2022 Volume 45 Issue 10 Pages 1581-1584
2022 Volume 45 Issue 10 Pages 1581-1584
Cellular senescence is an inherent tumor suppressive process, and cancer-targeted senescence induction represents an attractive anti-tumor strategy. Here, we show that a methoxyflavanone derivative (Perilla-derived methoxyflavanone, PDMF) from the Asian medicinal herb, Perilla frutescens, induces cellular senescence in A549 human adenocarcinoma cells but not in normal human bronchial epithelial (NHBE) cells. We also provide evidence that PDMF preferentially activates the p53–p21 pathway in A549 cells, and that p53 is essential for its pro-senescent activity.
Cellular senescence is an irreversible phenotypic change in cells, defined as a permanent cell cycle arrest via telomere erosion and/or limitation of cell division.1) In addition to this type of replicative senescence, exogenous stresses also induce premature senescence.2) Pathophysiologically, cellular senescence acts as a barrier against tumor initiation and progression.2) Thus, strategies to induce cancer cell senescence (pro-senescence therapy) have gained much attention, because of their ability to impede cancer progression, even in advanced and metastatic stages.3)
In addition to such therapeutic viewpoints, cancer prevention through senescence induction is an attractive anti-tumor strategy, wherein plant flavonoids and polyphenols have attracted much attention as compounds of interest.4) Perilla frutescens is an anti-inflammatory medicinal herb that also possesses anti-tumor potency.5) Recently, our group has identified a new flavanone derivative from P. frutescens, termed Perilla-derived methoxyflavanone (PDMF),6) and found that PDMF induces p53-mediated cell cycle arrest in A549 human lung adenocarcinoma cells.7) As p53 plays a central role in inducing cellular senescence,8,9) we assume that PDMF could also be effective in inducing cancer cell senescence. In the present study, we provide evidence that PDMF specifically induces cancer cell senescence in A549 lung cancer cells but not in primary normal human bronchial epithelial (NHBE) cells, and that this pro-senescent action of PDMF is accompanied by a selective activation of the p53–p21 pathway in A549 cells.
PDMF (8-hydroxy-5,7-dimethoxyflavanone, 99.6% purity) was chemically synthesized by Tokyo Chemical Industry (Tokyo, Japan). Etoposide was purchased from Sigma-Aldrich (MO, U.S.A.). Human lung adenocarcinoma A549 cells were purchased from RIKEN BRC (Tsukuba, Japan), and cultured under the described conditions.7) NHBE cells were obtained from Lonza (MD, U.S.A.), and cultured in BEGM™ medium (Lonza) at 37 °C with 5% CO2. Cell culture for senescence induction was performed as described.10) Briefly, A549 cells or NHBE cells (5000 cells) were seed in a 6-well plate or a collagen-coated 6-well plate for overnight pre-culture. Then both cells were cultured for 1 to 10 d upon stimulation with PDMF (200 µM) or etoposide (0.5 µM).
Senescence-Associated β-Galactosidase (SA-β-Gal) StainingExpression of SA-β-gal was visualized using a Senescence β-Galactosidase Staining Kit (Cell Signaling Technology, MA, U.S.A.). SA-β-gal+ senescent cells were counted in five different areas per well in three independent wells under a microscope (×300 magnific field), and the percentage of SA-β-gal+ cells among the total cells was calculated.
Western Blot AnalysisA549 cells or NHBE cells were stimulated with 200 µM PDMF or 0.5 µM etoposide for 4 d, and whole cell lysates were collected as described.7) The lysates were then subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and resolved proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Merck Millipore, Cork, Ireland). Immunoblotting was then performed as reported previously7) using the following rabbit primary antibodies from Cell Signaling Technology: anti-p53 polyclonal antibody (pAb, 1 : 1000 dilution), anti-phospho-p53 (Ser15) pAb (1 : 1000), anti-p21 monoclonal antibody (mAb, clone #12D1, 1 : 1000), or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mAb (clone #14C10, 1 : 5000). Positive signals were visualized by staining with a secondary peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) (Cell Signaling Technology) followed by detection using an ECL Western blotting Detection Reagent (Cytiva, Tokyo, Japan).
RNA InterferenceTransfection with p53 small interfering RNA (siRNA) under PDMF-co-stimulation was performed as described previously7) with minor modifications. Briefly, A549 cells (1.5 × 103 cells, supplemented with 200 µM PDMF) were transfected with 6 pmol of p53 siRNA (#106141, Ambion, TX, U.S.A.) or control siRNA (#AM4611) using Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific, MA, U.S.A.). On days 3 and 6, fresh siRNA reagents were added and the transfected A549 cells were cultured for 9 d upon stimulation with PDMF.
Statistical AnalysisStatistical analysis was conducted using one-way ANOVA with a post hoc Tukey–Kramer multiple comparison test, and p < 0.05 was considered as statistically significant.
We first tested whether PDMF induced cancer cell senescence in A549 cells, and found that stimulation with PDMF (200 µM, for 7 d) significantly increased the number of SA-β-gal+ senescent A549 cells (Fig. 1A). Dose response analysis indicated that pro-senescent potency of PDMF becomes plateau and most potent in 200 µM (Supplementary Fig. S1). Time-course kinetics analysis (1 to 10 d) revealed that the pro-senescent rate of PDMF was comparable to that of a positive control etoposide, a DNA-damaging agent (Fig. 1B). We also observed that no significant decrease in cell viability was seen in these experimental conditions (Supplementary Fig. S2). More importantly, we also found that PDMF failed to induce cellular senescence in NHBE cells, whereas etoposide normally induced SA-β-gal+ senescent NHBE cells (Figs. 2A, B). These data suggest that PDMF induces cellular senescence in A549 human lung cancer cells, but not in primary human lung epithelial cells.
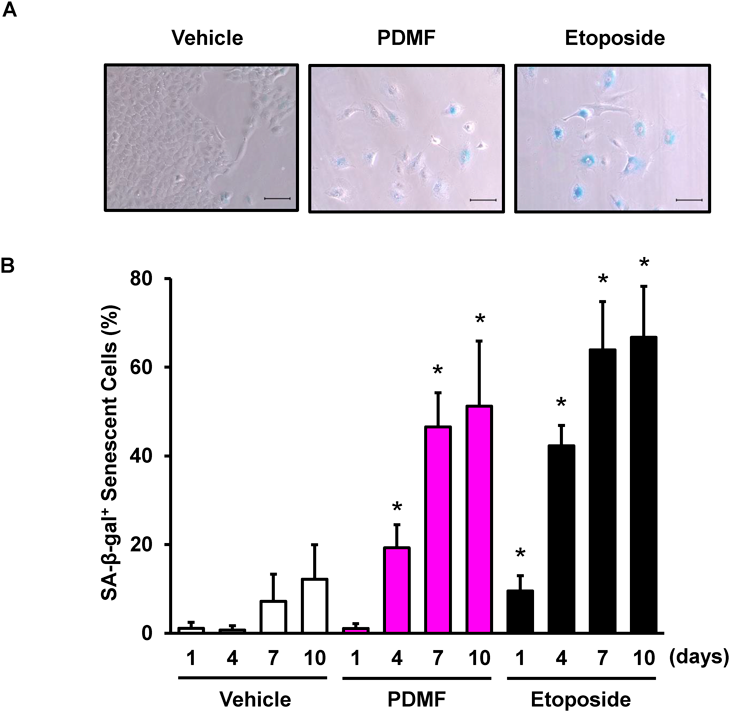
(A) Microscopic observation of SA-β-gal+ senescent A549 cells upon 7 d of stimulation with PDMF (200 µM) or etoposide (0.5 µM). Scale bars indicate 100 µm. (B) Time course kinetics of PDMF-driven senescence induction. Data are indicated as mean ± standard deviation (S.D.). Asterisks indicate significant differences as compared with the corresponding vehicle groups (p < 0.05).

(A) Microscopic inspection of SA-β-gal+ cells. NHBE cells were stimulated with PDMF (200 µM) or etoposide (0.5 µM) for 7 d. Scale bars indicate 100 µm. (B) Time course analysis of SA-β-gal+ NHBE cells upon stimulation with PDMF or etoposide. Data are indicated as mean ± S.D. Asterisks indicate significant differences (p < 0.05, vs. the vehicle groups).
As p53 and its transcriptional target, p21, play a crucial role in inducing cancer cell senescence,8,11) we next examined whether PDMF could activate the p53–p21 pathway in A549 cells as well as in NHBE cells. Western blot analysis indicated that stimulation with PDMF induced serine (Ser) phosphorylation of p53 at the Ser15 residue [p-p53 (Ser15)], and increased the protein expression levels of p53 and p21 in A549 cells (upper panels in Fig. 3A). However, the same PDMF stimulation failed to phosphorylate p53 and up-modulate p21 protein levels in NHBE cells (lower panels in Fig. 3A). In contrast to the differential actions of PDMF, stimulation with etoposide (shown as “Etopo.” in Fig. 3A) equivalently induced p53 phosphorylation in both A549 cells and NHBE cells. These results suggest that the cancer-selective pro-senescent potency of PDMF is accompanied by preferential activation of the p53–p21 pathway in A549 cells but not in NHBE cells. Since activation of p53 and subsequent induction of p21 are hallmark markers of cellular senescence,12) the fact that PDMF activates p53 and p21 (Fig. 3A) suggests that this compound fulfills its pro-senescent action accompanied by up-regulation of those bona fide senescence markers. Our preliminary transcriptome analysis also has indicated that PDMF induces CXCL-8/IL-8 and CCL2/MCP-1, both of which represent another senescence marker known as senescence-associated secretory phenotype (SASP)-associated chemokines (A. M. and S. K., unpublished data).12)

(A) A549 cells or NHBE cells were treated with PDMF (200 µM) or etoposide (0.5 µM) for 96 h. Western blot analysis was conducted for detecting serine 15-phosphorylated p53 (p-p53-Ser15), p53, or p21. GAPDH was detected as a loading control. (B) p53 is essential for the pro-senescent action of PDMF. A549 cells were transfected with p53 siRNA or negative control siRNA on days 0, 3, and 6. After 9 d culture upon stimulation with PDMF (200 µM), SA-β-gal+ senescent cells were counted. Data are indicated as mean ± S.D. Asterisks indicate significant differences compared with each group (p < 0.05).
To determine whether p53 is required for the pro-senescent action of PDMF, we also knocked down p53 in A549 cells upon stimulation with PDMF. Successful siRNA-mediated knockdown of p53 in A549 cells has already been established in our previous study.7) We found that p53 knockdown completely abolished the pro-senescent activity of PDMF (Fig. 3B), indicating that p53 is essential for the PDMF-driven cancer cell senescence in A549 cells.
The precise mechanism by which PDMF selectively activates p53 in A549 cells to induce cellular senescence requires further investigation. PDMF stimulation induced p53 phosphorylation in A549 cells but not in NHBE cells, whereas etoposide induced p53 phosphorylation in both cells (Fig. 3A), suggesting that PDMF may induce p53 phosphorylation via signaling pathways other than those involved in the DNA damage checkpoint. Indeed, our preliminary observations indicate that PDMF fails to activate ATM, a major p53 kinase that senses DNA damage (A. M. and S. K., unpublished data). Identification of PDMF-responsive p53 kinases is critical for elucidating the molecular basis by which PDMF induces cancer-specific cellular senescence. Another unsolved issue is why and how PDMF acts primarily on A549 cells, but not on NHBE cells; are there any differences in its cell surface binding, incorporation, and/or subcellular localization? Do PDMF-targeted molecules exist only in A549 cells? To address these issues, comparative cell imaging analysis of PDMF and identification of PDMF-binding targets are underway.
In summary, we showed that PDMF induces p53-driven cancer cell senescence in A549 human lung cancer cells, but not in primary NHBE cells. Further mechanistic elucidation of its cancer selectivity may provide insights into the development of pro-senescence therapies for cancers.
This work was supported by JSPS KAKENHI Grant No. 22K06661 (to S. K.), JST SPRING Grant No. JPMJSP2132 (to A. M.), and Mishima Foods Co., Ltd. (to S. K.).
N. H. and K. B. are employees of Mishima Foods Co., Ltd. The other authors declare no conflict of interest.
This article contains supplementary materials.