2022 Volume 45 Issue 12 Pages 1857-1861
2022 Volume 45 Issue 12 Pages 1857-1861
Various side effects associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in analgesia have been reported. Among the NSAIDs, celecoxib has fewer side effects and is often used in therapeutic applications. However, the effect of celecoxib on aged skin is unknown. In this study, we investigated the effects of celecoxib administration on the skin of aged mice. We analyzed a 40-week-old mouse model and a 10-week-old mouse as the control group. The animals were orally administered celecoxib for four consecutive days and then killed and dissected the day after the last dose. In aged mice treated with celecoxib, the water content of the stratum corneum, which is one of the markers of dry skin, was lower than that in the control and young mice groups. In addition, serum hyaluronic acid, creatinine, and inflammatory cytokines in the collected blood samples of aged mice were elevated compared to those in other mice groups, suggesting the onset of acute renal injury. Therefore, it was considered that acute renal injury occurred from the administration of celecoxib to aged mice, whereas dry skin developed by the promotion of inflammatory cytokine secretion and release into the bloodstream in this group.
In Japan, which is a super-aging society, the amount of non-steroidal anti-inflammatory drugs (NSAIDs) used is increasing every year, for a wide range of conditions, including immune diseases, arthritis, and headache. NSAIDs exhibit anti-inflammatory and analgesic effects by inhibiting prostaglandin (PG) production, which leads to the inhibition of the cyclooxygenase (COX)-dependent arachidonic acid metabolic pathway. COX has two isoforms: COX-1 and COX-2, with COX-1 being constantly expressed in the body. However, unlike COX-1, COX-2 is expressed in inflammatory tissues. Gastric ulcer is one of the side-effects of NSAID administration.1–3) Nonetheless, COX-2 selective inhibitors cause fewer gastrointestinal adverse effects than COX-1 inhibitors.1–3) Consequently, celecoxib, a COX-2 selective inhibitor, is safer and more preferred for use in the elderly population that generally has reduced physical function.4) Although celecoxib is frequently used because of its high safety, it has been associated with skin problems compared to COX-1 selective inhibitors, excluding the onset of drug eruption. The reported skin-related side effects include dermatitis, itching, and dry skin.5)
To date, no study has focused on the effect of celecoxib on the skin. In particular, since physical function is generally deteriorated in the elderly,6) the physiological function of their skin is also likely to decline. Therefore, this population is at a higher risk of developing skin-associated side effects from the use of celecoxib.
In this study, we aimed to investigate the effect of celecoxib on aged/elderly skin. Male mice (10- or 40-week-old) were evenly divided into two groups (four mice per group). One group was treated with celecoxib, and the other with saline. After continuous daily oral administration, transepidermal water loss (TEWL) and stratum corneum water content of the dorsal skin were measured. In addition, markers indicating kidney function and inflammatory cytokines in the plasma were measured. Subsequently, the expression of dry skin and its mechanism were investigated.
Specific pathogen-free (SPF) hairless mice (HOS:HR-1; 10- and 40-week old) were purchased from SLC (Hamamatsu, Shizuoka, Japan). The mice were housed under a 12-h light cycle at a constant temperature of 23 ± 2 °C and relative humidity of 55 ± 10%. The mice were fed laboratory chow (CE-2; Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum.
Experimental DesignMice were randomly selected (n = 4 per group) to receive oral administration of celecoxib (10 mg/kg, dissolved in 0.25% dimethyl sulfoxide; TOCRIS Bioscience, Avonmouth, Bristol, U.K.) once daily for four days. The celecoxib concentration used in this study was based on Liang et al.,7) and is the maximum dosing that does not impact the general health characteristics (weight loss and behavioral abnormalities) of mice following long-term administration. Control mice were administered the corresponding delivery vehicles according to the same schedule. This study strictly followed the recommendations and guidelines for the care and use of laboratory animals at Suzuka University of Medical Science (Approval No. 34). All surgical procedures were performed with every effort made to minimize animal suffering.
Measurement of TEWL and Water Content of Stratum CorneumOn Day 5, TEWL and water content of the stratum corneum in the dorsal skin of each mouse were measured. TEWL and water content of the stratum corneum are markers of skin permeability, and thus, reflect the barrier function of the skin; decreased TEWL and increased water content of the stratum corneum indicate dry skin.8–10) TEWL and water content of the stratum corneum were measured using a Tewameter TM300 (Courage + Khazaka Electronic GmbH, Cologne, Germany)11) and Corneometer CM825 (Courage + Khazaka Electronic GmbH),12) respectively. TEWL was recorded once the reading had stabilized, approximately 30 s after the probe was placed on the skin. The Corneometer probe was placed on the dorsal skin surface of each mouse to determine electrical capacitance, which was used as a marker for the water content in the animal’s stratum corneum. Values are presented as the average of three independent measurements.
Tissue Sectioning, and Hematoxylin–Eosin (H&E) StainingDorsal skin and jejunal tissue samples were isolated and fixed in phosphate buffered saline containing 4% paraformaldehyde (Wako Pure Chemical Corporation, Osaka, Japan). Fixed tissue specimens were embedded in frozen Tissue-Tek OCT Compound (Sakura Finetek, Tokyo, Japan) and sliced into 5-µm-thick sections. The skin and jejunal specimens were stained with H&E as per the established procedures for histological analysis prior to microscopic evaluation. To determine the overall skin thickness, 10 regions in which the skin appeared flat in the acquired images were randomly selected. The length from the outer layer of the epidermis to the border of the subcutis was measured and the average value was calculated.
Detection of Plasma Hyaluronic Acid, Tumor Necrosis Factor (TNF)-α, and Creatinine ConcentrationPlasma samples were collected on the last experimental day and levels of hyaluronic acid and TNF-α were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, U.S.A.) according to the manufacturer’s instructions. The optical density was measured using a microplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.). Plasma creatinine concentration was measured using a Creatinine Assay Kit (Cayman, Ann Arbor, MI, U.S.A.) according to the manufacturer’s instructions.
Statistical AnalysisAll data are presented as the mean ± standard deviation. Data were analyzed using Tukey’s test and differences were considered statistically significant at p < 0.05.
Following the celecoxib delivery vehicle administration to the control mice, no effect on body weight was observed (Fig. 1).
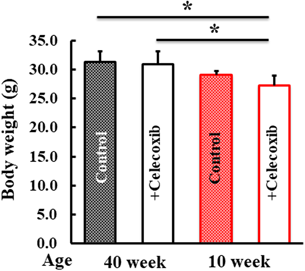
After celecoxib treatment, the body weights of the mice were measured. Values represent mean ± standard deviation (Tukey’s test, * p < 0.05, significant difference).
We examined various indicators of skin dryness. TEWL did not differ among the mice (Fig. 2). However, the capacitance of the dorsal skin in 40-week-old mice treated with celecoxib was lower than that in the control mice (Fig. 2). Moreover, this was absent in the 10-week-old mice (Fig. 2).
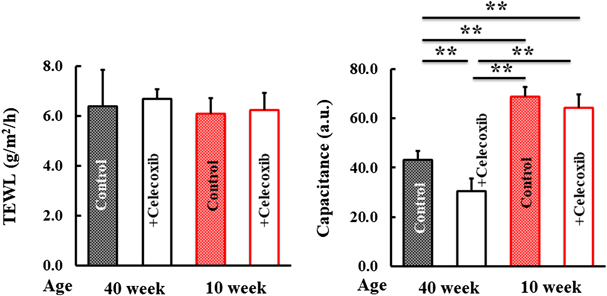
Trans epidermal water loss (TEWL) and capacitance results were compared. Values represent mean ± standard deviation (Tukey’s test, ** p < 0.01, significantly different).
H&E-stained skin and jejunal tissues were evaluated microscopically. The skin of celecoxib-treated 40-week-old mice was thicker compared to that of control mice (Figs. 3a, b, i). However, no difference was observed among the skin of 10-week-old mice (Figs. 3c, d, i). The jejunal tissue were not affected by celecoxib in both 10- and 40-week-old mice (Figs. 3e–h).
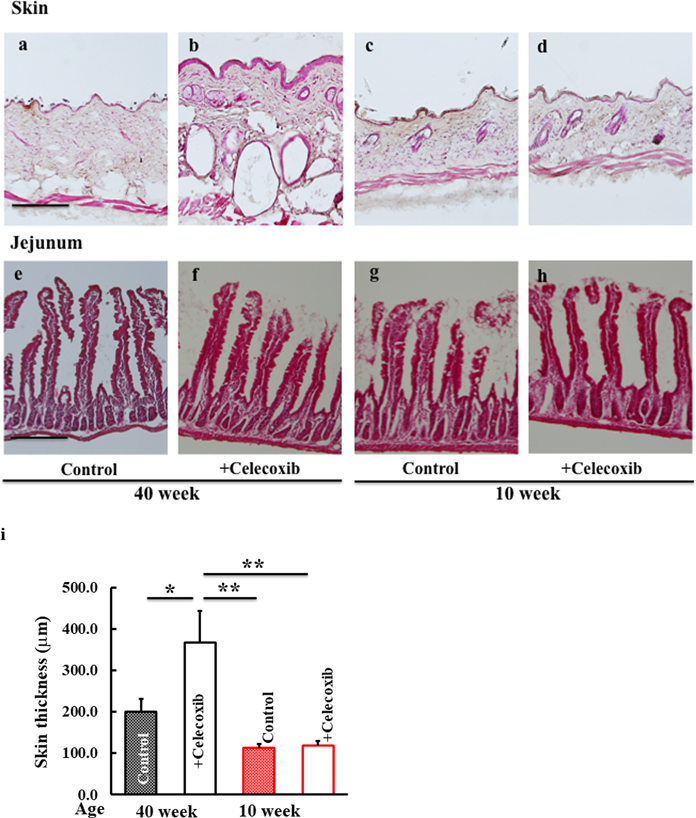
Skin (a–d) and jejunal (e–h) tissues of 40- and 10-week-old mice treated with celecoxib (b, d, f, h) compared with controls (a, c, e, g). To determine the skin thickness, the length from the outer layer of the epidermis to the border of the subcutis was measured (i). Scale bar = 100 μm.
Plasma concentrations of markers for inflammatory conditions were analyzed. Hyaluronic acid and TNF-α in the plasma of 40-week-old-mice increased following celecoxib treatment (Fig. 4). Creatinine, a kidney injury marker, was higher in 40-week-old mice treated with celecoxib than that in control mice. In contrast, these developments were not observed in the 10-week-old mice group (Fig. 5).
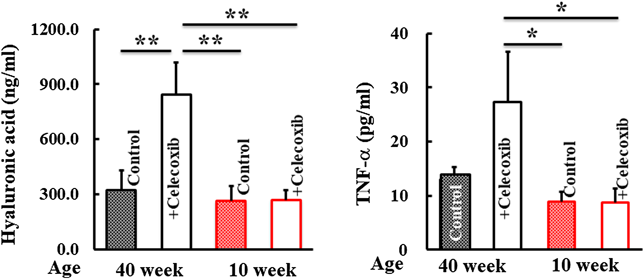
The concentrations of plasma hyaluronic acid and TNF-α were determined using enzyme-linked immunosorbent assay (ELISA) kits. Values represent mean ± standard deviation (Tukey’s test, * p < 0.05, ** p < 0.01, significantly different).
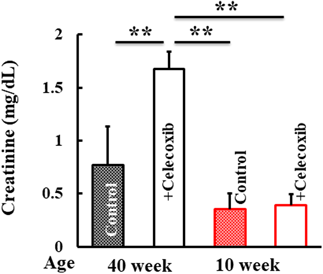
Creatinine concentration was measured in the plasma of 10- and 40-week-old mice treated with or without celecoxib. Values represent mean ± standard deviation (Tukey’s test, ** p < 0.01, significantly different).
In 10-week-old mice, there was no difference in skin dryness between the celecoxib and control groups. However, the 40-week-old mice exhibited a clear difference in the skin condition, based on the TEWL, corneometer, and H&E staining findings, following celecoxib administration when compared with the control group. In addition, increased plasma hyaluronic acid levels were observed only in the 40-week-old celecoxib group, indicating acute tissue inflammation. Thus, aging can be considered a risk factor to developing skin drying as a drug-related adverse effect of celecoxib. The loss of physical function due to aging might make the side effects of drug administration to be more evident.
Increases in blood creatinine and hyaluronic acid are observed in tissues with acute inflammation, suggesting the involvement of the kidneys.13) The effects of NSAIDs, particularly COX-2 selective inhibitors, on the kidneys have been reported.14) According to the drug-induced nephropathy clinical practice guidelines, NSAIDs, including COX-2 inhibitors, may cause ischemic nephropathy and drug-induced kidney injury (DKI) starting immediately after administration and frequently progressing until the 5th day.15) In this study, DKI was observed in aged mice, which is in agreement with previous reports.16,17) No renal disease was observed in young mice, suggesting that the decrease in renal function due to aging increased the adverse effects of celecoxib administration. As a side note, in celecoxib-treated 40-week-old mice, plasma hyalutonic acid increased, but skin hyaluronic acid levels remained unchanged (data not shown). Therefore, hyaluronic acid was considered to have no effect on skin capacitance.
Kidney damage leads to increased secretion of inflammatory cytokines from the organ.18) Consistently, blood levels of TNF-α were increased in this study, suggesting that this inflammatory cytokine affected the skin and contributed to skin dryness. However, the effects of celecoxib in aging mice may not be limited to the kidneys. Following the determination of Glutamic Pyruvic Transaminase and Glutamic Oxalacetic Transaminase levels, we observed that the effect of celecoxib administration on the liver was minimal (data not shown). Nevertheless, we did not investigate the effect of celecoxib on other organs, which requires further scrutiny in the future.
In summary, these results suggest that administration of celecoxib may induce inflammation in the kidneys of aged mice, and substances released from the kidneys may affect the skin and lead to dryness of the skin. However, the direct relationship between celecoxib/renal/dry skin is not clear and needs further investing.
Overall, the findings presented herein reflect the effect of celecoxib in a small sample of aging mice. However, a larger proportion of elderly humans are administered celecoxib, which may present as a better sample size statistically for such a study. Therefore, it is necessary to conduct clinical trials to elucidate the detailed mechanisms of the observed adverse effects in the human elderly population.
This study was supported by JSPS KAKENHI (Grant No. 18K06082).
The authors declare no conflict of interest.