2022 Volume 45 Issue 1 Pages 1-18
2022 Volume 45 Issue 1 Pages 1-18
Cellular Ca2+ signaling functions as one of the most common second messengers of various signal transduction pathways in cells and mediates a number of physiological roles in a cell-type dependent manner. Ca2+ signaling also regulates more general and fundamental cellular activities, including cell proliferation and apoptosis. Among ion channels, Ca2+-permeable channels in the plasma membrane as well as endo- and sarcoplasmic reticulum membranes play important roles in Ca2+ signaling by directly contributing to the influx of Ca2+ from extracellular spaces or its release from storage sites, respectively. Furthermore, Ca2+-gated ion channels in the plasma membrane often crosstalk reciprocally with Ca2+ signals and are central to the regulation of cellular functions. This review focuses on the physiological and pharmacological impact of i) Ca2+-gated ion channels as an apparatus for the conversion of cellular Ca2+ signals to intercellularly propagative electrical signals and ii) the opposite feedback regulation of Ca2+ signaling by Ca2+-gated ion channel activities in excitable and non-excitable cells.
The intracellular concentration of Ca2+ ([Ca2+]i) under resting conditions is generally maintained at 100 nM or lower regardless of the cell type. Ca2+ signaling involves increases in [Ca2+]i, which commonly occur spontaneously or in the early phases of cellular responses to various physiological stimuli.1) Ca2+ signaling functions as a fundamental second messenger in a number of cellular signal cascades in the human body and mediates many cell-specific functions and activities. These functions include contraction in muscles, transmitter release in synapses, hormone release in secretary cells, and the sensing of physical stimuli in sensory nerve endings. Ca2+ signaling also regulates basic cellular activities, such as gene expression, cell cycle regulation, cell proliferation, autophagy, focal adhesion, migration, and apoptosis (Fig. 1). However, excessive Ca2+ signaling occasionally induces cell damage and even cell death. One of the reasons why Ca2+ signaling mediates a large number of cellular functions is its extreme diversity in spaciotemporal features from local Ca2+ transients within 100 ms in duration and 500 nm in diameter, such as Ca2+ sparks, to sustained or oscillatory increases in [Ca2+]i for more than one minute.2)

The activation of G protein-coupled receptors (GPCR) stimulates phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 diffuses in the cytosol and interacts with IP3 receptors (IP3R) in the ER/SR membrane, which form Ca2+ release channels. The Ca2+ signal mediates fundamental cell activities, such as gene expression, cell cycle, mitosis, autophagy, and apoptosis. In excitable cells, the primary Ca2+ signal is due to Ca2+ influx through voltage-gated Ca2+ channels (VGCC) by the generation of an action potential. The Ca2+ that enters the cell activates the ryanodine receptor (RyR) Ca2+ release channel in the ER/SR for Ca2+ release. Specific cellular functions driven by Ca2+ signaling include transmitter release, contraction, and hormone secretion in excitable cells. Physical stimuli-gated ion channel (PSGC) and ligand-gated ion channels are also permeable to Ca2+ but Na+ as well. In non-excitable cells, Ca2+ influx is mainly due to receptor-operated Ca2+ channel (ROC) and store-operated Ca2+ channel (SOC). SOC mediated Ca2+ entry (SOCE) is activated by the depletion of Ca2+ storage sites via IP3 formation and subsequent Ca2+ release. (Color figure can be accessed in the online version.)
The source of [Ca2+]i increases in Ca2+ signaling is often Ca2+ influx through Ca2+-permeable channels in the plasma membrane (PM). A large Ca2+ concentration gradient across the PM produces a strong electromotive force for Ca2+ influx through voltage-gated Ca2+ channels (VGCC) and voltage-independent Ca2+ channels on the PM, with Ca2+ influx through the latter generally being markedly slower and smaller. VGCCs activated upon depolarization are a predominant Ca2+ influx pathway in excitable cells, but not in non-excitable cells. Another Ca2+ source for Ca2+ signaling is its release from intracellular storage sites. The main intracellular Ca2+ storage/release sites are the endo- and/or sarcoplasmic reticulum (ER and/or SR), on which the Ca2+ release channels, ryanodine receptors (RyRs) and inositol 1,4,5-trisphospate receptors (IP3Rs) are functional.1)
The present review overviews information accumulated for more than two decades on the physiological and pharmacological impact of i) Ca2+-gated ion channels as an apparatus for signal conversion from changes in [Ca2+]i to electrical signals, which are intercellularly propagative, and ii) the opposite feedback regulation of Ca2+ signaling in excitable and non-excitable cells by Ca2+-gated ion channels and concomitant membrane potential changes, which are emergent druggable targets.
The established classification and nomenclature of ion channels need to be referred to those presented by The International Union of Basic and Clinical Pharmacology (IUPHAR) (https://www.guidetopharmacology.org/).3) One of the practical classifications of ion channels may be that based on gating mechanisms. Table 1 proposes a classification of ion channels on the PM based on their gating mechanisms. RyRs and IP3Rs are channels in ER/SR membranes, but are also listed here because of their close involvement in the issues discussed in this review.
 |
The stars indicate the channels for which characteristics, physiological functions, and pathophysiological significance in related diseases are discussed in this review. The Ca2+-gated ion channels underlined in the list are major issues described. This classification and list shown here are not completely comprehensive, but clearly show ion channel functions. (Color figure can be accessed in the online version.)
In this classification, “Ca2+-gated ion channels” refer to channels for which an increase in [Ca2+]i is an essential factor for their activation. The Ca2+-gated K+ channel family consists of three subfamilies classified by channel conductance. Ca2+-activated K+ channels with large conductance (BK, Maxi-K, and KCa1.1 channels encoded by KCNMA1), intermediate conductance (IK and KCa3.1 channels encoded by KCNN4), and small conductance (SK) have 100 to 300 pS, 25 to 100 pS, and 2–25 pS, respectively. SK channels are further subclassified by genes into the following three channels: SK1 or KCa2.1 encoded by KCNN1, SK2 or KCa2.2 encoded by KCNN2, and SK3 or KCa2.3 encoded by KCNN3.
In these Ca2+-gated channels, intracellular Ca2+ binding with an α-subunit protein itself or an auxiliary Ca2+-binding protein, such as calmodulin, triggers channel activity. The direct binding of cellular Ca2+ with the α-subunit of BK channels or Ca2+-activated Cl− (TMEM16A, Ano-1) channels triggers channel activity. However, BK channel activity also strongly depends on membrane potential, particularly when the γ-subunit is included in the channel complex.4–6) Furthermore, TMEM16A activity is facilitated by membrane depolarization.7) IK and SK channels have a constitutive binding site in the α-subunit for the calmodulin molecule.8) The channel gating of IK and SK channels is voltage-insensitive and essentially depends upon a conformational change by Ca2+ binding to calmodulin in the channel complex.
In contrast, the voltage-gated K+ (Kv) channel interacting protein (KChIP) has been identified as the β-subunit of Kv4.2 and Kv4.3 in cardiac myocytes9,10) and the central nervous system (CNS). KChIP includes an EF hand motif and functions as a type of [Ca2+]i sensor to modulate the activity of Kv4.x in a Ca2+-dependent manner.11) Nevertheless, the channel gating of Kv4.x is essentially dependent on voltage. Moreover, ion channel activities are often modulated by phosphorylation in a Ca2+-dependent manner, mostly due to Ca2+-dependent protein kinases, such as calmodulin-dependent protein kinase II (CaMKII),12) while these channels are generally not included in Ca2+-gated ion channels.
2-2. The Activation of BK Channels by Ca2+ Sparks Functions as a Signal Convertor in the Negative Feedback Regulation of Ca2+ Signaling in Smooth Muscle Cells (SMCs)The physiological impact of local Ca2+ transients in a cell was initially visualized in 1993 as Ca2+ sparks in cardiac myocytes by line-scan microfluorimetry using a laser confocal microscope.13) Ca2+ sparks have been identified as an “elementary” Ca2+ release event under physiological conditions in cardiac myocytes. They arise from the spontaneous opening of a small number of RyRs acting in concert. In addition, the spontaneous opening of VGCC in the T-tubule of a junctional area closely facing RyRs in SR may elicit a Ca2+ spark via the Ca2+-induced Ca2+ release (CICR) mechanism. Ca2+ sparks with similar characteristics have been identified in skeletal muscle.14) A Ca2+ spark, defined as an “elementary Ca2+ event of excitation-contraction (E-C) coupling,” has been suggested to comprise the smaller release of Ca2+, Ca2+ quarks, through a single or few RyRs.15)
Spontaneous transient outward currents (STOCs) were initially observed in isolated voltage-clamped intestinal SMCs in 198616) and have since been recorded in almost all types of SMCs. The mechanism underlying STOCs was previously proposed to be BK channel activation by local Ca2+ transients.17) STOCs presumably driven by Ca2+ sparks were shown to contribute to muscle relaxation, but not contraction, in vascular SMCs.18) The simultaneous recording of STOCs using a whole-cell patch clamp technique and Ca2+ sparks by two-dimensional Ca2+ imaging using fast-scanning confocal fluorescent microscopy (Nikon RCM8000) clearly demonstrated a spatiotemporal relationship between Ca2+ sparks and the sequential generation of STOCs in a one-to-one manner in SMCs.19) Highly localized spontaneous Ca2+ release from the SR through RyR2 as a Ca2+ spark activates nearby BK channels and elicits STOC, which shifts the membrane potential to a hyperpolarizing direction. The inhibition of Ca2+ uptake in the SR using the Ca2+ ATPase inhibitor, cyclopiazonic acid, resulted in a transient enhancement in STOCs followed by their suppression.20,21) Ca2+ sparks and STOCs randomly occur in multiple cells in smooth muscle tissue and, thus, membrane hyperpolarization by STOCs spreads to electrically connected neighboring SMCs. Membrane hyperpolarization reduces VGCC activity to relax SMCs. The pharmacological blockade of BK channels in isolated arterial vessels depolarized SMCs and increased vessel tone. Therefore, Ca2+ sparks as Ca2+ signals in SMCs do not induce muscle contraction, but contribute to muscle relaxation via BK channel activation.22,23) The deletion of the BK channel β1-subunit gene induced hypertension in mice.24) Furthermore, the deletion of the BK α-subunit (BKα) gene resulted in smooth muscle dysfunction, including overactive bladder and incontinence25) as well as erectile dysfunction26) in mice. RyR2 is an essential component of Ca2+ sparks in cardiac myocytes and SMCs and RyR1 in skeletal muscle.22,27) The heterozygous knockout of the RyR2 gene (RyR2−/+) reduced the frequency and amplitude of STOCs in isolated SMCs from the urinary bladder and resulted in increased bladder tonus and incontinence.28)
Therefore, the physiological impact of Ca2+ sparks in SMCs markedly differs from those in cardiac and skeletal muscle cells, in which a Ca2+ spark is an elementary event underlying the synchronous release of Ca2+ during E-C coupling. In SMCs, the functional coupling between RyR2 in the SR membrane and BK channels in the PM converts local Ca2+ sparks to conductive membrane hyperpolarization. This conversion system plays a pivotal role in the negative feedback regulation of Ca2+ signaling in SMC tone under resting conditions29) (Fig. 2A, left).

A: Under resting conditions, a Ca2+ spark occurs as local Ca2+ release from the SR by the spontaneous opening of RyRs in the SR membrane. A Ca2+ spark signal is converted to membrane hyperpolarization by the activation of BK channels in the PM and suppresses VGCC activity. This reciprocal process forms the “negative feedback regulation of Ca2+ signaling” in distinct local areas (see B, upper) in a cell. During excitation with an action potential (AP), Ca2+ influx through VGCC elicits a Ca2+ hot spot as a larger Ca2+ signal by Ca2+-induced Ca2+ release from the SR through RyRs (see B, lower). BK channel activation by Ca2+ hot spots forms the AP repolarization phase and after-hyperpolarization, which reduce Ca2+ influx as “negative feedback regulation.” B: Upper panel (Resting): A transient increase in [Ca2+]i as a spark and a concomitant spontaneous transient outward current (STOC) were simultaneously recorded in voltage-clamped single urinary bladder SMCs. The Ca2+ image was obtained at the peak of the Ca2+ spark. Lower panel (Excitation): Ca2+ hot spots and associated membrane currents were elicited by depolarization from −60 to 0 mV. The initial inward VGCC current and subsequent large outward BK channel current were recorded with a large increase in [Ca2+]i. The Ca2+ image was obtained at the peak of the BK channel current. It is notable that an intensive Ca2+ hot spot occurred (lower panel) at the same local area of the Ca2+ spark site (upper panel). This research was originally published in J Physiol 2001, 534, 313–326,19) with permission from “John Wiley and Sons.” (Color figure can be accessed in the online version.)
Spatiotemporal analyses of the tow dimensional (2D) Ca2+ imaging of single cells with high time resolution using the voltage clamp technique have provided important insights into Ca2+ spark sites under resting conditions in SMCs. 1) The majority of Ca2+ sparks were frequently detected in distinct sites in a cell.19) 2) Each STOC occurred in a synchronous manner with a Ca2+ spark just beneath the PM (Fig. 2B, upper), but did not follow Ca2+ sparks located deep inside of the cell.19) 3) The frequency of STOCs was significantly lower and the resting membrane potential was shallower in SMCs from RyR2−/+ mice than from wild-type mice.28)
E-C coupling may occur in highly excitable smooth muscle tissues, such as the gastrointestinal tract, arterioles, bladder, vas deferens, ureter, and uterus. In contrast, physiologically quiescent SMCs, including large arteries, airways,30–32) and the iris sphincter,33) do not elicit action potentials under normal conditions, except when excitability is markedly increased by the blockade of K+ channels.34) The membrane excitability of SMCs was previously shown to markedly vary among tissues and was mainly dependent on the density of VGCC in the PM.35) The increase in [Ca2+]i upon an action potential in highly excitable SMCs originated from a few specific Ca2+ spark sites and immediately progressed to larger Ca2+ signals, so-called “Ca2+ hot spots.”36) These hot spot sites are located just beneath the PM.19,36) The initial increase in [Ca2+]i upon an action potential was shown to occur as several Ca2+ hot spots and quickly spread throughout the whole cell area.37) The CICR mechanism38) may be induced by the influx of Ca2+ via VGCC and following the release of Ca2+ through RyRs in SMCs. Therefore, Ca2+ signaling following an action potential rapidly occurs by two steps: Ca2+ hot spots in discrete Ca2+ spark sites (Fig. 2B) that then spread as Ca2+ waves throughout a cell to induce contraction. CICR may contribute to both steps of Ca2+ signaling.37,39) The Ca2+ hot spots that occur upon an action potential strongly activate BK channels to form a repolarizing phase and the after-hyperpolarization of an action potential in order to restore Ca2+ signaling and membrane excitability to resting levels (Fig. 2B, lower), indicating the negative feedback regulation of Ca2+ signaling by BK channels during excitation (Fig. 2A, right).
A large increase in [Ca2+]i as a hot spot is sequestrated due to extrusion by Na+-Ca2+ exchangers, pumping in by the SR, and uptake by mitochondria. The alternative confocal imaging of a Ca2+ hot spot in the cytosol and Ca2+ signaling in a nearby mitochondrion was obtained using the dual and alternative laser excitation system with a confocal fluorescent microscope (Nikon RCM8000). Mitochondrial Ca2+ uptake from a nearby Ca2+ hot spot was clearly demonstrated following an evoked action potential under the current clamp mode in urinary bladder SMCs.40) The mitochondrial increase in Ca2+ was markedly slower than the formation of Ca2+ hot spots and their spread. Ca2+ hot spots facilitate Ca2+ uptake by nearby mitochondria and presumably stimulate mitochondrial respiration.
Accumulated evidence suggests that SMCs have a specific Ca2+ microdomain for the conversion of intracellular local Ca2+ signals into electrical activity.22) Ca2+ hot spots were not clearly detected in SMCs treated with methyl-β-cyclodextrin,41) which destroys the structure of caveolae by removing cholesterol in the PM. The genetic deletion of caveolin-1 (Cav1), a molecule that is essential for the formation of caveolae, decreased coupling between Ca2+ sparks and STOCs in vascular SMCs.42) Caveolae on the PM appear to play a vital role in the formation of a Ca2+ microdomain presumably composed of VGCC, BK channels, and RyRs in SMCs.
The major RyR type that is functional in SMCs is RyR2. Among the 3 types of RyRs, the expression of RyR3 is widely, but not abundantly, detected in various cell types. However, RyR3 is neither predominant against RyR1 in skeletal myocytes or RyR2 in cardiomyocytes and SMCs.29) The tissue-specific predominant expression of RyR3 has been reported in a number of cell types in rodents, such as CA1 pyramidal neurons,43) some vascular SMCs,44) and uterine SMCs.45,46) Although the contribution of RyR3 to Ca2+ spark generation has been suggested in vascular SMCs,47) the co-expression of a RyR3 dominant-negative variant48) in SMCs indicates a more complex physiological situation.49) The phenotypes of RyR3 gene deletion in mice are CNS symptoms50,51) and atypical extraocular muscles52); however, reproductive functions remain unaffected in female rats.45) The physiological functions of RyR3 have not yet been elucidated in detail.
2-3. Single Molecular Imaging Analysis as a Potential Tool to Clarify the Molecular Basis for Microdomain Formation in the Ca2+ Signal Conversion SystemThe molecular mechanisms underlying the formation of Ca2+ microdomains as “an apparatus to convert local Ca2+ signals into electrical signals” have been clarified using single molecular imaging with total internal reflection fluorescent (TIRF) microscopy.53)
The labeling of a target molecule with a fluorescent protein, such as green/cyan/yellow fluorescent proteins (GFP, CFP, and YFP, respectively) and mCherry, by genetic engineering allows us to visualize the molecule as a single fluorescent particle in the TIRF field of vision. This visualization makes it possible to analyze the intermolecular relationships responsible for Ca2+ signal conversion in living cells as follows (Fig. 3).

A: The assembly of ion channel subunits can be examined by the photobleaching method. The BK channel α-subunit forms a functional channel as a tetramer and the γ-subunit interacts with the α-subunit in a one-to-one manner.56) B: Combined molecular movements suggest molecular complex formation.42) C: FRET analyses.54) D: BiFC methods provide indirect, but convincing evidence for the interaction between two molecules in living cells. Two compartments of the Venus fluorescent protein, VN and VC, may be reconstituted when they are located in close proximity (<10 nm) and form fluorescent particles in the TIRF field of vision. This strongly suggests a direct molecular interaction between two molecules (JP2 and cav1, in “D”) labeled with VN and VC, respectively.63) (Color figure can be accessed in the online version.)
(1) Analyses of subunit assembly formation. Functional BK channels are formed by tetrameric BKCa α-subunits.5) When a GFP-labeled BKCa-α-subunit is expressed in a cell and a single fluorescent particle is continuously exposed to excitation light, photobleaching occurs and quenching is performed in four steps, thereby confirming the tetrameric formation of BKCa-α.54) Moreover, the stoichiometry of a newly identified γ-subunit in the interaction with α-subunits can be assessed using the photobleaching method.55,56) The colocalization of α- and γ-subunits labeled with mCherry and GFP, respectively, can be detected as a single yellow particle (Fig. 3A). Based on a distribution analysis of GFP bleaching steps, the 1 : 1 stoichiometry of two subunits can be identified.56)
(2) Analysis of interactions between multiple molecules in a living cell. Single molecules of VGCC(Cav1.2)-mCherry and BKCa-α-GFP often colocalize on the PM and move as a single yellow particle (Fig. 3B). In addition, after cell fixation, the photobleaching method can be used to detect stoichiometry in a molecular complex. It was demonstrated that a VGCC molecule interacts with a BKCa-α subunit in a one-to-one manner,42) and up to four VGCCs with a single BK channel.
(3) Fluorescence resonance energy transfer (FRET) analyses. A FRET analysis is an established method for identifying the extremely close positioning (<40 nm) of two molecules labeled with fluorescent proteins.57) A direct interaction between Cav1-CFP and BKCa-α-YFP was suggested by FRET54) (Fig. 3C) and confirmed by co-immunoprecipitation. A single K+ channel molecule is composed of four α-subunits, typically as a homotetramer, but occasionally as a heterotetramer within a subfamily. The heterotetramer can also be identified by FRET analyses in a similar manner.58)
(4) Bimolecular fluorescence complementation (BiFC) as a powerful tool to identify the interaction between two molecules in a living cell. Venus, a GFP-derived fluorescent protein, is divided into two components, VN (the N-terminal fragment of Venus) and VC (the C-terminal fragment of Venus), which are not fluorescent, but may fluoresce by reassembling their contact with each other in close positioning (<10 nm).59–61) Junctophilin 2 (JP2), a bridging protein between the PM and SR,62) labeled with VN was co-expressed with Cav1 labeled with VC in HEK293 cells. VN-JP2 + VC-cav1 formed fluorescent particles in the TIRF field of vision, strongly suggesting a direct molecular interaction in living cells63) (Fig. 3D).
(5) Simultaneous recording of Ca2+ imaging and single molecular imaging. The TIRF method is suitable for Ca2+ spark imaging analyses because the correspondence of each spark to a STOC under simultaneous recording is clearer and more exact than that in confocal Ca2+ images. Moreover, the spatial configuration of a Ca2+ spark and Cav1 and JP2 can be obtained in a vascular SMC in which Cav1 and JP2 are expressed63) (Fig. 4A). Furthermore, after recording Ca2+ sparks, RyR2 in SR was stained using BODIPY-ryanodine64) (Fig. 4B).
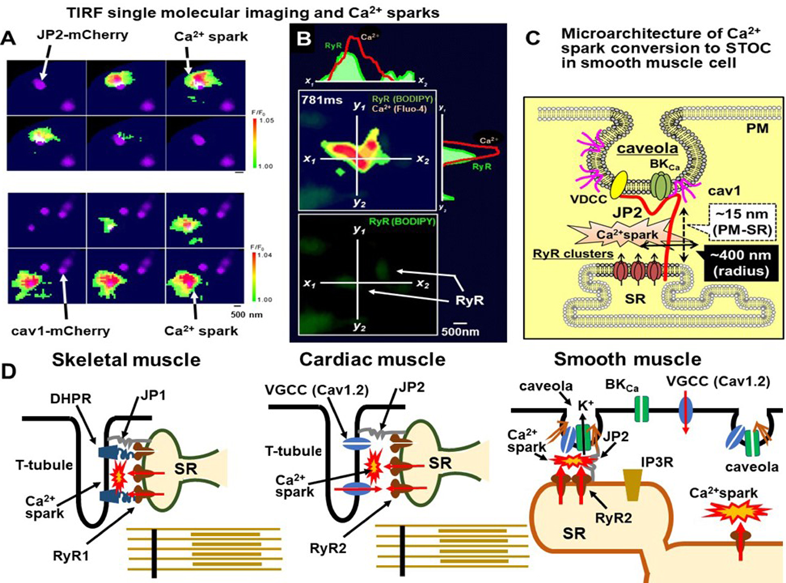
A: Ca2+ sparks were recorded in a cell, in which mCherry-labeled junctophilin-2 (JP2) or mCherry-labeled caveolin-1 (Cav1) was exogenously expressed. These molecules were carefully expressed at low levels in primary cultured vascular SMCs.63) B: Ca2+ sparks were recorded in a cell in which RyRs were stained by BODIPY-ryanodine. Unpublished observation by Dr. Hisao Yamamura, Nagoya City University, with permission from Dr. Yamamura. C: A summarized diagram of the Ca2+ microdomain for the effective conversion system from the Ca2+ spark signal to the STOC electrical signal.63) D: The different architectures for the Ca2+ spark microdomain in cardiac and skeletal myocytes are shown together with that in SMCs. (Color figure can be accessed in the online version.)
(6) Imaging of Ca2+ sparklets and subsequent CICR under voltage clamping. Since the depth of the TIRF field of vison in the vertical direction is up to approximately 200 nm from the bottom of the chamber, it is also suitable for observing the influx of Ca2+ through VGCC in the PM. A Ca2+ sparklet is a minute local increase in [Ca2+]i at the internal opening of a single or clustered VGCC (Cav1.2) in the PM. Ca2+ sparklets can be detected upon a short depolarization for 10 ms in voltage-clamped urinary bladder SMCs (not shown in figures).39) A Ca2+ sparklet was followed by a rapid Ca2+ wave that spread towards the PM from the inside of the cell. The Ca2+ wave induced by a Ca2+ sparklet was abolished by a treatment with ryanodine. These findings indicate that CICR via the activation of RyRs in the SR by Ca2+ sparklets is functional in SMCs.37,39)
Based on the findings obtained in single molecular analyses,42,63) the molecular interaction of Cav1 with JP2, VGCC, and BKα subunit leads to the accumulation of these molecules in caveolae (Fig. 4C). JP2 in caveolae maintains a close distance between caveolae and SR as the micro-space of a Ca2+ spark. Among four junctophilin family members, JP1 is specifically expressed in skeletal muscle to connect T-tubules and SR. JP2 is widely expressed in cardiac and smooth muscles.62) JP3 and JP4 are mainly distributed in the brain.62,65) It has been demonstrated that approximately 50% of all Ca2+ sparks occurred within 400 nm of JP2 or Cav1 in primary cultured SMCs from the mouse mesenteric artery.63) Furthermore, the removal of caveolae41,42) significantly reduced the coupling efficiency between Ca2+ sparks and STOCs in SMCs. Similar effects were observed after the knockdown of JP2.63) These findings indicate that the molecular interaction between Cav1 and JP2 is essential for the positioning of VGCC and BK channels near RyRs in spark sites in SMCs.63,66) The molecular assembly of the efficient apparatus for the Ca2+ signal convertor in SMCs is shown in Fig. 4C. The different architectures of the Ca2+ spark microdomains in cardiac and skeletal myocytes are also shown (Fig. 4D). The architecture of the junctional space at which Ca2+ sparks occur in SMCs is distinct from those of skeletal and cardiac myocytes67) because the T-tubule structure is absent in SMCs. As an alternative, caveolae play pivotal roles in the regulation of Ca2+ signaling during rest and excitation by forming Ca2+ microdomains in SMCs.29)
2-4. The Conversion of Ca2+ Signals to Electrical Pacemaker Activity in a Ca2+ Clock SystemThe heartbeat and gastrointestinal peristalsis are both driven by electrical signals, which occur in pacemaker cells and spread through specific impulse-conducting systems to muscle cells in these tissues. In the gastrointestinal and urinary systems, spontaneous Ca2+ oscillations in the pacemaker cells, c-kit-immunopositive interstitial cells of Cajal (ICC) or ICC-like cells, can be converted to pacemaking electrical oscillations (slow wave). This signal conversion is due to the activation of Ca2+-gated Cl− channels, which have been identified as TMEM16A.68–70) “Clockwork Ca2+ release” from the ER in ICCs is mediated by IP3Rs and/or RyRs in a manner that is dependent on the ICC types in tissues.71)
The “Ca2+ clock” function of Ca2+ release from the SR was originally described as part of the pacemaking mechanism together with the “membrane clock” due to the voltage- and time-dependent ion channel kinetics of the sinoatrial (SA) node cells of the heart.72) In SA node cells, the activation of Na+-Ca2+ exchange by local Ca2+ release from the SR through RyR2, as Ca2+ sparks or similar Ca2+ transients, contributes, at least partly, to the slow depolarization that constitutes pacemaker potential73) (Fig. 5A). The Na+-Ca2+ exchange current in cardiac myocytes clearly exhibits electrogenicity.74) While the Na+-Ca2+ exchange current in SMCs is small and may not have electrophysiological functions,75) the contribution of this current in ICC-like cells in the lower urinary tract has been suggested.76) The major Ca2+ clock mechanism in ICCs in the gastrointestinal tract is considered to be spontaneous Ca2+ release through IP3Rs in the ER membrane.77) However, Ca2+ release mediated by RyRs has also been suggested to make a significant contribution to the Ca2+ clock in ICC-like cells in the lower urinary tract.78) In the cardiac SA node and urinary tract, RyRs are major Ca2+ release channels for the Ca2+ clock, while IP3Rs may contribute to coupling between the Ca2+ clock and membrane clock.73) Previous studies reported that ICCs in the gastrointestinal79,80) and urinary tracts expressed RyR2 and/or RyR3 in addition to IP3Rs (Fig. 5B). In an organized gut motility system, slow waves in ICCs spread as pacemaker potentials with associated action potentials along ICCs and to electrically connected SMCs. The pacemaking mechanism due to the “Ca2+ clock” functions more specifically in the ICCs of the gastrointestinal tract than in those of the SA node, in which a combination with the membrane clock appears to be essential. Moreover, ICC-like cells and telocytes both often exhibit spontaneous or evoked Ca2+ oscillations in a number of tissues outside the gastrointestinal tract, including the lower urinary tract.78,81,82)

B: Schematic diagram of the pacemaking mechanism in ICCs. Spontaneous Ca2+ release through IP3Rs and/or RyRs activates the TMEM16A channel to induce a slow wave as the pacemaker potential in ICCs. The slow wave with action potentials spreads along ICCs and connected SMCs. C: The model of ICC pacemaking activity was reconstituted in HEK293 cells. (I) The overexpression of RyR3 induced spontaneous Ca2+ oscillations. (II) Co-expression of the TMEM16A channels with RyRs converted Ca2+ oscillations to a periodical depolarization-like slow wave. (III) The co-expression of SK2 channels with RyR3 converted Ca2+ oscillations to periodical hyperpolarization. (IV) The further expression of connexin 43 with SK2 and RyR3 showed propagated periodical hyperpolarization (Recordings are not shown in this figure). Ca2+ imaging represents Ca2+ oscillations in HEK293 cells expressing RyR3 + SK2. Following an increase in [Ca2+]i in the initiation cell, the elevation was propagated to the neighboring cell presumably due to the propagation of membrane hyperpolarization, which was blocked using a SK2 channel blocker (not shown). This research was originally published in Biochemical Biophysical Research Communication, 2019, 510, 242–247, with permission from Elsevier. (Color figure can be accessed in the online version.)
Pacemaking depolarization by TMEM16A channel activation induces the influx of Ca2+ through VGCC, which elicits CICR via RyR activation. This is a positive feedback mechanism that facilitates Ca2+ signaling in ICCs.71) The conversion of discrete Ca2+ clock events to the electrical slow wave is an essential step for the initiation and propagation of pacemaker activity through multicellular organs in order to accomplish synchronized physiological functions, such as peristatic motion. A simulation model of Ca2+ oscillations by clockwork Ca2+ release and subsequent signal conversion can be reconstituted in HEK293 cells by the heterologous co-expression of RyR3 and Ca2+-gated ion channels, i.e., the SK2 channel or TMEM16A80,83) (Fig. 5C). The overexpression of RyR3 elicited periodical oscillations to a similar frequency as ICCs. The incorporation of Ca2+-gated ion channels converted Ca2+ oscillations to electrical slow waves. The co-expression of TMEM16A or SK2 channels resulted in depolarizing or hyperpolarizing slow waves, respectively. The additional expression of connexin 43, which forms gap junction in ICCs, facilitated multicellular electrical coupling (not shown in Fig. 5).83) This simulation demonstrated that the conversion of Ca2+ oscillations to slow waves with cell-to-cell propagation can be reconstituted as a model of Ca2+ clock-dependent pacemaker activity by the combined expression of critical elements in the heterologous system and may provide valuable knowledge for fitting to a theoretical simulation.
TMEM16A is highly expressed in ICCs, but is also distributed in SMCs, epithelial cells,84) nasal and olfactory neurons,85) and the pineal body.86) It is often overexpressed in cancer cells.87,88) In some vascular and airway SMCs,89) Ca2+ sparks couple with both BK and TMEM16A channels. A Ca2+ spark elicits a STOC and a spontaneous inward current (STICs).90) STICs contribute to leading the resting membrane potential in a depolarizing direction in SMCs. However, the regulation of membrane potential by BK channels is generally more dynamic than that by TMEM16A, presumably due, at least in part, to the single channel conductance of the BK channel (approx. 200 pS), which is approximately 80-fold higher than that of TMEM16A (approx. 2.7 pS).86) The cell-dependent reversal potential of Cl− channels may also be a factor contributing to the involvement of TMEM16A channels in cellular electrical activities, including the resting membrane potential91) A STOC and a STIC was often recorded as a continuous signal and the STOC always anteceded the STIC,89) presumably due to different kinetics of BK and TMEM16A channels activated by a single Ca2+ spark. Although the architectural involvement of TMEM16A channels in Ca2+ microdomains has not yet been elucidated in detail, an interaction with the actin cytoskeleton92) and a close relationship with RyRs93) have been suggested. The physiological impact of TMEM16A in Ca2+ signaling and contraction has been emphasized in vascular SMCs94,95) and other SMC tissues, such as the internal anal sphincter.96)
Spontaneous and/or nicotine-induced Ca2+ oscillations, which are regulated by BK channel activity, have been detected in some primary cultured pineal cells of the rat.97,98) TMEM16A and TMEM16B channels, presumably as a heterodimer, contribute to Ca2+ signaling, which regulates melatonin secretion,99) suggesting the potential contribution of these channels to circadian rhythms.
The major Ca2+ influx pathway in excitable cells is VGCC activated by membrane depolarization. In many highly excitable cells, an action potential is initiated by voltage-gated Na+ channel activation. The subsequent activation of VGCC triggers Ca2+ signaling and signal cascades. On the other hand, the activation of VGCC is essential for action potentials in SMCs. Na+ channels are expressed at low levels in some types of SMCs in rodents, including the ureter100) and uterus101); however, their functional contribution is negligible or small. In any type of excitable cell, membrane depolarization itself facilitates VGCC activity and Ca2+ influx, presumably because of the non-inactivating component (window current) of VGCC.102) This is also the case in SMCs that are electrically quiescent under physiological conditions, such as those in large arteries and airways.103)
In non-excitable cells, which do not elicit action potentials, the functional expression of voltage-gated Na+ channels and VGCC is extremely low or absent. The major Ca2+-permeable channels in these cells are transient receptor potential (TRP) channels and Ca2+ release-activated calcium (CRAC) channels, which are abundantly expressed in most cell types, including cancer cells.104) The TRP channel group consists of 10 family members and more than 50 subfamily members and the distribution of its channels widely varies depending on the cell type.105) The characteristics of gating mechanisms, ion permeability, and physiological functions also widely vary among TRP channels and are emergent druggable targets.106) Many TRP channels are non-selective to Na+ and Ca2+, while CRAC channels shows higher selectivity to Ca2+. TRP and CRAC channels are both gated in non-voltage-dependent manners. The crosstalk of TRP channels with Ca2+-gated ion channels is an emergent issue of importance, particularly in non-excitable cells, but is not discussed in this review.107)
CRAC channels are responsible for store-operated calcium entry (SOCE), which is activated by the depletion of Ca2+ stores. Functional CRAC channels consist of two sets of molecules, Orai (Orai 1, 2, or 3) and STIM (STIM 1 or 2).108) STIM belongs to the CRAC channel family and is distributed on the ER membrane to sense the Ca2+ concentration on the luminal side of the ER. The depletion of Ca2+ in the ER by Ca2+ release through IP3Rs induces the aggregation of STIM, which results in the formation of oligomers on the ER membrane that directly interact with the Orai protein cluster on the cell membrane. The interaction with STIM activates Orai to function as the Ca2+-permeable channel responsible for SOCE.109)
In in vitro experiments, SOCE can be induced by the addition of Ca2+ to the extracellular solution after Ca2+ store depletion by simultaneous treatments with thapsigargin, a specific inhibitor of SR Ca2+ pumps, and an exposure to Ca2+-free solution (Fig. 6A). In t-BBEC177, a cell line derived from the bovine brain capillary endothelium, the increase in [Ca2+]i by SOCE, which was activated in this manner, was measured using the fluorescent Ca2+ indicator Fura-2 under the voltage clamping. SOCE appeared to be larger at a holding potential of −80 mV than at 0 mV and was completely blocked by La3+, a non-selective blocker of Ca2+ influx.110) Therefore, SOCE through CRAC channels was apparently increased by membrane hyperpolarization. Similar hyperpolarization-induced increases in [Ca2+]i have been detected in other non-excitable cells, such as airway epithelial cells111) and chondrocytes.112) Membrane hyperpolarization increases the driving force for Ca2+ influx through these non-voltage-gated Ca2+-permeable channels. The Ca2+ influx rate through TRP channels is markedly slower than that through VGCC. Since Ca2+ influx through CRAC channels is even slower, it does not induce significant membrane depolarization. TRP and CRAC channels are both potential targets of drug discovery for a number of diseases.106,108)

A: SOCE as a Ca2+ influx pathway in non-excitable cells was examined in t-BBEC177, a cell line derived from the bovine brain capillary endothelium. The increase in [Ca2+]i by SOCE was detected by fura-2 applied from a recording patch pipette under voltage clamping. The addition of Ca2+ to the extracellular solution after Ca2+ storage depletion by a treatment with thapsigargin in Ca2+-free solution elicited a slow, but large increase in [Ca2+]i due to SOCE. The increase in [Ca2+]i was strongly dependent on the membrane potential (b, c, d, and e) and completely blocked by La3+ (d). Ca2+ influx by SOCE was enhanced by membrane hyperpolarization. B: The regulation of Ca2+ signaling by the membrane potential operated in the opposite directions in excitable and non-excitable cells. To reduce Ca2+ signaling for therapy under pathophysiological conditions, the Ca2+ influx pathway and regulation of the membrane potential by K+ channel modulation are both druggable targets; however, the effects of modulators are in opposite directions in excitable and non-excitable cells. This research is a part of Ph.D. Thesis of Dr. Hiroaki Kito at Nagoya City University, 2014, with permission from Dr. Kito. (Color figure can be accessed in the online version.)
Regardless of whether a cell is excitable or non-excitable, membrane hyperpolarization is generally attributed to K+ channel activation. In excitable cells, membrane hyperpolarization reduces the open probability of VGCC and suppresses Ca2+ signaling and mediated cellular activities. In contrast, membrane hyperpolarization by K+ channel activation facilitates the influx of Ca2+ through TRP and/or CRAC channels in non-excitable cells. Therefore, the regulation of membrane potential by a K+ channel modulator results in Ca2+ signal changes in opposite directions in excitable and non-excitable cells (Fig. 6B). This opposite regulation is a key issue in drug development targeting for K+ channel modulators with respect to tissue-selective drug actions and side effects.
3-2. Physiological Impact of Ca2+-Gated Ion Channels in the Regulation of Ca2+ Signaling in Non-excitable CellsThe functional importance of TRP channels as receptor-operated Ca2+ channels has been extensively examined since the middle of 1990’s. The Orai-STIM channel complex was identified as a molecular entity of the CRAC channel that is responsible for SOCE in immune cells in 2006.113) These channels, particularly the CRAC channel, provides a pivotal Ca2+ influx pathway in non-excitable cells. It is notable that membrane hyperpolarization facilitates the influx of Ca2+ through these non-voltage-gated channels.
In a chondrocyte-like cell line derived from chondrosarcoma, in which VGCC are not functionally expressed, the stimulation of histamine H1 receptors induced oscillatory Ca2+ signaling and synchronous membrane hyperpolarization112) (Fig. 7A). Ca2+ signaling by histamine consists of biphasic components, namely, initial phasic and smaller sustained components. The phasic component was mainly due to Ca2+ release via IP3 formation. The sustained component was due to the influx of Ca2+ through TRP and CRAC channels. The sustained Ca2+ signal was extensively enhanced by membrane hyperpolarization from 0 to −60 mV under voltage clamping. Oscillatory membrane hyperpolarization was significantly suppressed by paxilline, a selective blocker of BK channels, indicating the contribution of BK channels to the positive feedback of Ca2+ signaling via membrane hyperpolarization.61,112) Single molecular imaging using the BiFC method can visualize the process of CRAC channel activation, which involves the oligomerization and translocation of the STIM protein to junctions with the PM and the interaction of STIM with Orai to form a cluster of functional CRAC channels60) (Fig. 7B). The architectural interaction between the Orai-STIM channel complex and IP3Rs is a hot issue.114,115)

A: The application of 1 µM histamine induced oscillatory Ca2+ signaling and synchronous membrane hyperpolarization (a). Ca2+ signaling by histamine consisted of biphasic components. The phasic and subsequent tonic components were due to Ca2+ release and Ca2+ influx, respectively. The sustained component of the Ca2+ signal was extensively enhanced by membrane hyperpolarization from 0 to −60 mV under voltage clamping. The BK channel block by paxilline reduced the sustained component, suggesting the contribution of BK channels to the positive feedback regulation of Ca2+ signaling.112) B: The addition of Ca2+ after storage depletion by histamine or thapsigargin in Ca2+-free solution caused the movement of STIM1-GFP and Orai1-mCherry in single molecular imaging in the TIRF field of vision. They aggregated together and formed clusters of active CRAC channels, as shown by punctate yellow dots. This study was originally published in Cell Calcium, 2015, 57, 337–347,60) with permission from Elsevier. (Color figure can be accessed in the online version.)
The positive feedback regulation of Ca2+ influx via CRAC and/or TRP channels by Ca2+-gated K+ channels has been reported in a wide range of non-excitable cells. In a chondrocyte-like cell line, histamine-induced Ca2+ signaling was potentiated by the positive feedback mechanism mainly due to BK channels and SOCE and facilitated extracellular matrix formation and inflammatory prostaglandin production.116) The contribution of Ca2+-gated K+ channels to positive feedback is not limited to BK channels, it also includes IK and SK channels. IK channels have been shown to play a central role in the positive feedback regulation of Ca2+ signaling via IP3-induced Ca2+ release and CRAC channels in T lymphocytes117,118) while Kv1.3 channels also have obligatory roles in T cell-mediated immune responses.
In the t-BBEC177 cell line, the stimulation of P2Y1 and P2Y2 receptors by ATP induced Ca2+ release via the formation of IP3 and also Ca2+ influx, presumably via TRPC1 and TRPC2.110) This Ca2+ signaling activated SK2 channels, and subsequent membrane hyperpolarization enhanced Ca2+ entry through TRPC and CRAC channels.119) This positive feedback mechanism of Ca2+ signaling via SK2 channel activation (Figs. 8A, B) facilitated cell proliferation (Fig. 8A). In addition, some ATP-stimulated cells (approximately 25%) elicited stable and extensive hyperpolarization close to the K+ equilibrium potential (approximately −80 mV). Inward rectifier K+ (Kir2.1) channels were highly expressed in these hyperpolarized cells.120) Furthermore, apoptotic cell death was frequently observed in these cells. ER stress induced by the application of tunicamycin also resulted in the overexpression of Kir2.1 and apoptosis.121) The positive feedback of Ca2+ signaling by SK2 was required for the overexpression of Kir2.1. In contrast, milder stress by hypoxia also increased the expression of Kir2.1, but facilitated cell proliferation.122) The up-regulated expression of dynamin 2 was shown to be responsible for the enhanced trafficking of Kir2.1 to the PM123) (Fig. 8A). Net cellular turnover, which results from the balance between cell death and proliferation, may be essential for maintaining healthy blood–brain barrier (BBB) functions. The contribution of SK2 channels to the positive feedback regulation of Ca2+ signaling may have a physiological impact on the homeostasis of the BBB and CNS.

A: In the t-BBEC177 cell line, Ca2+ signaling induced by ATP activated SK2 channels and subsequent membrane hyperpolarization, which, in turn, enhanced Ca2+ entry through TRP and CRAC channels. This positive feedback regulation of Ca2+ signaling by SK2 channels enhanced cell proliferation and presumably contributed to BBB homeostasis. In approximately 25% of ATP-stimulated cells, stable and extensive hyperpolarization to −80 mV was observed. In these hyperpolarized cells, the expression of Kir2.1 channels and rate of apoptosis were high. Net cellular turnover, which results from the balance between cell death and proliferation, may be essential for maintaining healthy BBB functions. B: Ca2+-gated K+ channels play central roles in negative and positive feedback regulation in excitable and non-excitable cells, respectively, and their completely opposite functions are emergent targets for drug discovery. (Color figure can be accessed in the online version.)
Therefore, Ca2+-gated K+ (BK, IK, and SK) channels play central roles in the positive and negative feedback regulation of Ca2+ signaling in non-excitable and excitable cells,124) respectively (Fig. 8B), under physiological and pathophysiological conditions and, thus, are potential targets of drug discovery.124,125)
The BK (Maxi-K, slo1, KCa1.1) channel is encoded by a single gene (KCNMA1), but is widely expressed across excitable and non-excitable tissues,126) and is the most highly expressed in the brain127,128) and muscle, particularly smooth muscle.5,129) BK channelopathy due to gain- and loss-of-function mutations has been identified in 16 mutations, which mainly result in neurological symptoms, such as epilepsy, ataxia, mental retardation, and chronic pain.130) A large number of splice variants of α-subunits,61,126,131) variants of β1–β4,132) and newly identified γ-subunits133) have contributed to the diversity of the physiological properties of BK channels.134,135)
The BK channel is a factor in the pathogenesis of CNS diseases, including epilepsy.136) However, in contrast to several Kv channels, the up-regulation of BK channel activity may be involved not only in decreases, but also in increases in CNS excitability. Therefore, it currently remains unclear whether BK channel modulators may be developed for epilepsy and ataxia therapy.128) BK channel openers were shown to exert protective effects against acute ischemic stroke by preventing Ca2+ overload and subsequent neuronal cell death in the CNS after ischemic stroke in model animals137); however, promising openers were not sufficiently effective in clinical trials. Nevertheless, the therapeutic potential of modulators of BK channels, as well as IK and SK channels, in nervous system disorders has been emphasized.124)
In contrast, the BK channel has consistently been an attractive drug target for the treatment of diseases due to smooth muscle hypercontractility, including hypertension, overactive bladder, incontinence, asthma, chronic obstructive pulmonary disease, and threatened premature labor.138–140) Similar to ATP-sensitive K+ channel openers, such as nicorandil,141) minoxidil,142) (levo)cromakalim143,144) KRN2391,145) and KRN4884,146) the development of BK channel openers targeting these diseases has been extensively challenged, but not yet successful in clinical trials.138,147) The main undesired effects of BK channel openers are facial flushing, migraine, and postural hypotension.147) Therefore, the BK channel has potential as a target for migraine therapy.148)
Several chemical compounds that exhibit separate bioactivities have been reported as BK channel openers and include nordihydroguaiaretic acid,149,150) tamoxifen, and DiBAC4(3).151) DiBAC4(3) and related voltage-sensitive oxonol dyes are specific openers of BKαβ1 and BKαβ4, but are not effective for BKα alone or BKαβ2. Since the tissue distribution of the BK channel mainly depends on the specific expression of the β-subunits, β1–β4,135) selectivity to β-subunits is a promising strategy for drug development.
BK channels are not expressed in the PM of the myocardium, while a splice variant of the BKα subunit is functionally expressed in the inner mitochondrial membrane (BKmito).131,152) Among mitochondrial K+ channels, which are emergent targets of drug discovery,153) the protective effects of ATP-sensitive K+ channels have been well established.154) The opening of K+ channels in mitochondria reduces the transmembrane potential and subsequent Ca2+ uptake and overload. This mimics an ischemic preconditioning effect and protects the myocardium from injury.155) Similarly, BKmito channel opening by 17β-estradiol156) or pimaric acid (see below) exerts cardioprotective effects.157) The BKmito channel is a hot target for therapy for cardiac ischemic injury.152) 17β-Estradiol may directly enhance BK channel activity, while BK channel expression is regulated by androgenic hormones in sexual organs158) and the amygdala.127) Various ion channels are expressed in the mitochondrial inner layer and K+ channels are promising drug targets.153)
Although none of the BK channel openers assessed to date are currently on the market, endogenous ω-3 polyunsaturated fatty acids, including docosahexaenoic acid and eicosapentaenoic acid, induce the opening of BK channels.159) Anandamide, an endogenous agonist of cannabinoid (CB) receptors, also opens BK channels,160) which is not mediated by CB1 or CB2 receptor activation. The modulation of BK channels by lipids,161) cholesterol,162) and alcohol163) are emergent topics for both health and disease. Some chemical components of natural products, such as dehydrosoyasaponin-I, quercetin magnolol, and maxikdiol, have been shown to induce the opening of BK channels.139,147) Pimaric acid and pimarane compounds from pine resin and seeds have also been identified as potent BK channel openers.164,165) Pimaric acid activates the single channel activity of BKmito channels and exerts protective effects against ischemic cardiac injury.157) It also potentiates some types of Kv channels166,167) and the TMEM16A channel,168) presumably through its effects on the voltage dependence of voltage sensors. A previous study reported that pimarane compounds improved special learning in model mice of Alzheimer’s disease.169) However, it has been pointed out that seed compounds for BK channel openers with druggable chemical structures are still poor.139)
4-2. IK and SK Channel-Related Diseases and Modulator PharmacologySickle cell anemia, which is due to a heterozygous gain-of-function mutation in the IK channel (SK4/KCa3.1/KCNN4) in red blood cell membranes, is a well-known channelopathy.170) Senicapoc, a selective IK channel blocker, was developed as therapy for Sickle cell disease, but is not on market because of insufficient efficacy in patients.125) The IK channel contributes to volume regulation by K+ efflux in red blood cells and also in immune cells, such as B cells, T cells, macrophages, mast cells, and microglia. IK channels play a central role in the positive feedback regulation of Ca2+ signaling and subsequent responses in immune cells,117) while voltage-gated Kv1.3 channels are also essential for the activation of immune responses.171) A novel dominant-negative spliced variant of human IK channels, which lacks the N-terminal domain by an alternative splicing event, was shown to be significantly expressed in human lymphoid tissues.172) IK channel activity may be modulated by this alternative splicing event under some inflammatory conditions. Among non-excitable cells other than immune cells, the expression of IK channels has been reported in vascular endothelial cells, osteoblasts,173) and cancer cells.165) IK channel expression has been widely detected in excitable cells, including CNS neurons,124) and some types of SMCs.174)
Multiple inflammatory diseases have been targeted by IK channel modulators in animal model diseases, including asthma, atherosclerosis, ischemic stroke, and renal and cardiac fibrosis.175) Moreover, senicapoc has been provided for investigator-initiated repurposing clinical trials in the US, which include Alzheimer’s disease and stroke therapy by the targeting of microglial IK channels.176)
In a mouse model of inflammatory bowel disease (IBD), IK channels were shown to play a role in the enlargement of mesenteric lymph nodes.177) Pharmacological IK channel blockade significantly attenuated the severity of IBD and reduced increases in the expression of IK channels and the production of Th1 cytokines in CD4+ T-lymphocytes.177) IK channel activity has been shown to regulate inflammatory cytokine production in CD44+ T cells via epigenetic modifications by histone deacetylases (HDAC2 and HDAC3).178) A treatment with an IK channel blocker was found to be beneficial in the recovery phase.179) Although the pharmacological blockade of KCa3.1 may reduce the risk of developing IBD, the possibility of a double-edged sword has also been suggested.180)
IK channels are engaged in the pathogenesis of delayed-type hypersensitivity (DTH) in the auricular lymph node CD44+ T lymphocytes of oxazolone-induced DTH model mice.181) The up-regulation of IK channels participates in CD44+ T-lymphocyte proliferation in the nodes of DTH model mice. These changes were suppressed by a treatment with an IK channel blocker. Therefore, IK channels have potential as a target for therapeutic interventions for allergy diseases, such as DTH.
An experimental stromal hyperplasia animal model of the rat by the implantation of a urogenital sinus corresponds to clinical benign prostatic hyperplasia (BPH).182) IK channels were highly expressed in human BPH samples. Furthermore, an in vivo treatment with TRAM-34, another selective IK channel blocker, significantly suppressed increases in implanted urogenital sinus weights. Therefore, IK channel blockers may be a novel therapeutic option for BPH.
In recent years, the IK channel is often referred to as “oncochannel” or “cancer-associated channel” because its overexpression has been detected in solid tumors,183) such as breast cancer, lung adenocarcinoma, oral squamous cell sarcoma, hepatocellular carcinoma, glioblastoma, colorectal cancer, and prostate cancer.184) The up-regulated expression of the IK channel is not only a marker of cancer progression stages, but is also a therapeutic target because Ca2+ signaling in tumor cells is expected to promote proliferation, migration, and invasion. Since the pharmacological blockade of IK channels is often effective in animal models and in vitro experiments, this channel is an attractive target for cancer therapy.171) On the other hand, cancer immunity may be modulated by IK channel modulators, suggesting difficulties with assessments of drug efficacy.
SK channels are closely involved in the regulation of action potential firing frequencies in neurons, particularly in the CNS. They are also functionally expressed in the cardiovascular system, and involved in endothelium-derived hyperpolarization in vasculature and, in part, the repolarization of cardiac action potentials.185) The peptide toxin from bee venom, apamin, is a highly selective blocker of SK (SK1, SK2, and SK3) channels. In addition to senicapoc and TRAM-34, new blockers of IK channels are highly selective against SK channels.125) SK channel-selective blockers have also been developed; however, the mechanisms underlying the selectivity among SK channels have not yet been elucidated. The prototype opener, 1-ethylbenzimidazolinone, exhibited low selectivity to IK and SK channels and exerted blocking effects on VGCC.186) More selective openers of the IK channels, SKA-111 and SKA-121 have recently been developed.125) To date, no modulators of IK or SK channels are on the market; however, drug development targeting on IK and SK channels modulators remains a hot issue.
4-3. TMEM16A-Related Diseases and Modulator PharmacologyAmong the TMEM16 (anoctamin protein, ANO) family, the main molecular entity of Ca2+-gated Cl− channels is TMEM16A, while TMEM16B and F may also be responsible for channel activity, at least in a few types of cells.187) The functional expression and pathophysiological significance of TMEM16A, known as ANO1, have been described in detail in ICCs and implicated in gastrointestinal motility disorders.7) TMEM16A plays important roles in several types of SMCs to maintain tonus, particularly in vascular SMCs, presumably via STIC activities. Pharmacological analyses revealed that the application of TMEM16A blockers reduced the tone of isolated vascular preparations and, correspondingly, systemic blood pressure in spontaneously hypertensive rats, indicating the potential of antihypertensive therapy using TMEM16A blockers.188,189)
Since the TMEM16A channel is highly expressed in the epithelium, it is regarded as an emergent therapeutic target for epithelium-originating diseases, such as asthma, diarrhea,190) and cystic fibrosis.191) TMEM16A and other types of Cl− channels also play key roles in the regulation of resting membrane potential in epithelium-derived t-BBEC177192) and its cellular functions.193,194) In a chondrocyte-like cell line as a non-excitable cell, TMEM16A and other types of Cl− channels193,195,196) are essential for the regulation of resting membrane potential and cellular functions. In addition, the overexpression of TMEM16A has been found in various cancers and may have potential as an emergent target of drug discovery.197,198)
Traditional Cl− channel blockers, including niflumic acid and anthracene-9-carboxcylic acid, exhibit low selectivity to TMEM16A channels. The BK channel activators NS1619 and isopimaric acid augmented TMEM16A channels as well,168) indicating the overlap of channel opening effects.199) A new generation of TMEM16A blockers, CACCinhA01, T16Ainh-A01, and TMinh-23, are sufficiently selective for use as pharmacological tools to investigate the contribution of TMEM16A to physiological functions.200,201) Drug development targeting on activators and blockers of the TMEM16A channel represents another hot issue.202)
The physiological impact of the conversion of Ca2+ signals to electrical activity by functional interactions with Ca2+-gated ion channels in the PM needs to be emphasized from two aspects. (1) The conversion from Ca2+ sparks to STOCs in SMCs changes the resting membrane potential and, thus, enforces a positive or negative feedback mechanism by enhancing or suppressing, respectively, the further influx of Ca2+ in reciprocal relationships. Positive or negative feedback is dependent on the combination of Ca2+-gated ion channels and Ca2+ entry pathways. The entry pathway, in turn, depend on excitable or non-excitable cells, in opposite ways. (2) Conversion may initiate pacemaking electrical signals as the Ca2+ clock, which propagates from cell to cell for tissue synchronization. Another step in the reciprocal relationship between Ca2+ signaling and Ca2+-gated channels is the Ca2+-dependent regulation of Ca2+-gated ion channel expression. These reciprocal relationships are an emergent issue and include promising targets for drug discovery, such as Ca2+ entry pathways. The concept of the Ca2+ clock has been and will be more extensively incorporated into the long-term periodical regulation system for circadian rhythms, including circadian expression changes in Ca2+-permeable channels and Ca2+-gated ion channels.203,204) The reciprocal relationship between Ca2+ signaling and Ca2+-gated ion channels is a hot issue in cancer research because these channels are often overexpressed in tumor cells. Therefore, the reciprocal interaction of Ca2+ signaling with Ca2+-gated ion channels is a promising target in drug discovery, including microRNAs for the expression control of related ion channels.88,205)
This review of the author’s work was written by the author upon receiving the 2018 Pharmaceutical Society of Japan Award.
The author expresses sincere gratitude to his supervisors: the late Dr. Minoru Watanabe Emeritus Professor Nagoya City University, the late Dr. Yutaka Kasuya Emeritus Professor The University of Tokyo, Dr. Hideomi Fukuda Emeritus Professor The University of Tokyo, Dr. Tadao Tomita Emeritus Professor Nagoya University, and Dr. Wayne Giles Emeritus Professor University of Calgary Canada. The author deeply thanks all the staff and collaborators in the research group at the Department of Molecular and Cellular Pharmacology, Nagoya City University. Collaborators outside the research group in the department, who took part in the studies described in this review are also appreciated. Studies driven by the author were mainly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS).
The author declares no conflict of interest.