Abstract
Estrogen deficiency during menopause causes a variety of neurological symptoms, including depression. The edible Lion’s Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (HE), is a medicinal mushroom that has the potential for a neuroprotective effect and ameliorating neurological diseases, such as depression, anxiety, and neurodegenerative diseases. HE contains phytoestrogens, including daidzein and genistein. However, the ameliorating effect of HE on menopausal symptoms is not well understood. Here we investigated the impact of methanol extract of the HE fruiting body on depressive-like behavior in postmenopausal model rats. The activation of estrogen receptor alpha (ERα) causes body weight loss and uterine weight gain. Body weight gain and uterine weight loss by estrogen deficiency in ovariectomized (OVX) rats were reversed with 17β-estradiol (E2) but not with HE. Thus, the phytoestrogens in HE may hardly activate ERα. Estrogen receptor beta (ERβ) is expressed in the brain, and activation of ERβ ameliorates menopausal depressive symptoms. Notably, depressive-like behavior in OVX rats evaluated in forced swim test was reduced by administration of not only E2 but also HE for 92 d. Long-term activation of ERα increases the risk of breast and uterine cancers. HE, therefore, may be effective in treating menopausal depression without the risk of carcinogenesis caused by ERα activation.
INTRODUCTION
During menopause, women experience a decrease in estrogen levels and various symptoms that negatively impact a woman’s QOL. Menopausal symptoms include depression as well as hot flashes and osteoporosis. The risk of developing depression increases by approximately 2.5 times with menopause.1) Hormone replacement therapy (HRT) is used to treat menopausal symptoms, particularly effectively treating autonomic imbalances such as hot flashes, sweating, and tachycardia. For menopausal depression, antidepressants are generally the first choice for treatment.2,3) HRT may also be used to treat menopausal depression,4–6) although some reports indicate that its effectiveness is limited.7–9) In cases where selective serotonin reuptake inhibitors (SSRIs) are used for depression in women over 50 years of age, the therapeutic effect of SSRIs can be significantly enhanced when used in combination with HRT.10) In addition to psychiatric symptoms, HRT may also improve cognitive function.11–14) Regarding adverse events of HRT, HRT with 17β-estradiol (E2) increases the risk of cancers such as breast and endometrial cancer, albeit very slightly.15,16)
In recent years, extensive research on medicinal mushrooms has been conducted to assess their potential for treating various conditions as safer alternatives to drug-dependent therapies. We previously showed a beneficial effect of Pleurotus eryngii for treating menopausal memory impairment and depression by using ovariectomized (OVX) rats.17) Hericium erinaceus (HE) is known as Lion’s Mane mushroom or yamabushitake in Japan. HE is a medicinal mushroom that has the potential for treating various conditions, including depression, anxiety,18) neurodegenerative diseases,19,20) dementia, cognitive impairment,21–23) diabetes,24) cancer,25,26) and spinal cord injury.27) It has also been shown to promote neurite outgrowth and nerve regrowth and to have a neuroprotective effect.28–30)
HE contains phytoestrogens. Two isoflavones, daidzein and genistein, have been isolated from HE mycelium.31) Polysaccharides and secondary metabolites such as erinacines, hericerins, hericenones, resorcinols, steroids, mono- and diterpenes, and volatile aromatic compounds have also been isolated from HE.32) Hericenone, a bioactive compound, shows an antidepressant-like effect. Hericenones C and D can induce the synthesis of nerve growth factors and stimulate neurogenesis in the hippocampus.21,33–35) HE, therefore, has the potential to improve the neurological symptoms of menopause. However, the ameliorating effect of HE on menopausal depression remains unclear. This study investigated the ameliorative effect of HE fruiting body on menopausal depression using menopausal model rats.
MATERIALS AND METHODS
Experimental AnimalsFemale Wistar rats (10 weeks old) were purchased from Japan SLC (Hamamatsu, Japan) and used for the experiment after two weeks of acclimatization. Rats had ad libitum access to a regular chow (MF®; Oriental Yeast, Tokyo, Japan) and tap water until the start of the experiment. Rats were kept in a temperature- and humidity-controlled room (23 ± 1 °C, 55 ± 5% humidity) with a 12-h light/dark cycle. The animals were cared for under the guidelines established by the University of Shizuoka. All animal experiments were pre-approved by the animal ethics committee of the University of Shizuoka (Approval No. 156168).
Methanol ExtractionFreeze-dried fruiting body powder of 2 kg HE was extracted with 8 L methanol (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) at 50 °C for 24 h. After filtration through filter paper (No. 2, Advantec, Tokyo, Japan), the residue was extracted with 8 L methanol again in the same manner. After removing the solvent with an evaporator, 560 g of HE extract was obtained. A voucher specimen of the mushroom HE (KLU-M 1232) was deposited in the Herbarium of the University of Malaya, Malaysia. Chemical profiles of compounds in methanol extract of HE was shown in Supplementary Figs. S1–4.33)
Ovariectomy and AdministrationBilateral ovariectomies were carried out under anesthesia with a mixture of butorphanol tartrate (Meiji Seika Pharma, Tokyo, Japan, 2.5 mg/kg body weight), medetomidine hydrochloride (FUJIFILM Wako Pure Chemical Corporation, 0.375 mg/kg body weight), and midazolam (FUJIFILM Wako Pure Chemical Corporation; 2 mg/kg body weight). Similar operations were carried out in the sham-operated rat group except for the ovariectomy. Two days later, the administration of HE or E2 (FUJIFILM Wako Pure Chemical Corporation) was started. HE was administered by feeding with a chow containing 1% HE extract prepared by a contractor (Oriental Yeast, Tokyo, Japan). E2 was given by drinking water containing 1 mg/100 mL E2, first prepared as 100 mg/mL in dimethyl sulfoxide and then diluted to 1 mg/100 mL with tap water. Rats had ad libitum access to the chows and drinking water. Rats were dissected on day 98 after ovariectomy, and uterine weight was measured.
Morris Water Maze TestThe Morris water maze test was performed 87 d after the ovariectomy. A pool 130 cm in diameter was filled with water (22 ± 2 °C), and a transparent escape platform was submerged in the pool. In the training session, rats were released into the water and trained to find the platform for up to 40 s. In the case of failure, rats were guided to the platform by hand. Rats were kept on the platform for 10 s and then picked up. Trials were repeated four times a day for five days. The release position of the rats was randomly changed every time. Rats with clearly different behavior during the training process were not used. In the probe test session, rats were released into the pool after platform removal. The pool was virtually divided into four sections: east, west, south, north, and south. The time the rat stayed in the quadrant where the platform had been placed was measured for 60 s using a video tracking system (ANY-maze Ver. 5.3; Muromachi Kikai, Tokyo, Japan).
Forced Swim TestThe forced swim test was performed 94 d after the ovariectomy. Each rat was placed for 15 min in an acrylic cylinder (20 cm in diameter, 50 cm in height) filled with water (22 ± 2 °C) to 22 cm. After 24 h, the rat was placed for 5 min in the cylinder again. Behavior was recorded with a video camera. The time the rats were passively floating in the water was measured as immobility time.
Statistical AnalysisThe following multiple comparisons were used: one-way ANOVA followed by Dunnett’s multiple comparison test or two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test. Statistical analysis was performed by GraphPad Prism (GraphPad Software, La Jolla, CA, U.S.A.). Outliers were excluded based on the Smirnov–Grubbs test. Data are shown as means with a standard error of the mean.
RESULTS
HE Extract Did Not Affect Daily Food Intake and Body Weight after OvariectomyBilateral ovariectomies were carried out in 12-week-old rats. Two days later, HE extract and E2 were administered for 94 d (Fig. 1A). The daily food intake was recorded at 21, 49, and 77 d after the ovariectomy (Table 1). OVX rats had a significantly higher food intake than those in the sham-operated rats (two-way repeated-measures ANOVA: F2,18 = 15.3, p = 0.0013 for treatment; F2,18 = 15.5, p = 0.0001 for days). HE extract did not affect this increased food intake after ovariectomy. The daily intake of HE extract was approximately 140–155 mg throughout the entire period.
Table 1. Effect of HE Extract on Daily Food Intake in OVX Rats
| Days after OVX | Sham (g/d) | OVX (g/d) | OVX + HE (g/d) |
|---|
| 21 | 14.0 ± 0.3 | 15.3 ± 0.3* | 15.5 ± 0.1* |
| 49 | 13.8 ± 0.4 | 14.9 ± 0.3 | 14.6 ± 0.2 |
| 77 | 12.1 ± 0.7 | 13.9 ± 0.4** | 14.0 ± 0.3** |
* p < 0.05, ** p < 0.01 vs. sham (two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test).
Estrogen deficiency in OVX rats resulted in significantly higher body weight gain than the sham-operated rats after 30 d from the ovariectomy (Fig. 1B). On day 72 after ovariectomy, the increased body weight of OVX rats was not significantly affected by HE extract but was reduced to the level of the sham-operated rats by E2 (one-way ANOVA, F3,36 = 36.7, p < 0.0001) (Fig. 1C).
HE Extract Did not Affect Uterine Weight after OvariectomyOn day 96 after ovariectomy, uterine weight was lower than in the sham-operated rats (Fig. 2). The decreased uterine weight by the ovariectomy was not significantly affected by HE extract administration but was increased by E2 administration (one-way ANOVA, F3,35 = 68.1, p < 0.0001).
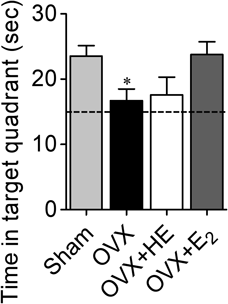
HE Extract Did Not Affect Memory Impairment after OvariectomyHippocampus-dependent spatial memory was assessed by the Morris water maze on day 87 after ovariectomy. In the training session, latency time to platform arrival was decreased over the course of the training but was not significantly different in each treatment group (two-way repeated-measures ANOVA: F3,120 = 1.16, p = 0.342 for treatment; F4,120 = 32.3, p < 0.0001 for days, data not shown). In the probe test session, time spent in the target quadrant was significantly reduced by ovariectomy (Fig. 3). Memory impairment in OVX rats was not significantly improved by the administration of HE extract but tended to be improved by E2 (one-way ANOVA, F3,28 = 3.34, p = 0.033).
HE Extract Ameliorated Depressive-Like Behavior after OvariectomyDepression was assessed as immobility time in the forced swimming test on day 94 after ovariectomy. The OVX rats showed the longest immobility among the four groups during a 5-min test period (Fig. 4A). Total immobility time was significantly increased by ovariectomy (Fig. 4B). This extended immobility time in OVX rats was decreased to the level of sham-operated rats by the administration of HE extract as well as E2 for 92 d (one-way ANOVA, F3,36 = 5.53, p = 0.0031).
DISCUSSION
In the present study, we examined the effects of HE on menopausal depression using a menopausal rat model that exhibits depressive-like behavior. Our results suggested that HE administration for 92 d ameliorates depressive-like behavior in OVX rats evaluated in the forced swim test. Concerning the carcinogenic risk of estrogen receptor alpha (ERα) activation, no significant activation of ERα, as assessed by body and uterine weight, was detected after HE administration at least for 92 d.
Clinical studies have demonstrated that the consumption of HE-containing cookies for four weeks during menopause improves the center for epidemiologic studies depression scale (CES-D), a scale for detecting depression.36) However, no significant effect was detected compared to the placebo group. In the present study, we investigated the ameliorating effect of HE on menopausal depression using a menopausal animal model. OVX rats exhibit depressive-like behavior due to estrogen deficiency.37) We confirmed that depressive-like behavior in OVX rats, as assessed by the forced swimming test, was ameliorated by administration of HE as well as E2.
Phytoestrogens such as daidzein and genistein have been isolated from HE.31) Phytoestrogens can cross the blood-brain barrier, and both daidzein and genistein bind up to 5 and 20 times more strongly to estrogen receptor beta (ERβ) than to ERα, respectively.38–41) ERβ is expressed at relatively high levels in the prostate, ovary, lung, bladder, brain, uterus, and testis.42) In the brain, ERβ expressed in the dorsal raphe, hippocampus, and medial amygdala is associated with depression.41,43,44) Activation of ERβ reduces depressive-like behavior during the forced swim test.45,46) The improvement of depressive symptoms by ERβ activation is due to promoting serotonin synthesis by induction of tryptophan hydroxylase and brain-derived neurotrophic factors synthesis.47–50) Thus, it is presumed that activation of ERβ is partially responsible for the effectiveness of HE in ameliorating depression-like behavior. However, since HE contains many bioactive substances, we cannot rule out the possibility that there are other causes than activation of ERβ for the ameliorative effects of HE.
On the other hand, activation of ERα induces a loss of body weight and a gain in uterine weight. ERα is abundantly expressed in the uterus, testis, pituitary, ovary, kidney, epididymis, and adrenal gland.42) In this study, the HE extract did not significantly affect body weight gain and uterine weight loss by estrogen deficiency in OVX rats. Thus, the phytoestrogens in HE may hardly activate ERα. Long-term activation of ERα induces breast cancer, uterine cancer, and cardiovascular disease.51,52) In addition, activation of ERβ attenuates the growth of cancer cells.53,54) Thus, HE is considered to have a low risk of cancer due to ERα activation.
In the present study, the HE methanolic extract did not ameliorate the hippocampal-dependent spatial memory impairment in the OVX rats. Previously, it was reported that dietary supplementation of HE improved the recognition memory in the novel object recognition test and increased neurotransmission at the hippocampal mossy fiber-CA3 synapse in wild-type mice.55,56) However, HE did not affect the hippocampal-dependent spatial memory evaluated using a Y maze and an object location task,56) which is consistent with our findings.
There are limitations to this study. First, there is no direct evidence that the improvement of depressive-like behavior in OVX rats is caused by ERβ activation with HE. In order to prove the direct involvement of ERβ activation in the ameliorative effect of HE, animal experiments using ERβ knockout mice, ERβ agonists (e.g., diarylpropionitrile), and ERβ antagonists (e.g., PHTPP) will be required. Second, HE is not entirely free of activation to ERα. Since genistein does not affect uterine weight, the activating effect of genistein on ERα is not strong. However, as previously reported, phytoestrogens can bind not only to ERβ but also to ERα.40) Third, we cannot conclude from the change in body weight that there is no activating effect of HE on ERα. Body weight is more strongly affected by ERα activation than ERβ, but ERβ activation has been reported to affect adiposity and body weight.57,58) Since ERβ agonists do not affect uterine weight, uterine weight is considered more appropriate for evaluating activity against ERα.58,59) Fourth, since we do not have a group of sham-operated rats administered with HE, we cannot properly evaluate whether the improvement effect of HE is limited to the depression caused by estrogen deficiency. As ERβ is also expressed in the male brains, the activation of ERβ by HE may positively affect the neurological function in males as well.
In summary, depressive-like behavior in OVX rats evaluated in the forced swim test was reduced by HE administration for 92 d. Since HE hardly activates ERα, HE may be effective in treating menopausal depression with a low risk of carcinogenesis caused by ERα activation.
Acknowledgments
This research was supported by the Japan Society for Menopause and Women’s Health under the JMWH Bayer Grant and the Asia-Oceania Collaborative Research Grants under The Kanae Foundation for the Promotion of Medical Science. We are grateful to Philip Hawke of the University of Shizuoka Scientific English Program for English language editing and the University of Shizuoka Oriental Medicine Research Club and Herbal Medicine Research Club for the daily support for the experiments. We thank lab members in the Department of Biochemistry for helpful discussions.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol. Rev., 115, 291–313 (2008).
- 2) Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann. Epidemiol., 4, 214–220 (1994).
- 3) Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J. Affect. Disord., 74, 85–96 (2003).
- 4) Gartrell NK, Koh AS, Becker C, LaVoy A, Rosen S, Thiemann S. Prevalence of hormone replacement therapy and antidepressant use in peri- and postmenopausal women. J. Gend. Specif. Med., 4, 60–63 (2001).
- 5) Bertschy G, De Ziegler D, Bianchi-Demicheli F. Mood disorders in perimenopausal women: hormone replacement or antidepressant therapy? Rev. Med. Suisse, 1, 2155–2156, 2159–2161 (2005).
- 6) de Novaes Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch. Gen. Psychiatry, 58, 529–534 (2001).
- 7) Cohen LS, Soares CN, Poitras JR, Prouty J, Alexander AB, Shifren JL. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am. J. Psychiatry, 160, 1519–1522 (2003).
- 8) Gentile S. The role of estrogen therapy in postpartum psychiatric disorders: an update. CNS Spectr., 10, 944–953 (2005).
- 9) Low LF, Anstey KJ. Hormone replacement therapy and cognitive performance in postmenopausal women—a review by cognitive domain. Neurosci. Biobehav. Rev., 30, 66–84 (2006).
- 10) Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J. Womens Health (Larchmt), 14, 609–616 (2005).
- 11) Koundi KL, Christodoulakos GE, Lambrinoudaki IV, Zervas IM, Spyropoulou A, Fexi P, Sakkas PN, Soldatos CR, Creatsas GC. Quality of life and psychological symptoms in Greek postmenopausal women: association with hormone therapy. Gynecol. Endocrinol., 22, 660–668 (2006).
- 12) Schmidt PJ. Depression, the perimenopause, and estrogen therapy. Ann. N. Y. Acad. Sci., 1052, 27–40 (2005).
- 13) Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J. Clin. Psychiatry, 63 (Suppl. 7), 45–48 (2002).
- 14) LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA, 285, 1489–1499 (2001).
- 15) Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet, 360, 942–944 (2002).
- 16) Liu Y, Ma L, Yang X, Bie J, Li D, Sun C, Zhang J, Meng Y, Lin J. Menopausal hormone replacement therapy and the risk of ovarian cancer: a meta-analysis. Front. Endocrinol. (Lausanne), 10, 801 (2019).
- 17) Minami A, Matsushita H, Horii Y, Ieno D, Matsuda Y, Saito M, Kanazawa H, Ohyama Y, Wakatsuki A, Takeda A, Hidari KI, Sabaratnam V, Suzuki T. Improvement of depression-like behavior and memory impairment with the ethanol extract of Pleurotus eryngii in ovariectomized rats. Biol. Pharm. Bull., 36, 1990–1995 (2013).
- 18) Chiu CH, Chyau CC, Chen CC, Lee LY, Chen WP, Liu JL, Lin WH, Mong MC. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3beta signaling in mice. Int. J. Mol. Sci., 19, 341 (2018).
- 19) Diling C, Tianqiao Y, Jian Y, Chaoqun Z, Ou S, Yizhen X. Docking studies and biological evaluation of a potential beta-secretase Inhibitor of 3-hydroxyhericenone F from Hericium erinaceus. Front. Pharmacol., 8, 219 (2017).
- 20) Kushairi N, Tarmizi NAKA, Phan CW, Macreadie I, Sabaratnam V, Naidu M, David P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol., 104, 153–162 (2020).
- 21) Kawagishi H, Zhuang C. Compounds for dementia from Hericium erinaceum. Drugs Future, 33, 149–155 (2008).
- 22) Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother. Res., 23, 367–372 (2009).
- 23) Saitsu Y, Nishide A, Kikushima K, Shimizu K, Ohnuki K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res., 40, 125–131 (2019).
- 24) Chaiyasut C, Sivamaruthi BS. Anti-hyperglycemic property of Hericium erinaceus—A mini review. Asian Pacific Journal of Tropical Biomedicine, 7, 1036–1040 (2017).
- 25) Ashour A, Amen Y, Allam AE, Kudo T, Nagata M, Ohnuki K, Shimizu K. New isoindolinones from the fruiting bodies of the fungus Hericium erinaceus. Phytochem. Lett., 32, 10–14 (2019).
- 26) Wang XL, Xu KP, Long HP, Zou H, Cao XZ, Zhang K, Hu JZ, He SJ, Zhu GZ, He XA, Xu PS, Tan GS. New isoindolinones from the fruiting bodies of Hericium erinaceum. Fitoterapia, 111, 58–65 (2016).
- 27) Wong KH, Naidu M, David RP, Abdulla MA, Abdullah N, Kuppusamy UR, Sabaratnam V. Functional recovery enhancement following injury to rodent peroneal nerve by lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae). Int. J. Med. Mushrooms, 11, 225–236 (2009).
- 28) Samberkar S, Gandhi S, Naidu M, Wong KH, Raman J, Sabaratnam V. Lion’s mane, Hericium erinaceus and tiger milk, lignosus rhinocerotis (Higher Basidiomycetes) medicinal mushrooms stimulate neurite outgrowth in dissociated cells of brain, spinal cord, and retina: an in vitro study. Int. J. Med. Mushrooms, 17, 1047–1054 (2015).
- 29) Shimbo M, Kawagishi H, Yokogoshi H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res., 25, 617–623 (2005).
- 30) Jang HJ, Kim JE, Jeong KH, Lim SC, Kim SY, Cho KO. The neuroprotective effect of Hericium erinaceus extracts in mouse hippocampus after pilocarpine-induced status epilepticus. Int. J. Mol. Sci., 20, 859 (2019).
- 31) He J, Fan P, Feng S, Shao P, Sun P. Isolation and purification of two isoflavones from Hericium erinaceum mycelium by high-speed counter-current chromatography. Molecules, 23, 560 (2018).
- 32) Friedman M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem., 63, 7108–7123 (2015).
- 33) Phan CW, Lee GS, Hong SL, Wong YT, Brkljaca R, Urban S, Abd Malek SN, Sabaratnam V. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct., 5, 3160–3169 (2014).
- 34) Ratto D, Corana F, Mannucci B, Priori EC, Cobelli F, Roda E, Ferrari B, Occhinegro A, Di Iorio C, De Luca F, Cesaroni V, Girometta C, Bottone MG, Savino E, Kawagishi H, Rossi P. Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients, 11, 715 (2019).
- 35) Ryu S, Kim HG, Kim JY, Kim SY, Cho KO. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain. J. Med. Food, 21, 174–180 (2018).
- 36) Nagano M, Shimizu K, Kondo R, Hayashi C, Sato D, Kitagawa K, Ohnuki K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res., 31, 231–237 (2010).
- 37) Minami A, Matsushita H, Ieno D, Matsuda Y, Horii Y, Ishii A, Takahashi T, Kanazawa H, Wakatsuki A, Suzuki T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric, 19, 568–573 (2016).
- 38) Barnes S, Prasain J, D’Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver CM. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct., 2, 235–244 (2011).
- 39) Chen LR, Ko NY, Chen KH. Isoflavone supplements for menopausal women: a systematic review. Nutrients, 11, 2649 (2019).
- 40) Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology, 139, 4252–4263 (1998).
- 41) Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol. Biochem. Behav., 86, 407–414 (2007).
- 42) Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology, 138, 863–870 (1997).
- 43) Estrada CM, Ghisays V, Nguyen ET, Caldwell JL, Streicher J, Solomon MB. Estrogen signaling in the medial amygdala decreases emotional stress responses and obesity in ovariectomized rats. Horm. Behav., 98, 33–44 (2018).
- 44) Lu H, Ozawa H, Nishi M, Ito T, Kawata M. Serotonergic neurones in the dorsal raphe nucleus that project into the medial preoptic area contain oestrogen receptor beta. J. Neuroendocrinol., 13, 839–845 (2001).
- 45) Sasayama D, Sugiyama N, Yonekubo S, Pawlak A, Murasawa H, Nakamura M, Hayashi M, Ogawa T, Moro M, Washizuka S, Amano N, Hongo K, Ohnota H. Novel oestrogen receptor beta-selective ligand reduces obesity and depressive-like behaviour in ovariectomized mice. Sci. Rep., 7, 4663 (2017).
- 46) Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol. Biochem. Behav., 78, 523–529 (2004).
- 47) Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J. Comp. Neurol., 468, 347–360 (2004).
- 48) Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17beta-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav. Brain Res., 227, 100–108 (2012).
- 49) Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci., 22, 3251–3261 (2002).
- 50) Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol. Neurobiol., 18, 87–123 (1998).
- 51) Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology, 142, 4751–4757 (2001).
- 52) Andruska N, Zheng X, Yang X, Helferich WG, Shapiro DJ. Anticipatory estrogen activation of the unfolded protein response is linked to cell proliferation and poor survival in estrogen receptor alpha-positive breast cancer. Oncogene, 34, 3760–3769 (2015).
- 53) Bossard C, Busson M, Vindrieux D, Gaudin F, Machelon V, Brigitte M, Jacquard C, Pillon A, Balaguer P, Balabanian K, Lazennec G. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLOS ONE, 7, e44787 (2012).
- 54) Saji S, Hirose M, Toi M. Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother. Pharmacol., 56 (Suppl. 1), 21–26 (2005).
- 55) Brandalise F, Cesaroni V, Gregori A, Repetti M, Romano C, Orru G, Botta L, Girometta C, Guglielminetti ML, Savino E, Rossi P. Dietary supplementation of Hericium erinaceus increases mossy fiber-CA3 hippocampal neurotransmission and recognition memory in wild-type mice. Evid. Based Complement. Alternat. Med., 2017, 3864340 (2017).
- 56) Rossi P, Cesaroni V, Brandalise F, Occhinegro A, Ratto D, Perrucci F, Lanaia V, Girometta C, Orru G, Savino E. Dietary supplementation of lion’s mane medicinal mushroom, Hericium erinaceus (Agaricomycetes), and spatial memory in wild-type mice. Int. J. Med. Mushrooms, 20, 485–494 (2018).
- 57) Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet., 4, e1000108 (2008).
- 58) Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R. Estrogen receptor-β-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J. Biol. Chem., 285, 31292–31303 (2010).
- 59) Weigt C, Hertrampf T, Zoth N, Fritzemeier KH, Diel P. Impact of estradiol, ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity. Mol. Cell. Endocrinol., 351, 227–238 (2012).