2021 Volume 44 Issue 5 Pages 599-604
2021 Volume 44 Issue 5 Pages 599-604
The purine nucleotide ATP is a fundamental unit in cellular energy metabolism. Extracellular ATP and its metabolites are also ligands for a family of receptors, known as purinergic receptors, which are expressed ubiquitously in almost every cell type. In the immune system, extracellular ATP and its signals regulate the migration and activation of immune cells to orchestrate the induction and resolution of inflammation. In this review, we provide an overview of purinergic receptors and their downstream signaling related to macrophage activation. We also discuss the roles of purinergic signaling for macrophage functions in physiological and pathological conditions.
Purines and pyrimidines are the scaffold constituents of nucleosides and nucleotides. They are ubiquitous molecules that perform important functions as the basic units of nucleic acids, allosteric modulators, energy intermediates and coenzymes.1) Among the earliest mediators of cell-to-cell communication during development, ATP is a reactive compound that participates in a large number of biochemical reactions.2) Recently, researchers have intensively studied the extracellular messenger roles of ATP in inflammation and immunity. Many inflammatory conditions have been linked to the extracellular release of nucleotides, particularly ATP.
For many years, whether there is any meaningful amount of ATP and its metabolites in the extracellular space was in question. The use of sophisticated techniques to measure the extracellular ATP concentrations in whole tissues has provided solid evidence that ATP levels in the interstitium of healthy tissues are only in the nanomolar range, whereas much higher levels (hundreds of micromoles per liter) in stimulated or diseased tissues can be detected.3) Intracellular ATP is released in response to extracellular biochemical signals or mechanical stimulation, such as host–pathogen interactions, shrinking, osmotic swelling and physical disruption.4–7) ATP release is mediated by multiple types of membrane channels, such as connexin and pannexin hemi-channels, maxi-anion channels, and the purinergic receptors.8–10) Other than releasing through channels, non-excitatory cells release ATP by exocytotic mechanisms in response to various stimuli, similar to what neurons do when depolarization occurs.11) Under inflammatory conditions, ATP is released passively following cellular stress or cell death, and extracellular ATP transduces signaling through membrane-bound purinergic receptors to exert its functions. In this review, we focus on the distribution of purinergic receptors in macrophages. We also summarize the current understanding of the role of purinergic signaling in diverse inflammatory responses.
Purinergic receptors are divided into P1 and P2 receptors.12) P1 receptors are receptors for adenosine, whereas P2 receptors are the ATP receptors. P2 receptors can be further divided into the metabotropic P2Y receptor (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–P2Y14) and P2X receptor (P2X1–7) subfamilies. P2Y receptors are coupled via G-proteins (Gq/11, Gs, or Gi/o) to Ca2+ flux, cyclic adenosine monophosphate regulation, and activation of the extracellular signal-regulated protein kinase (ERK) pathway.13) P2X receptors are homo- or heterotrimeric ion channels. Transmembrane fluxes of mono- (Na+ and K+) and divalent (Ca2+) ions are mediated by P2X receptors.12–14) Physiological and pathological processes, including wound healing, tissue homeostasis, neurodegeneration, immunity, and inflammation, are modulated by purinergic signaling. Purinergic receptors are expressed on almost all cell types.
Macrophages are hematopoietic cells involved in immune responses, such as cytokine or chemokine production and phagocytosis. Many types of P2X and P2Y receptors are expressed on macrophages, indicating that these receptors play key roles in immune responses. Macrophages display marked phenotypic heterogeneity throughout the body, so macrophages in different tissues may express different sets of purinergic receptors.15)
Extracellular purines and pyrimidines have been implicated in controlling the movement of macrophages.16) Apoptotic cells release ATP, which is known as a long-range “find-me” signal to recruit macrophages and motile monocytes. Once in the tissue, these cells can clear dying cells and promote the restoration of homeostatic tissue function17) (Fig. 1). The recruitment of macrophages and monocytes to apoptotic cells depends on P2Y2 receptor in vitro and in vivo.18) Furthermore, the migration of microglia to extracellular ATP is dependent on Gi/o-coupled P2Y12 in vitro.19,20)

ATP released from dead or dying cells acts as a “find-me” signal to attract macrophages. P2Y receptors on macrophages detect low concentrations of ATP and induce cell migration. Macrophages then clear cellular debris, thereby promoting tissue repair. (Color figure can be accessed in the online version.)
Extracellular ATP also guides macrophage migration in an indirect manner. Chemoattractants, such as C5a and N-formyl-methionylleucyl-phenylalanine, induce the extracellular release of ATP, thereby amplifying the chemoattractant gradient.21–24) This response is called the purinergic feedback loop.25) Furthermore, ATP stimulation of macrophages generates lamellipodial membrane protrusions to promote chemotaxis via autocrine/paracrine signaling involving the receptors P2Y2 and P2Y12.23,24) Therefore, extracellular ATP functions as both a direct and indirect chemoattractant, and regulates the inflammatory immune response in compromised tissues.
All P2X receptors are cation-selective channels. The P2X receptors are equally permeable to Na+ and K+, with considerable Ca2+ permeability and have significantly lower affinities for ATP than the P2Y receptors.26,27) P2Y receptors provoke intracellular mobilization of Ca2+ stores at low concentrations of extracellular ATP (micromolar), whereas P2X channels open in the presence of higher ATP concentrations (10 µM to mM). When ATP binds to a P2X receptor, the gate opens within milliseconds to allow cation flow across the membrane.28) Upon binding, the pore diameter increases from approximately 8 to 40 Å. Ionic conditions affect P2X receptor responses to ATP. In the case of P2X7 receptor (P2X7R), replacing Na+ with K+ greatly increases ATP responsiveness, suggesting that an altered ionic condition in damaged tissues has a biological role.29) Pannexin-1, the transmembrane channel, mediates the influx of large molecules following P2X7R stimulation.30)
P2X4R and P2X7R are predominantly expressed on microglia and macrophages and trigger the release of inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α.31) Additionally, P2X4R induces the secretion of C-X-C motif chemokine 5 by human macrophages.32) P2X7R also promotes the expression of chemokine C-C-motif ligand 3 in mouse microglial MG-5 cells and chemokine C-X-C-motif ligand 2 in mouse microglia cells.33,34) Moreover, prostaglandin E2 is also released in response to P2X7R agonists in human and mouse macrophages.35) In addition to ion fluxes, several intracellular signal transduction pathways are activated downstream of P2X4R and P2X7R via the following molecules: ERK; phospholipase (PL)A2, PLC, and PLD; and acidic and neutral sphingomyelinases.12,36) P2X7R stimulation also potently activates the nuclear factor-kappaB (NF-κB), nuclear factor of activated T cells c1, hypoxia inducible factor-1α (HIF-1α), and phosphatidylinositol 3-kinase (PI3K)/AKT/GSK-3β pathways.12,37) Furthermore, P2X7R contributes to IL-1β release mediated by the NOD-like receptor protein 3 (NLRP3) inflammasome (Fig. 2).
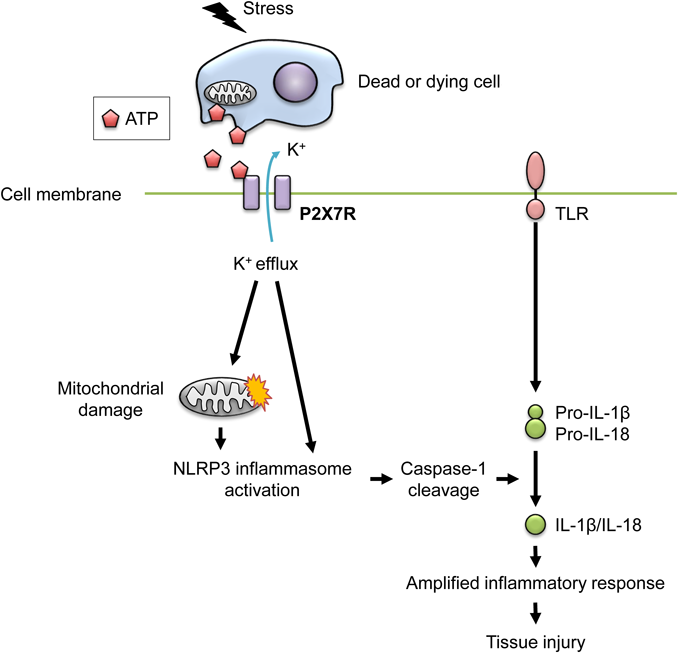
High concentrations of ATP released from dying cells potently stimulate P2X7R on macrophages, thereby triggering potassium efflux from the cytosol to the extracellular space. Potassium efflux causes the activation of NLRP3 inflammasome directly or indirectly by inducing reactive oxygen species (ROS) production from mitochondria. The activated NLRP3 inflammasome induces the maturation and release of the pro-inflammatory cytokines IL-1β and IL-18, leading to the induction of tissue injury. High concentrations of ATP can also cause cellular stress, such as cell death, which may inhibit macrophage-mediated phagocytosis. (Color figure can be accessed in the online version.)
NLRP3 inflammasome activation is an important event caused by P2X7R engagement.12) The NLRP3 inflammasome is a protein complex composed of NLRP3, apoptosis-associated speck-like protein containing a CARD, and caspase-1. High concentrations of extracellular ATP activate P2X7R, thereby inducing potassium efflux from the cytosol to the extracellular space.38) The consequent decrease in intracellular potassium induces the NLRP3 inflammasome activation. Potassium efflux triggers the association of NLRP3 with NEK7, a member of the family of mammalian NIMA-related kinases, leading to NLRP3 inflammasome assembly and activation.39) Furthermore, the decrease in intracellular potassium concentration also causes mitochondrial damage, leading to the production of reactive oxygen species (ROS), which can also stimulate the NLRP3 inflammasome.38,40,41) The activated NLRP3 inflammasome induces self-cleavage of immature caspase-1 (p45) to generate mature caspase-1 (p20). Subsequently, mature caspase-1 sequentially cleaves immature pro-IL-1β to generate mature IL-1β, which is released into the extracellular spaces.42) The NLRP3 inflammasome also mediates maturation of IL-18 by caspase-1. P2X7R expression is required for in vivo IL-1β release in mouse models. No IL-1β was detected in the peritoneal fluid of P2X7R-deficient mice that had been intraperitoneally injected with lipopolysaccharide (LPS).43) Tumor-bearing P2X7R-deficient mice also demonstrated decreased levels of serum and intratumoral IL-1β, when compared with wild-type mice.44)
A number of studies in humans and mice provide conclusive evidence that purinergic signaling in immune cells plays a major role in inflammatory immune responses. Micromolar concentrations of extracellular ATP mediate a rapid elevation of intracellular Ca2+ in myeloid cells, such as macrophages.45) Purinergic signals also drive inflammatory gene expression upon engagement of toll-like receptor 4 by LPS. The broad contribution of purinergic signaling to inflammation has now been established in a wide range of pathological conditions.
Microglia are the tissue-resident macrophages in the central nervous system, which are especially sensitive to extracellular ATP- and adenosine diphosphate-stimulated chemotaxis and morphological changes.19) Local tissue damage results in increased extracellular levels of ATP. Microglia respond to tissue damage through their P2Y receptors, which promote dynamic cellular morphological changes, thereby prompting them to rapidly move into the damaged area. This response is highly sensitive to ATP-hydrolyzing enzymes and purinergic receptor antagonists.46) ATP-induced activation of microglia-associated P2X4R contributes to the pathophysiology of comorbid pain and depression.47) In addition, the ATP-microglial P2X7R axis is involved in the development of tauopathies and amyotrophic lateral sclerosis.48,49)
Liver resident macrophages, Kupffer cells, detect extracellular ATP released from damaged or dead hepatocytes via P2X7R and may contribute to the development of inflammatory responses in liver diseases, such as non-alcoholic fatty liver disease and acetaminophen-induced hepatotoxicity.50,51)
Purinergic signals are also crucial for initiating inflammation in other types of myeloid cells. Contact allergens trigger the accumulation of ATP, which further mediates pro-inflammatory cytokine production by myeloid cells and subsequent sensitization. P2X7R deficient mice show no sensitization to contact allergens and do not release pro-inflammatory cytokine IL-1β in response to ATP and LPS. P2X7R on antigen-presenting cells is critically involved in the development of graft-versus-host disease.52) Antigen-presenting cells also potentiates airway inflammation through exogenous ATP-mediated activation of P2X7R. In asthmatic patients, rapid accumulation of ATP in the lung is triggered by allergic challenges.53) Mice with experimentally induced asthma have also shown ATP accumulation in lung.53) The administration of ATP-hydrolyzing enzymes in the airways greatly reduces eosinophil infiltration, T-helper 2 cytokine production, and bronchial hyper-reactivity. Mouse studies have suggested the potential involvement of dendritic cell-associated P2X4R and P2X7R in the pathogenesis of allergen-induced airway inflammation.54,55) Purinergic signals are also involved in cigarette smoke-induced inflammation and emphysema.56) Mice deficient in P2X7R are remarkably resistant to lung inflammation caused by exposure to cigarette smoke. Mast cells expressing P2X7R play a central role in the initiation of the inflammatory cascade during intestinal inflammation.57) The degranulation of mast cells is augmented by Ca2+ influx through P2X7R, leading to inflammation. In cardiovascular diseases, the plasma level of extracellular ATP increases during atherogenesis. Extracellular ATP causes atherosclerosis and vascular inflammation through stimulation of the P2Y2 receptor and P2X7R.58,59) Extracellular ATP has also been detected at high levels in the synovial fluid of patients with rheumatoid arthritis (RA), and P2X7R-deficient mice show lower incidence and severity of RA.60)
P2X4R on non-myeloid cells, such as epithelial cells and fibroblasts, also responds to extracellular ATP and is critically involved in the development of ischemic acute kidney injury, liver fibrosis, and RA.61–63)
P2X7R has attracted great interest, owing to its therapeutic potential shortly after it was cloned and shown to trigger inflammatory cytokine release. Several pharmaceutical companies have synthesized P2X7R inhibitors that have gone into phase I and II clinical trials for the treatment of chronic inflammatory diseases.64,65) So far, efficacy of blocking P2X7R has been tested in more than 30 clinical studies in the context of chronic obstructive pulmonary disease (COPD), osteoarthritis, RA, inflammatory pain and Crohn’s disease (CD). Currently, ClinicalTrials.gov lists eight studies that are underway to investigate the effects of P2X7R blockade on chronic allograft vasculopathy and RA, as well as to determine the suitability of P2X7R as a biomarker. One study is listed in the European Clinical Trial Register to evaluate the use of a P2X7R antagonist in RA. In general, no serious safety concerns have been raised about the P2X7R antagonists tested thus far; the main side effects reported have been dizziness, headache, diarrhea, and nausea.66) Although they have a favorable safety profile, the clinical efficacy of the P2X7R antagonists has been disappointing in RA, knee osteoarthritis, and COPD. However, encouraging results have been obtained in CD.67) Preclinical studies using P2X7R radioligands suitable for positron emission tomography have shown that P2X7R could be labeled with these tracers in mouse, rat, and monkey brains.68–70) The effects of P2X7R blockade in neuroinflammation and neurodegenerative diseases is under investigation. Therefore, controlling purinergic signaling still appears to be an excellent pharmacological potential to modulate the function of macrophage in different diseases.
Recent studies have shown that ATP functions not only as the primary unit of energy in cells, but also as a basic constituent of inflammatory microenvironments. These findings have greatly increased our understanding of the molecular mechanisms underlying tissue damage and repair mediated by the immune system. Among the purinergic receptors expressed on immune cells, including macrophages, P2X7R has emerged as a critical player in inflammation; it is regarded as a potential target for the control of the immune response. Chemical compounds targeting this receptor need to be further developed and tested for their ability to treat inflammatory conditions in human patients.
This work was supported, in part, by the Japan Society for the Promotion of Science KAKENHI (Grant Nos. 18KK0229 and 19H03371 to T.S.); Ministry of Education, Culture, Sports, Science, and Technology KAKENHI (Grant No. 17H06415 to T.S.); Kobayashi Foundation (to T.S.); Kato Memorial Bioscience Foundation (to N.T.); and GSK Japan Research Grant (to N.T.).
The authors declare no conflict of interest.