2022 Volume 45 Issue 10 Pages 1510-1517
2022 Volume 45 Issue 10 Pages 1510-1517
Royal jelly (RJ) has beneficial effects on human health, and some of these effects are reported to be the result of its estrogenic activity; however, chemicals with estrogenic activities may disrupt physiological estrogen signaling leading to adverse effects on human health. Thus, clarification of the mode of action of RJ is needed. Here, we investigated whether the estrogen-like actions of RJ are induced via estrogen receptors (ERs)–mediated genomic actions by using an in vitro reporter assay in human choriocarcinoma JEG3 cells and an estrogen-responsive reporter (E-Rep) mouse line that can be used to sensitively detect transactivation of ERs in multiple organs simultaneously. In the in vitro reporter assay, ERs–dependent transcriptional activity was significantly increased by 17β-estradiol (E2) treatment at concentrations of 1 nM and above, confirming that the assay was highly responsive to estrogen; however, RJ did not exhibit any agonist activity via either the α or β form of ER. Similarly, in E-Rep mice, E2 showed significant ERs–dependent genomic action in 17 tissue types including uterus and mammary gland, whereas RJ did not. Thus, unlike endocrine-disrupting chemicals, the estrogen-like activity of RJ is unlikely to be due to genomic actions via ERs.
Estrogens are steroid hormones that regulate various physiological processes, and the actions of estrogens are typically mediated by estrogen receptors (ERs), α and β, which are nuclear receptors acting as transcription factors. The binding of estrogens to ERs produces an estrogen–ER transcriptional complex in which the receptor is converted to its functionally active form.1,2) The estrogen–ER transcriptional complex then binds to estrogen-responsive elements (EREs) in the promoter regions of downstream genes to induce gene expression. In addition to their ER-mediated genomic actions, estrogens have non-genomic actions; for example, they activate G protein-coupled receptor 30 (GPR30) on the plasma membrane, and they rapidly induce intracellular mitogen-activated protein kinase signaling.3,4) Therefore, the physiological mechanisms of estrogen signaling are complicated. The genomic actions of ERs are important for various physiological processes; however, their unforeseen actions can induce various negative effects, such as reproductive abnormality, hormone-mediated cancers, and alteration of neuronal development, and so on, as can be seen in the case of endocrine-disrupting chemicals (EDCs).5–7)
Royal jelly (RJ), a yellowish material secreted from the hypopharyngeal glands of worker bees of the European honeybee (Apis mellifera), is the principal food source of the queen honeybee, and it consists of water (50–60%), proteins (18%), carbohydrates (15%), lipids (3–6%), mineral salts (1.5%), and vitamins.8) RJ is widely used in the manufacture of cosmetics and health foods because of its beneficial effects on human health. A randomized placebo-controlled, double-blind trial revealed that the ingestion of RJ for six-month improved glucose tolerance, erythropoiesis, and mental health in humans.9) Also, animal study has demonstrated the improvement of the stress-induced depression-like behavior in RJ-treated mice.10) Moreover, RJ has been shown to have anti-microbial,11) hepatoprotective,12) insulin-like,13) neurotrophic,14) anti-allergic,15) and myeloprotective and anti-tumor16) activities. One of the mechanisms for the beneficial effects of RJ is thought to be its estrogenic activity. For example, RJ has been shown to decrease bone loss in an ovariectomized rat model of osteoporosis caused by estrogen deficiency.17) Other in vitro and in vivo studies have also reported the estrogenic properties of RJ.18–20) However, many of these reports of the beneficial estrogen-like effects of RJ are limited to specific tissues or cell types, and the detailed mode of action remains unclear. In addition, chemicals with estrogenic actions may disrupt physiological estrogen signaling leading to adverse effects on human health like EDCs. Therefore, studies to clarify the mechanism of action of RJ are needed.
The current standard approach for elucidation of the in vivo estrogenic activity of a chemical is the uterotrophic bioassay in rodents (Organisation for Economic Cooperation and Development (OECD) Test Guideline (TG) 440).21) However, a major drawback of this approach is that it allows for the detection of estrogenic activity only in uterine tissues. Previously, we established an estrogen-responsive transgenic reporter mouse (E-Rep mouse), in which the luciferase gene luc2 was expressed under the control of EREs and demonstrated that these mice can be used to evaluate the estrogenic properties of chemicals in multiple organs and tissues simultaneously, not only in uterine tissue.22) For example, using E-Rep mice, we reported that 2,3,7,8-tetrachlorodibenzo-p-dioxin, a known EDC, exerts estrogenic effects in the liver and kidney and anti-estrogenic effects in the pituitary gland within the same individual,22) and that triphenyl phosphate, an organo-phosphate flame retardant, exerts anti-estrogenic effects in the uterus and pituitary gland.23) An additional advantage of our E-Rep mice is that they can be used to detect ER–ERE-dependent genomic actions, which is useful for elucidating the mechanisms underlying the biological effects of chemicals with estrogenic activity.
Here, we examined whether the estrogen-like actions of RJ are induced via ERs-mediated genomic actions by using both an in vitro reporter assay and our previously developed E-Rep mice.
17β-Estradiol (E2) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). D-Luciferin was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). RJ (Lot. 100909B-2) was kindly provided by API Co., Ltd. (Gifu, Japan) as a freeze-dried form consisting of protein (39.4%), 10-hydroxy-2-decenoic acid (10H2DA) (6.0%), water (2.1%). No denaturation or degradation of the components of the freeze dry RJ powder has occurred due to heating and drying. RJ used in the current study was produced by Apis mellifera in Jiangsu Province, China, and the bees have access to rapeseed.
In Vitro Dual-Luciferase Reporter AssayPlasmid construction and detailed protocols of dual-luciferase reporter assays are described in a previous study.24) Briefly, human choriocarcinoma JEG-3 cells (ATCC HTB-36) were obtained from the American Type Culture Collection (Manassas, VA, U.S.A.) and cultured in minimal essential medium (Invitrogen, Carlsbad, CA, U.S.A.) containing 2 mM l-glutamine, 0.1 mM minimal essential medium nonessential amino acid solution (Invitrogen), and 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere containing 5% CO2. The cells (3 × 104 cells/well) were seeded in 24-well plates 24 h before transfection. Then, the cells were transfected at 37 °C with pGL4.23-ERE (a plasmid that expresses Firefly luciferase under the control of EREs) (20 ng/well), pGL4.74 (a Renilla luciferase expression plasmid) (0.2 ng/well), and pSVhERα (a human ERα expression plasmid) (5 ng/well) for ERα agonist assay, and with pGL4.23-ERE (10 ng/well), pGL4.74 (0.1 ng/well), and pSVhERβ (a human ERβ expression plasmid) (10 ng/well) for ERβ agonist assay, using Lipofectamine reagent (Invitrogen). At 24 h after transfection, E2 and RJ were dissolved in dimethyl sulfoxide (DMSO) and added to the cultures (final DMSO concentration 0.1% (v/v)) and the cells were cultured in medium containing 1% charcoal-stripped FBS for another 24 h at 37 °C. After incubation, cells were lysed in Passive Buffer (Promega, Madison, WI, U.S.A.), and the cell lysate were assayed for luciferase activity by using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was determined as the fold induction of the control after normalization against Renilla luciferase activity.
AnimalsFemale E-Rep mice (ICR;B6D2-Tg(ERE-Luc)5Tysn)22) were used for the present in vivo experiments. Male E-Rep mice were used for breeding, and the strain was maintained by mating with female ICR mice (SLC, Hamamatsu, Japan). All animals were maintained under a 12–12-h light–dark cycle (lights off at noon) and a constant temperature (23 ± 2 °C). Food and water were provided ad libitum. All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of Gifu Pharmaceutical University (Gifu, Japan). All efforts were made to minimize both the number of animals used and the pain and distress they experienced.
Animal TestingThe in vivo experiments were conducted according to OECD TG 440 (uterotrophic bioassay in rodents) protocol.21) Bilateral ovariectomy was performed in isoflurane-anesthetized female E-Rep mice at 8 weeks of age (designated as day −7). To eliminate the effects of phytoestrogens contained in the diet, AIN-93M purified diet (CLEA Japan, Tokyo, Japan) was used. At 7 d post-ovariectomy (day 0), the mice were started on AIN-93M alone (control group), AIN-93M containing E2 at various concentrations (0.01, 0.1, 1, and 10 ppm), or AIN-93M containing 4% (w/w) freeze-dried RJ, and feeding with the test diet was continued for 7 consecutive days (days 0–7).
At the end of treatment (on day 7), the mice were euthanized by a lethal overdose of isoflurane, and the uterus and other tissues (pituitary gland, kidney, muscle, fat, adrenal gland, small intestine, thymus, liver, stomach, lung, large intestine, heart, pancreas, brain, spleen, and mammary gland) were isolated and weighed. For the uterotrophic bioassay, although both wet and blotted (i.e., after removal of the luminal contents) uterus weight are evaluated in OECD TG440, the results of a validation study have shown that there is no difference in the data obtained with only one of the weights25); therefore, we evaluated only the blotted weights in the present study. The mammary gland was fixed with 4% paraformaldehyde, and transferred sequentially to 10% and 20% sucrose solution. The mammary gland samples were embedded in tissue freezing medium and frozen in liquid nitrogen for immunohistochemistry. Other tissues were frozen at −80 °C until use in the luciferase assay.
Luciferase Assay for TissuesTissue samples were homogenized in lysis buffer (1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), 2 µg/mL aprotinin (FUJIFILM Wako, Osaka, Japan), and 2 µg/mL leupeptin (Peptide Institute, Inc., Osaka, Japan)) by using a micro-homogenizer. Then, 10 µL of the tissue lysate was incubated with 50 µL of luciferin substrate solution (20 mM tricine (pH 7.8) (Nacalai Tesque), 2.67 mM MgSO4, 0.1 mM ethylenediaminetetraacetic acid, 33.3 mM dithiothreitol (Nacalai Tesque), 0.27 mM CoA (Oriental Yeast Co., Ltd., Tokyo, Japan), 0.53 mM adenosine triphosphate (Oriental Yeast Co., Ltd.), and 0.47 mM D-luciferin (Sigma-Aldrich)). Luciferase activity was measured with a Mithras LB940 luminometer (Berthold Technologies, Wildbad, Germany), and values are presented as relative light units (RLU)/µg protein.
Immunohistochemical Analysis of Mammary GlandCryosections (16 µm) of the sampled mammary glands were generated and mounted onto microscope slides and then dried. The slides were then washed in 0.1% Tween-20-phosphate-buffered saline (PBS), treated with 0.5 µg/mL proteinase K, washed in 0.1% Tween-20–PBS, and blocked with 0.1% bovine serum albumin (BSA)–PBS for 1 h. After blocking, the slides were incubated with rabbit anti-myc tag (abcam, Cambridge, U.K., ab9106, RRID:AB_307014, 1 : 500) diluted in 0.1% BSA–PBS overnight at 4 °C. After washing in 0.1% Tween-20–PBS, the slides were incubated with Alexa Fluor 633 goat anti-rabbit immunoglobulin G (IgG) (Invitrogen, Carlsbad, CA, USA, A-21070, RRID:AB_2535731, 1 : 500) diluted in 0.1% BSA–PBS for 1 h at room temperature. After washing in 0.1% Tween-20–PBS, the slides were incubated with 4′,6-diamidino-2-phenylindole (DAPI) diluted in PBS for 5 min and washed again with 0.1% Tween-20–PBS. Finally, the slides were enclosed in mounting medium and observed under a fluorescence microscope (BZ-8000, Keyence, Tokyo, Japan) and the images were analyzed by BZ analyzer (Keyence).
StatisticsStatistical differences were determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. All results were performed with SPSS 15.0 J for Windows (SPSS, Inc.). Values with p < 0.05 (one-sided test) were considered statistically significant.
It has been shown that RJ activates EREs in in vitro reporter gene assays.18) Therefore, to determine which form of ER, α or β or both, is involved in ERE activation by RJ, we conducted a dual-luciferase reporter assay using human choriocarcinoma JEG3 cells co-transfected with full-length ERα- or ERβ-expression plasmids and ERE-reporter plasmids.
First, we confirmed that the assay was sensitive to E2. In JEG3 cells co-transfected ERα-expression plasmids and ERE-reporter plasmids, treatment with E2 at concentrations of 0.01, 0.1, 1, 10, and 100 nM increased ERα-dependent luciferase activity by 1.22-, 1.67-, 2.58-, 2.81-, and 3.65-fold, respectively, with the increases being statistically significant at concentrations of 1 nM and higher (Fig. 1A). Similarly, in JEG3 cells co-transfected ERβ-expression plasmids and ERE-reporter plasmids, treatment with E2 at concentrations of 0.1, 1, 10, and 100 increased ERβ-dependent luciferase activity by 48.6-, 120, 109-, 128-, and 158-fold, respectively, with the increases being statistically significant at concentrations of 1 nM and higher (Fig. 1B). Thus, we concluded that the assay was indeed sensitive to estrogen stimulus.

JEG-3 cells were co-transfected with pGL4.23-ERE plasmids, pGL4.74 plasmids, and either pSVhERα (A, C) or pSVhERβ (B, D) plasmid. The cells were then treated with various concentrations of 17β-estradiol (E2) (A, B), or with various concentrations of royal jelly (RJ) or with 100 nM E2 (C, D). Finally, Firefly and Renilla luciferase activities were measured by using a luminometer. Relative Firefly luciferase activity is shown as the fold induction of the vehicle control after normalization against Renilla luciferase activity, and as the mean ± S.D. of quadruplicate cultures. * p < 0.05 vs. vehicle control by one-way ANOVA followed by Dunnett’s multiple comparisons test.
We then examined the agonist activity of RJ against ERs in JEG3 cells. In JEG3 cells co-transfected ERα-expression plasmids and ERE-reporter plasmids, treatment with RJ at concentrations of 0.3, 3, and 30 µg/mL had no effect on ERα-dependent luciferase activity (Fig. 1C). A similar lack of effect was seen in JEG3 cells co-transfected ERβ-expression plasmids and ERE-reporter plasmids treated with 0.3 or 3 µg/mL RJ (Fig. 1D). Thus, we concluded that RJ does not have any agonist activity against ERs in this in vitro reporter assay system.
Contrary to the present results, RJ is reported to inhibit the binding of E2 to both ERα and ERβ.18) However, the inhibitory activity of RJ is approximately 10000-fold weaker than that of diethylstilbestrol, a synthetic estrogen, and the results are insufficient evidence that the components of RJ bind directly to ERs. The same study also showed that RJ activates EREs in in vitro reporter gene assays18); however, we did not find that to be the case in the present study. The reasons for the differences between the results of the current study and those of the previous study are not clear; however, there are two possible explanations. First, we used pGL4.23 plasmid containing the luc2 gene, which has more than 90% fewer putative transcription binding sites26) compared with the older vector used by the previous study,18) which would mean less non-specific luciferase activity was detected in the current study. Second, we used plasmids expressing the two different forms of ER, whereas the previous study did not used any plasmids expressing ERs.18) In the absence of ERs expression plasmids, it is not possible to determine which form of ER, α or β or both, is involved in ERE activation. In contrast, by using ERs expression plasmids, we were able to more accurately detect ER-dependent transcriptional activation. These differences indicate that the in vitro system used in the present study is much better designed, in terms of detecting ERs-dependent luciferase activity with less non-specific luciferase expression, than that used in the previous study18) and that our system accurately captured the estrogenic action potential of RJ.
Evaluation of the Estrogenic Activity of RJ in VivoAlthough our in vitro reporter assay showed that RJ has no agonist activity against either form of ER, the in vivo estrogenic activity of a chemical is dependent not only on its affinity for ER but also on its pharmacokinetics and metabolic activation as well as the presence of any tissue-specific proteins, such as transcriptional co-activators or co-repressors that interact with the ER transcriptional complex. Therefore, the estrogenic activity of a chemical must be considered for all the different tissues in the body at the same time. The uterotrophic bioassay (OECD TG440), which is based on a physiological phenomenon in which uterine weight increases in response to chemicals with estrogenic activity, has been proposed as an in vivo screening test to evaluate the estrogenic properties of chemicals.25,27) Therefore, the following in vivo experiments were basically performed in accordance with the protocol of the OECD TG440.21)
To investigate whether RJ has estrogenic activity in vivo, E-Rep mice were exposed to 4% (w/w) RJ or 0.01, 0.1, 1, or 10 ppm E2 (as positive controls) for 7 d, and then estrogenic activity was evaluated. Because the actual ingestion route of RJ as health food is oral intake, we evaluated the effects of oral administration of RJ on E-Rep mice. We confirmed that neither the body weight nor the feed intake of the mice changed significantly during the treatment period, indicating that neither E2 nor RJ treatment induced systemic toxicity (Fig. 2). The actual exposure dose of RJ, calculated from the mean food intake (4.65 g) and mean body weight (31.1 g) of the mice in the 4% (w/w) RJ-treated group, was about 5.98 g/kg-body weight (b.w.)/d. It is reported that oral administration of RJ at 2 g/kg-b.w./d for 2 d induces anti-fatigue effects in five-week-old male ddY mice.28) Moreover, oral administration of RJ at 0.005–0.05 g/kg-b.w./d for 6 weeks (5 d per week) is reported to inhibit the development of atopic dermatitis–like skin lesions induced in female NC/Nga mice by repeated application of the hapten picryl chloride.29) Therefore, the 4% RJ used in the present study afforded the same or higher concentration of RJ compared with those used in these previous studies. In addition, the actual exposure dose of E2 calculated from the mean food intake (4.11 g) and mean body weight (31.7 g) of the mice exposed to 1 ppm E2 was about 130 µg/kg-b.w./d, which is comparable with the dose at which luciferase induction was observed in E-Rep mice subcutaneously administered E2 in a previous study (100 µg/kg-b.w./d).22) Together, these findings indicate that the RJ concentration used in the present study was appropriate for investigating the physiological effects of RJ.

Ovariectomized female E-Rep mice were fed AIN-93M alone (control group) or AIN-93M containing the indicated concentrations of 17β-estradiol (E2) or 4% w/w royal jelly (RJ) for 7 consecutive days. Body weight and food intake was measured once each day. Data are shown as means ± S.D. (n = 5–7).
We next examined the change in the uterine weight in the E-Rep mice exposed to 4% RJ or to 0.01, 0.1, 1, or 10 ppm E2 for 7 d, in accordance with the protocol of the OECD TG440 (Fig. 3). In mice exposed to 1 or 10 ppm E2, uterine weight was significantly (p < 0.05) increased from 0.039 g (control) to 0.193 and 0.274 g, respectively, indicating that the bioassay was sensitive to the estrogenic activity of E2 (Fig. 3). In contrast, in mice exposed to RJ, no change in uterine weight was observed. These results indicate that uterotrophic bioassay was not able to detect any estrogenic activity of RJ.
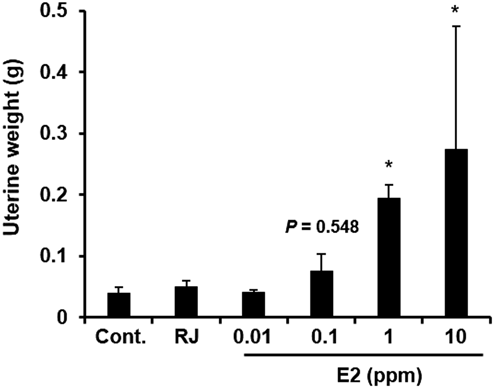
Ovariectomized female E-Rep mice were fed AIN-93M alone (control group) or AIN-93M containing the indicated concentrations of 17β-estradiol (E2) or 4% w/w royal jelly (RJ) for 7 consecutive days, after which uterine weight was measured. Data are shown as mean ± S.D. (n = 4–7). * p < 0.05 vs. control by one-way ANOVA followed by Dunnett’s multiple-comparisons test.
Because the bioassay using an increase in uterine weight did not detect any estrogenic activity of RJ, we repeated the experiment but this time used a luciferase-based assay to detect ERs-dependent genomic actions in various tissues. The luciferase activity in the uterus of E-Rep mice was significantly (p < 0.05) increased from 16.1 (control) to 425 RLU/µg protein after exposure to 0.1 ppm E2, and tended to be increased to 156 (p = 0.106) or 167 (p = 0.085) RLU/µg protein, although not significantly so, after exposure to 1 or 10 ppm E2, respectively (Fig. 4A). These findings suggest that this luciferase assay was more sensitive than the uterotrophic bioassay, which detected the estrogenic activity of E2 at a concentration of 1 ppm or more (Fig. 3). Despite this improved sensitivity, no luciferase activity was detected in the uterus of mice exposed to RJ (Fig. 4A).

Ovariectomized female E-Rep mice were fed AIN-93M alone (control group) or AIN-93M containing the indicated concentrations of 17β-estradiol (E2) or 4% w/w royal jelly (RJ) for 7 consecutive days, after which luciferase activity in the indicated tissues was measured by using a luminometer. Data are shown as relative light units (RLU)/µg protein and as mean ± S.D. (n = 4–7). * p < 0.05 vs. control by one-way ANOVA followed by Dunnett’s multiple comparisons test.
We then examined the luciferase activity in 15 other tissues (pituitary gland, kidney, muscle, fat, adrenal gland, small intestine, thymus, liver, stomach, lung, large intestine, heart, pancreas, brain, and spleen) (Figs. 4B–P). In the pituitary gland, which is one of the most responsive organs to estrogen, luciferase activity was significantly increased by exposure to 0.1 or 10 ppm E2 and tended to be increased, although not significantly so, by exposure to 1 ppm E2 compared with control; in contrast little luciferase expression was detected in the pituitary gland of mice exposed to RJ (Fig. 4B). Similarly, the induction of luciferase activity was detected in all of the other tissues examined by exposure to at least 10 ppm E2, indicating that all of the tested tissues showed estrogen-responsiveness (Figs. 4C–P). However, little or no luciferase activity was detected in any of the tissues after exposure to RJ. Thus, we conclude that RJ exert little estrogenic effects on tissues via ER-ERE–dependent genomic actions.
The mammary gland is another very responsive organ to estrogen; however, because the mammary gland is surrounded by adipose tissue, it is technically difficult to isolate it as a single tissue and evaluate its luciferase activity. Therefore, to investigate whether RJ has estrogenic activity in mammary gland, we performed an immunohistochemical analysis of myc-tagged luc2 protein (Fig. 5). Induction of myc-tagged luc2 protein expression was detected in the mammary gland of E-Rep mice exposed to 1 or 10 ppm E2; however, no myc-positive signal was detected in mice exposed to RJ, indicating that RJ has no estrogenic activity in mammary gland. Thus, taking this finding together with those in the other tissues, we conclude that RJ exert little or no its estrogenic effects via ER–ERE-dependent genomic action.

Ovariectomized female E-Rep mice were fed AIN-93M alone (control group) or AIN-93M containing the indicated concentrations of 17β-estradiol (E2) or 4% w/w royal jelly (RJ) for 7 consecutive days, after which immunostaining for myc-tagged luciferase protein was performed using rabbit anti-myc antibody (red) in the mammary gland. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining (blue). Scale bars: 500 µm.
The literature contains reports indicating that RJ has osteoinductive,30) anti-inflammatory,31) anti-skin aging,32,33) anti-microbial,11) hepatoprotective,12) insulin-like,13) neurotrophic,14) anti-allergic,15) and myeloprotective and anti-tumor16) activities. Some of these beneficial effects of RJ are thought to be related to its estrogenic activity. A randomized controlled clinical trial involving 90 married, postmenopausal women (age, 50–65 years) showed that vaginal treatment with RJ was effective at improving symptoms associated with sexual and urinary problems and at improving QOL, which the authors attributed to the estrogenic properties of RJ.34) In addition, there are several reports showing that RJ exhibits weak estrogenic activity in vitro and in vivo. For example, it has been reported that subcutaneous injection of 1 g/kg-b.w. RJ induces mRNA expression of vascular endothelial growth factor, an estrogen-responsive gene, in rat uterus and that RJ activates EREs in in vitro reporter gene assays and enhances cell proliferation in human breast cancer MCF-7 cells.18) Moreover, oral administration of 4% (w/w) RJ in a diet to female SAMR1 mice for 9 weeks is reported to result in an increase in bone ash content and up-regulation of procollagen I α1 mRNA expression, as induced by E2.19) In addition, subcutaneous injection of 1 g/kg-b.w./d RJ for 3 d in 20-d-old female Sprague Dawley rats is reported to result in mild hypertrophy of the uterine luminal epithelium.20) However, the estrogen-like effects of RJ reported in these studies are limited to specific tissues and cell types, and it is unclear whether these effects are directly induced by ER-mediated genomic actions. In contrast to these previous findings, in the present study in E-Rep mice we found that RJ has little or no estrogenic activity via ER-mediated genomic actions in various tissues including the uterus. Given that we confirmed that the evaluation systems used in the present study could sensitively detect ER-mediated genomic actions, we conclude that the previously reported estrogen-like effects of RJ in animal and cellular models, and the beneficial effects of RJ in humans, are induced via ER genomic action-independent mechanisms. The implication is that, unlike EDCs, RJ has little potential to induce adverse effects by disrupting the genomic actions of ERs.
RJ used in the current study was produced by Apis mellifera in Jiangsu Province, China, and contains 6.0% 10-hydroxy-2-decenoic acid (10H2DA). 10H2DA is known as one of the major medium-chain fatty acids contained in RJ. It has been reported that subcutaneous injection of 1 g/kg-b.w./d 10H2DA for 3 d in immature Sprague Dawley rats results in mild but statistically significant hypertrophy of the uterine luminal epithelium without increasing uterine weight, concluding that 10H2DA contributes to the estrogenic effects of RJ.20) In the present study, the mice were calculated to have been exposed to 0.36 g/kg-b.w./d 10H2DA. Although the routes of administration are different, the dose of 10H2DA is only about three times different between our study and the previous study (1 g/kg-b.w./d).20) However, neither uterine weight nor luciferase activity were increased in the current study in E-Rep mice. Therefore, the mild hypertrophy of the luminal epithelium reported in the previous study20) may be the result of other modes of action of 10H2DA rather than estrogenic action. Another study demonstrated that exposure of ovariectomized mice to 0.04 g/kg-b.w./d 10H2DA for 4 weeks suppresses osteoclastogenesis by inhibiting nuclear factor-κB (NF-κB) signaling.35) Therefore, this inhibitory action of 10H2DA on NF-κB signaling may be one possible mechanism of not only the osteoinductive effects but also the other beneficial effects of RJ. In addition, it remains possible that the estrogen-like effects of RJ, such as the suppression of bone loss in an osteoporosis model rat,17) are mediated by non-genomic actions of GPR30 (plasma membrane ER) and subsequent activation of phosphatidylinositol-3 kinase (PI3K)/Akt signaling,36,37) because PI3K/Akt signaling is reported to be involved in osteoblast differentiation.38) Further studies are needed to clarify the detailed molecular mechanisms in the estrogen-like effects of RJ.
Here, we investigated the estrogenic activity of RJ using both an in vitro reporter assay system and an estrogen-responsive reporter mouse, and found that RJ exhibited little or no estrogenic activity in either system. These results suggest that the previously reported beneficial effects of RJ on human health are not the result of ER-mediated genomic actions, and that oral ingestion of RJ has little potential to induce adverse effects by estrogen disruption, as in the case of EDCs.
This study was financially supported by a Grant-in-Aid for Scientific Research (B) (18H03381) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; the Long-range Research Initiative of the Japan Chemical Industry Association; and a Grant-in-Aid for Research on Risk of Chemical Substances (21KD1004) from the Ministry of Health, Labour and Welfare, Japan. We thank API Co., Ltd., (Gifu, Japan) for providing the royal jelly and information pertaining to its components.
The authors declare no conflict of interest.