibited any antimicrobial activity against any of the bacteria tested (Table 4). No increase in antimicrobial activity due to cyclization was observed in myticalin A6 derivatives, suggesting that examination of antimicrobial activity using a linear derivative is suitable.
The antimicrobial activity of (X-Pro-Lys)7 (p) against S. aureus was significantly reduced (Table 4). This result indicates that the guanidino group present in the side chain of Arg plays an important role in the expression of antimicrobial activity.
(Trp-Pro-Arg)7 (q) exhibited a slight decrease in antimicrobial activity against S. aureus and weak antimicrobial activity against the Gram-negative bacteria E. coli and P. aeruginosa (Table 4). The lipophilicity was increased by increasing the number of indole rings via the side chain of Trp residues. The increase in lipophilicity is also illustrated by the fact that the retention time (tR) of myticalin A6 (3–23)-OH (h) was 16.0 min, but the tR of (Trp-Pro-Arg)7 (q) was longer, at 17.3 min, in HPLC analysis (Table 2). These results suggest that the increased lipophilicity enhances the activity against the outer membrane of Gram-negative bacteria.
In (Tyr-Pro-Arg)7 (r), the antimicrobial activity against S. aureus was further reduced, although not completely abolished, whereas no antimicrobial activity against Gram-negative bacteria was observed (Table 4).
These results demonstrate that in the myticalin A6 derivatives synthesized in this study, the indole group of Trp residues plays a greater role in the expression of antimicrobial activity against E. coli and P. aeruginosa than the phenol group of Tyr residues. Currently, our group is conducting further structure–activity relationship studies aimed at the synthesis of useful antimicrobial peptides.
Finally, the lower antimicrobial activity of peptides (X-Pro-Lys)7 (p), (Trp-Pro-Arg)7 (q), and (Tyr-Pro-Arg)7 (r) compared with that of myticalin A6 (3–23)-OH (h) indicated that these derivatives are not suitable for use as basic model peptides.
In the present study, myticalin A6 (a) exhibited high antimicrobial activity against S. aureus and less-potent antimicrobial activity against E. coli, but it was not active against P. aeruginosa. Our data revealed that the central X-Pro-Arg repeat sequence portion is important for the expression of myticalin A6 (a) antimicrobial activity. Myticalin A6 (3–23)-OH (h), which corresponds to (X-Pro-Arg)7, exhibited the same level of antimicrobial activity as myticalin A6 (a).
CD spectroscopy measurements were performed to investigate the secondary structure of myticalin A6 (a) and its derivatives. The CD spectrum of myticalin A6 (3–23)-OH (h) did not clearly indicate an α-helix or a β-sheet structure (Fig. 1). Furthermore, the CD spectra of myticalin A6 (6–26)-OH (j) and myticalin A6 (16–26)-OH (m) in water deviated from those of myticalin A6 (a) and myticalin A6 (3–23)-OH (h) as the chain length of the peptides decreased (Fig. 1). The lack of antimicrobial activity of myticalin A6 (16–26)-OH (m), which has a short X-Pro-Arg repeat sequence, suggests that the secondary structure indicated by the CD spectrum of myticalin A6 (3–23)-OH (h) is important for the expression of antimicrobial activity.
We also investigated the effect of cyclization on antimicrobial activity, but no increase in activity was observed with the cyclized myticalin A6 derivatives, so further structure–activity relationship studies will be conducted using a linear derivative.
Derivatives (p–r), synthesized in consideration of their potential use as model peptides, were deemed unsuitable for that purpose because their antimicrobial activity was lower than that of myticalin A6 (3–23)-OH (h). (X-Pro-Lys)7 (p) exhibited a marked decrease in antimicrobial activity, demonstrating the importance of the Arg side chain. In addition, (Trp-Pro-Arg)7 (q) exhibited increased antimicrobial activity against E. coli and P. aeruginosa, which are Gram-negative bacteria. The increase in lipophilicity of (Trp-Pro-Arg)7 (q) is thus thought to enhance activity against the outer membrane of Gram-negative bacteria.
The results obtained in this study provide basic knowledge regarding antimicrobial agents useful against drug-resistant bacteria such as MRSA. Further structure–activity relationship studies are currently underway in our group.
The authors declare no conflict of interest.