2021 Volume 44 Issue 7 Pages 920-925
2021 Volume 44 Issue 7 Pages 920-925
Type I platelet-activating factor-acetylhydrolase (PAF-AH) forms a complex consisting of two catalytic subunits (α1 and/or α2) with a regulatory subunit (β). Although this protein was discovered as an enzyme that degrades an acetyl ester linked at the sn-2 position of platelet-activating factor (PAF), its physiological function remains unknown. In this study, to examine whether knockout mice lacking the catalytic subunits of this enzyme showed a different phenotype from that of wild-type mice, we measured and compared the body weights of knockout mice and control mice. The body weights of knockout mice were significantly increased compared to those of the control mice during 6 to 20 weeks from birth. Food intake was also significantly increased in knockout mice compared with control mice during these periods. Since a decrease in testis weight was reported in the knockout mice, we expected a decrease in testosterone levels. We measured and compared the amounts of testosterone in the serum and testis of knockout and control mice using liquid chromatography-tandem mass spectrometry, and found that testosterone levels in both the serum and testis were significantly decreased in the knockout mice compared with the control mice. These results suggest that a deficiency of type I PAF-AH catalytic subunits causes an increase in body weight, in part, due to reduced testosterone levels in male mice.
Platelet-activating factor-acetylhydrolase (PAF-AH) is an enzyme that hydrolyzes an acetyl group linked at the sn-2 position of platelet-activating factor (PAF). Three types of isoforms have been identified: intracellular types I and II and a plasma type.1) Type I and II PAF-AHs are localized in the cells and tissues, while plasma-type PAF-AH circulates in the blood with lipoproteins including low density lipoprotein (LDL) and high density lipoprotein (HDL). Although these proteins have been discovered as the enzymes that degrade PAF and convert to an inactive metabolite, lysoPAF, these enzymes also hydrolyze esters of phospholipids other than PAF. Their functions also remain unknown, although many speculations about intracellular PAF-AHs2,3) and plasma-type PAF-AH have been suggested.4) Among these enzymes, type II PAF-AH and plasma-type PAF-AH share similar protein structures and substrate specificities. Human type II PAF-AH shares 43.0% amino acid sequence identity with plasma-type PAF-AH.5) Both enzymes preferentially degrade oxidatively modified phospholipids,6) suggesting that these enzymes play a role in preventing the accumulation of toxic oxidized phospholipids in the cellular membrane and lipoprotein surface. In contrast, type I PAF-AH has no homology with the other two enzymes and its substrate specificity is relatively different. Of the substituents at the sn-2 position of PAF derivatives, type I PAF-AH degrades only the acetyl group, while other enzymes show hydrolytic activity toward propionyl and butyryl groups, which are generated by non-enzymatic oxidation. Therefore, it is thought that the role of type I PAF-AH is different from that of other enzymes that remove oxidized phospholipids. Type II and plasma-type PAF-AHs are monomeric proteins, while type I PAF-AH forms a complex consisting of two catalytic subunits (α1 and/or α2) with a regulatory subunit (β). Analysis of the crystal structure of the β subunit in complex with the α2/α2 homodimer demonstrated that the quaternary structure consisting of the α2/α2 homodimer and the two β subunits.7) The β subunit is encoded by LIS1, a causal gene of the Miller–Dieker lissencephaly. Researchers’ interest has focused on the role of the enzyme activity of the type I PAF-AH complex in neuronal migration during brain development. Interestingly, switching of catalytic subunits from the α1/α2 heterodimer to α2/α2 homodimer is observed in rat neuronal cells during the postnatal stage.8)
In addition to brain development, type I PAF-AH possibly plays a role in spermatogenesis. Koizumi et al.9) and Yan et al.10) independently reported that testicular size at the age of 5-weeks and 9-weeks, respectively, was remarkably reduced in α2−/− male mice, as compared with the wild-type male mice. There is no study about embryonic stage at which these abnormalities occur in these knockout mice, although α2 deficiency-induced testicular atrophy is an interesting phenomenon in reproductive medicine. α1−/−/α2−/− male mice are sterile because of severe impairment of spermatogenesis.9,10) In the mouse testis, α2 is expressed in all spermatogenic cells, whereas α1 is expressed only in spermatogonia. These observations suggest that the deletion of both catalytic subunits induces a marked loss of germ cells at an early spermatogenic stage.9,10) To date, male infertility is the only abnormal phenotype detected in α1−/−/α2−/− mice. On the other hand, Sugatani et al. recently reported that increased body weight and enhanced adiposity were observed in PAF-receptor knockout mice.11) The increase in body weight in PAF-receptor knockout mice is explained by low energy expenditure due to a reduction in the ability to use uncoupling protein 1 (UCP1)-mediated thermogenesis.11) Furthermore, cellular thermogenesis is upregulated in 3T3-L1 adipocytes treated with c-PAF,12) a non-metabolizable PAF-receptor agonist. Based on these results, we expected that the body weight would decrease in type I PAF-AH knockout mice, as compared with wild-type mice because the amount of PAF is increased due to the deficiency of PAF-degrading enzyme. However, contrary to expectation, we observed in this study that the body weight was higher in the α1−/−/α2−/− mice than in wild-type mice.
We investigated the mechanism by which the body weight was increased in α1−/−/α2−/− mice compared with the wild-type mice and speculated that a deficiency of type I PAF-AH catalytic subunits caused an increase in body weight, in part, due to reduced testosterone levels in male mice. Furthermore, the increased body weight of α1−/−/α2−/− mice suggests that type I PAF-AH acts on the substrates different from PAF.
Type I PAF-AH α1- and/or α2-deficient mice were donated by Dr. Hiroyuki Arai, University of Tokyo.9) Male c57BL/6N mice were purchased from Crea Japan, Inc. (Tokyo, Japan). Genotypes of α1 and α2 were determined by PCR analysis using wild-type (WT) and knockout (KO) primers, the sequences of which are listed in Table 1. Mice were housed in the Central Experimental Animal Center, Teikyo University, and maintained with 12-h light-dark cycles in controlled environmental rooms with free access to water and standard chow (CRF-1; Crea Japan). All experimental procedures involving animals were carried out according to the protocols approved by the Teikyo University Institutional Animal Care and Use Committee. Body weight and food intake were measured once a week for 6–20 weeks. At 20 weeks of age, mice were sacrificed and various organs were isolated and weighed. Mice were anesthetized using an isoflurane, and whole blood was drawn by cardiac puncture. After bleeding, organs were perfused with 0.5 M KCl and the weights were measured. Obtained organs were frozen in liquid nitrogen soon after the measurement of weights. Whole blood was left at room temperature for 30 min, centrifuged, and the resulting supernatant was used as a serum sample.
| Gene | Genotype | Sequence | |
|---|---|---|---|
| Forward primer | Reverse primer | ||
| α1 | Wild-type | 5′-AGCAAGCCCACGCCTGTGCAAGAC-3′ | 5′-ACTTCGGGTTCCTTGTCTTTGCTG-3′ |
| Knockout | 5′-TGGGAAGACAATAGCAGGC-3′ | 5′-GGCTCAGGTGTAGGTAATCA-3′ | |
| α2 | Wild-type | 5′-AAAGAACCAGATGTGCTGTTTGT-3′ | 5′-CTCCCCCAATTCCAAAATTAAGAG-3′ |
| Knockout | 5′-TGGGAAGACAATAGCAGGC-3′ | 5′-GCAAAACAAGAGACTGGGTG-3′ | |
The testis sample was combined with 24-fold volume of SET Buffer: 250 mM sucrose, 1 mM ethylenediaminetetraacetic acid (EDTA) and 10 mM Tris–HCl (pH 7.4) and then homogenized with 10 up-down strokes using a Potter–Elvehjem glass-Teflon homogenizer at 1000 rpm. Then, 200 µL of the serum sample and tissue homogenate were transferred into a 5-mL glass tube with 100 µL of the internal standard, 13C-testosterone (5 ng/mL).
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)LC-MS/MS analysis was carried out using the triple quadrupole mass spectrophotometer TSQ quantum ultra (Thermo Fisher Scientific, Waltham, MA, U.S.A.) with an ultra performance liquid chromatography (UPLC) system and an autosampler. Chromatographic separation was achieved at 40∘C on a 1.7 µm ACQUITY UPLC BEH C18 (Waters, Milford, MA, U.S.A.) reversed-phase column (2.1 × 50 mm). The eluent flow rate was 200 µL/min and the eluents were 0.1% formic acid in water (eluent A) and acetonitrile (eluent B). The following gradient profile was used: 0–2 min; 10% B; 2–10 min, 10–80%B; 10–13 min, 80%B. The autosampler temperature was held at 10∘C, and the injection volume was 20 µL. A divert valve was used to direct the eluent flow into the mass spectrometer from 6.5 to 8.5 min after injection. The following ionization conditions were used: electrospray ionization (ESI), sheath gas pressure of 50 arb, sheath gas temperature of 270∘C, and capillary voltage of 4000 V. The collision energies for the quantitation ions of both unlabeled and 13C-labeled testosterone used as an internal standard (IS) were 23 and 27 eV, respectively. Detection was performed by selected reaction monitoring (SRM) in the positive mode. The ion transitions of the m/z values were 289→109 for testosterone and 292→112 for IS. The LC-MS/MS raw data were processed using analytical software (X-Calibur QUAN Browser ver.2.0–2.1).
Real-Time PCRTotal RNA was obtained from liver, brown adipose tissue (BAT) and white adipose tissue (WAT) using ISOGEN reagent (Nippon Gene, Tokyo, Japan). cDNA was synthesized from total RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, U.S.A.). The resulting cDNA was subjected to a quantitative real-time PCR using SYBR Select Master Mix (Thermo Fisher Scientific) and 7500 Fast Real-Time PCR System (Applied Biosystems). Relative mRNA expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Primer sequences for UCP1 and other genes were synthesized based on a previous report12) and website at https://pga.mgh.harvard.dus/primerbank/, respectively.
Data StatisticsValues are expressed as mean ± standard deviation (S.D.). Significance was assessed by the Student’s t-test. Alternatively, a nonparametric analysis was performed using the Mann–Whitney U test. A probability of the less than 5% was considered to be significant.
To examine the differences in phenotypes, we first compared the body weights between male mice deficient in type I PAF-AH catalytic subunits and control male mice. The body weights of knockout mice were significantly increased compared to those of the control mice between 6 to 20 weeks from the birth, as shown in Figs. 1 and 2. Food intake was also significantly increased in knockout mice compared to control mice (Fig. 3). We examined whether the expression of leptin, an adipocyte-derived hormone, was changed in the WAT of the knockout mice by real-time PCR. No significant differences were observed in leptin mRNA levels in WAT (Supplementary Fig. S1). Therefore, deficiency in the catalytic subunits of type I PAF-AH does not affect leptin-mediated differences in food intake.

Photograph shows the increased body size of knockout mice at 15 weeks of age.
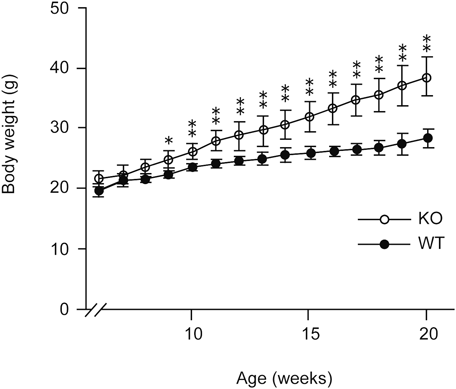
Body weight was measured in the knockout mice and wild type mice at 6–20 weeks of age. Data are expressed as mean ± standard deviation (S.D.) (n = 5 in each group). * p < 0.05 and * p < 0.01.

Weekly food intake was measured in the knockout mice and wild type mice at 7–20 weeks of age. Data are expressed as mean ± S.D. (n = 5 in each group). * p < 0.01.
We examined the difference in the tissue weights at 20 weeks between the knockout mice and control mice. It has been reported that testis weights of 5-week-old α1−/−/α2−/− mice are approximately 35% lower than those of control mice.9) We also confirmed that 20-week-old α1−/−/α2−/− mice lost an average of 51% of their testis weight (Fig. 4). Among tissue weights, WAT and liver were significantly heavier in α1−/−/α2−/− mice by 12.5 and 8.1%, respectively, in comparison to control mice (Fig. 5). Although we measured the lipogenic enzymes (glycerol-3-phosphate acyltransferase 1 (GPAT1) and 3(GPAT3)) and the lipolytic enzymes (lipoprotein lipase (LPL) and hormone sensitive-lipase (HSL)), there was no significant difference between α1−/−/α2−/− mice and control mice (Supplementary Fig. S1).

Testis weight was measured in knockout mice and wild type mice at 20 weeks of age. Data were expressed as mean ± S.D. (n = 5 in each group). * p < 0.01.

Testis weight was measured in the knockout mice and wild type mice at 20 weeks of age. Data are expressed as mean ± S.D. (n = 5 in each group). * p < 0.05
We expected that the testosterone concentration would decrease in mice deficient in catalytic subunits of type I PAF-AH, because testis weights were lower in the knockout mice than in the control mice. LC-MS/MS analysis revealed that testosterone concentration in the serum was remarkably reduced in the mice deficient in the catalytic subunits of type I PAF-AH compared with the control mice (Fig. 6). Additionally, testosterone concentration in the knockout mice was reduced not only in the serum but also in the testis (Fig. 6), suggesting that deficiency of the catalytic subunits of type I PAF-AH caused a reduced production of testosterone.

Testosterone levels were measured at 20 weeks of age by LC-MS/MS as described in Materials and Methods. Data are presented by box-and-whisker plot. The end of the box are upper and lower quartiles presented as mean ± S.D. (n = 5 in each group). (n = 9 in each group for serum and 15 in each group for testis). * p < 0.05.
Among the three types of PAF-AHs identified as PAF-degrading enzymes, the exact physiological role of type I PAF-AH remains unknown, although many findings suggest that type I PAF-AH is involved in various physiological and pathological functions including brain development, spermatogenesis, and cancer pathogenicity.1) Recently, Sugatani et al. reported that PAF-receptor knockout mice showed increased body weight and enhanced adiposity compared to wild-type mice.11) This body weight phenotype of the PAF-receptor knockout mice was explained by the lack of PAF/PAF receptor signaling, which regulates energy expenditure.12) Based on this observation, we expected that body weight gain would be decreased in type I PAF-AH knockout mice because the amount of PAF is increased due to the deficiency of PAF-degrading enzymes. However, contrary to expectations, body weight gain was increased in type I PAF-AH knockout mice, as compared with the control mice. Therefore, increased body weight gain in type I PAF-AH knockout mice is thought to be caused by a different mechanism from that in PAF-receptor knockout mice. All three types of PAF-AHs show broad substrate specificity.1) These enzymes show little discrimination between an ester and an ether at the sn-1 position of PAF. Type I PAF-AH has significant phospholipase A1 activity, as shown by the fact that the specific activity toward the PAF analogue containing acetyl group at sn-1 is comparable to that toward PAF. In addition, this enzyme preferentially hydrolyzes octyl acetate and triacetylglycerol.13) Furthermore, this enzyme acts on even aspirin (acetylsalicylic acid), which is structurally unrelated to PAF.14) The substrate specificity of type I PAF-AH is different from that of other PAF-AHs, namely, type II and plasma-type PAF-AHs, which show similar substrate specificity. Both enzymes show hydrolytic activity toward PAF analogues containing sn-2 acyl groups longer than acetyl group, whereas hydrolytic activity toward these PAF analogues is very low in type I PAF-AH. Given that type I PAF-AH shows broad specificity toward lipids and other molecules containing acetyl groups, it is possible that type I PAF-AH produces unknown bioactive metabolites that could regulate body weight gain. However, since we do not have any evidence that metabolites produced by type I PAF-AH is involved in the regulation of body weight, further study is needed.
BAT functions as a tissue to generate energy in the form of heat.15) The ability of BAT to produce heat depends on the expression of the BAT-specific protein, uncoupling protein 1 (UCP-1). Adipose tissue-specific overexpression of UCP-1 protects against diet-induced obesity in mice.16) Increased body weight gain in PAF-receptor knockout mice is explained by the mechanism by which PAF/PAF receptor signaling upregulates the expression of UCP-1 mRNA.11) However, there was no significant difference in UCP-1 mRNA expression between type I PAF-AH knockout mice and control mice (Supplementary Fig. S1). Therefore, a mechanism different from the upregulation of PAF/PAF receptor signaling is associated with body weight gain in α1−/−/α2−/− mice. One of the possible mechanisms for increased body weight gain in α1−/−/α2−/− mice is correlated with the effect of reduced testosterone levels. There are many reports in which low testosterone level increases body weight in several kinds of animal. Hypogonadism increases the risk of obesity through the increase of body fat, in particular visceral fat17) and testosterone treatment reduces the amount of visceral fat18) in men. Androgen deprivation therapies including orchiectomy19) and gonadotropin-releasing hormone (GnRH) agonist20) for human prostate cancer promotes the obesity. Castration-induced testosterone deficiency caused body weight gain in male minipigs.21) Androgen receptor (AR) knockout mice have a late-onset obesity phenotype, with a 20–40% increase in the body weight compared with control mice.22,23) Testosterone-treated mice exhibited reduced body weight gain, as compared with the control mice.24) Hypogonadism after castration causes body weight gain and abdominal obesity in high-fed mice.25) Despite many evidences obtained in men and experimental animal models, the mechanism by which decrease in testosterone caused obesity remains largely unknown. It seems likely that body weight gain in α1−/−/α2−/− mice is attributed to increase in food intake rather than reduced energy expenditure, because UCP-1 mRNA level is unchanged between α1−/−/α2−/− mice and control mice. In fact, it has been reported that daily food intake is significantly higher ad libitum fed male minipigs after castration.21) Fan et al. demonstrates that activation of AR in the hypothalamus directly enhances signaling induced by adipocyte-derive hormone leptin which suppress food intake in male mice.26) Thus, the mechanism by which low testosterone level increases food intake in male mice could be explained by suppression of leptin function in central nervous system. In humans, testosterone treatment is known to reduce body weight in obese men with testosterone deficiency, as described above. Moreover, many epidemiologic studies have established that low testosterone levels are associated with human diseases related to obesity. In this study, low testosterone levels were observed in the mice deficient in the catalytic subunits of type I PAF-AH. Although variation in the human PAF-AH type I subunits is not fully assessed in the human body, expression of α2 subunits in the human erythrocytes is variable among individuals.14) Therefore, low expression of the catalytic subunits of type I PAF-AH would cause reduced levels of testosterone and lead to further pathophysiological states, including obesity. In this study, we observed obesity caused by decreased level of testosterone in male mice. Sato et al. reported that neither heterozygous nor homozygous female AR knockout mice developed obesity.27) Therefore, obesity caused by decreased level of testosterone might be male-specific effect in mice.
In conclusion, we found that the body weight is increased in the mice deficient in the catalytic subunits of type I PAF-AH via a reduction in the levels of serum testosterone level. We speculate that a deficiency of type I PAF-AH catalytic subunits causes body weight increase, in part, due to reduced testosterone levels in male mice. In addition, there is the possibility that type I PAF-AH is associated with the regulation of serum testosterone.
Authors would like to thank Katsuhiro Yoshimoto and Mariko Fujiwara, Pharma Cluster Co., Ltd., for their helpful advice.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.