2022 Volume 45 Issue 12 Pages 1832-1838
2022 Volume 45 Issue 12 Pages 1832-1838
SMTP-7, a fungal metabolite, is reported to have a high degree of availability for the ischemia–reperfusion (IR)-induced acute kidney injury (AKI) model. Cisplatin, a widely used anticancer drug, has serious side effects, such as AKI. Hence, we aimed to examine the effect of SMTP-7 on cisplatin-induced AKI in this study. Significant increases in blood urea nitrogen (BUN) and serum creatinine (Scr) were observed at 72 h after the intravenous infusion of cisplatin (20 mg/kg). Histologically, necrosis and dilatation (hyaline casts) as well as regeneration were observed in proximal tubules. SMTP-7 inhibited the elevation on BUN and Scr caused by cisplatin dose dependently. The efficacy of SMTP-7 was notable when the drug was administered on the day after cisplatin treatment, whereas the repeated administration of the drug did not result in an enhanced efficacy. Moreover, 10 mg/kg of SMTP-7 considerably ameliorated tubular necrosis and dilatation. The cisplatin treatment also caused an up-regulation of tumor necrosis factor-α (TNF-α) mRNA expression prior to the elevation of the levels of BUN and Scr. Administration of SMTP-7 (10 mg/kg) at 24 h after the cisplatin infusion alleviated the up-regulation of TNF-α mRNA expression. These findings suggest that SMTP-7 exhibits a renoprotective effect against cisplatin infusion based on the inhibition of the expression of pro-inflammatory cytokines such as TNF-α and may be expected a new effective drug for the treatment of cisplatin-induced AKI.
Recently a rise in the prevalence of renal failure is observed in the world. Although number of studies are carried out, there are few effective drugs for the therapy of renal failure. Renal failure is classified with acute kidney injury (AKI) and chronic kidney disease (CKD). AKI is characterized by a rapid decline in the renal function, progressing over minutes or days.1) Moreover, AKI is associated with high morbidity and mortality. Recently, AKI has been reported to increases the risk for progression to CKD.2) In CKD patients not undergoing treatment, an end-stage renal disease may ensue, requiring renal transplant or dialysis. Based on the prevalence and unfavorable prognosis of AKI, developing new strategies to combat this disease is urgently needed. AKI can be caused by ischemia, drugs, toxins, and sepsis. It is known that about 20% of the cases of AKI among hospitalized patients are drug-induced AKI.3) In particular, cisplatin, a widely used chemotherapeutic drug for various cancers, often induces AKI, and this side effects are limiting factor in chemotherapy.4) The pathological condition of cisplatin-induced AKI is acute proximal tubular necrosis; notably oxidative stress and inflammation play a role in the development of the pathological condition.5)
We reported that SMTP-7, a novel metabolite derived from the fungus Stachybotrys microspore,6,7) improved renal function in the ischemia–reperfusion (IR)-induced AKI model.8) This compound has thrombolytic, anti-inflammatory, and anti-oxidative activities without serious side effects9–15) and is currently being evaluated for its clinical efficacy for acute ischemic stroke.7,16) Inflammation also involves progression of renal injury induced by IR and pro-inflammatory cytokines within the kidney play a key role.17) Based on these reports, we inferred that SMTP-7 might also be useful and represent a new therapeutic strategy against cisplatin-induced AKI.
The study sought to evaluate whether SMTP-7 could exert protective effects against cisplatin-induced AKI. This is the first study on how SMTP-7 affects AKI caused by cisplatin.
Male ddY mice weighing 25–35g were obtained from Tokyo Laboratory Animals Science Co., Ltd. (Tokyo, Japan). They were housed in an air-conditional room (room temperature: 23 ± 2 °C, relative humidity: 50 ± 20%, 12-h light–dark cycle) with free access to standard laboratory diet and water.
All experiments were according to the e regulations of the Committee of Animal Care and Welfare of Showa University.
ReagentsSMTP-7 was kindly supplied from TMS Co., Ltd. (Tokyo, Japan). Cisplatin was obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Both drugs were dissolved in saline (cisplatin: 1 or 2 mg/mL, SMTP-7: 0.01. 0.1 or 1 mg/mL). Blood urea nitrogen (BUN) and serum creatinine (Scr) were determined using a QuantiChrom™ urea assay kit (BioAssay systems, Hayward, CA, U.S.A.) and LabAssay ™ creatinine kit (FUJIFILM Wako Pure Chemical Corporation), respectively. All other reagents were commercially available with the highest grade.
Cisplatin-Induced AKIUnder anesthesia (inhalation of 1.0 to 1.5% isoflurane, FUJIFILM Wako Pure Chemical Corporation), animals received a continuous intravenous infusion of 10 or 20 mg/kg of cisplatin for 15 min intervals. Identical volumes of saline were also infused in a negative control group. After 24, 48, or 72 h of the administration, blood samples were collected. These samples centrifuged at 3000 rpm for 15 min and serum was separated for the determination of BUN and Scr.
Treatment of Cisplatin-Induced AKI with SMTP-7Cisplatin (20 mg/kg) was intravenously infused for 15 min into the mice. SMTP-7 was also intravenously infused for 15 min at a dose of 10 mg/kg immediately, or at 24 or 48 h after the cisplatin infusion. The control group was given cisplatin and vehicle. On the other hand, the negative control group received neither cisplatin nor SMTP-7. In the case of repeated dosing, SMTP-7 was infused at 24 and 48 h after the cisplatin infusion. A dose–response relationship was determined using 0.1, 1, or 10 mg/kg of SMTP-7 at 24 h after the cisplatin administration. At 72 h, blood samples were obtained and kidneys were isolated. Renal dysfunction was evaluated based on the serum levels of BUN and Scr as well as histological examinations of the kidneys.
Histological ExaminationsAfter 72 h of the cisplatin infusion, kidneys were isolated and fixed in 10% formalin neutral buffer solution (FUJIFILM Wako Pure Chemical Corporation), then followed by paraffin embedding. Paraffin embedded kidneys were sectioned into 3 µm thickness, and stained with hematoxylin (Merk, Darmstadt, Germany) and eosin (FUJIFILM Wako Pure Chemical Corporation). A photomicroscope (OLYMPUS BHS, Olympus, Tokyo, Japan) connected with digital camera (OPLYMPUS digital camera E-330, OPLYMPUS) was utilized in determination of histological changes. The images were acquired at a 50- or 200-fold magnification, and histological changes were graded semiquantitatively based on the percentages of damaged tubules in the cortex under blinded evaluations. The histopathological scores are as follows; 0, no remarkable changes; 1, less than 10% of the field; 2, 10–20% of the field; 3, 20–50% of the field; 4, more than 50% of the field.
Real-Time RT-PCRTotal RNA extraction, reverse transcription, and real-time RT-PCR were performed as previously described.18) The primer sequences of β-actin were as follows.
The forward primer: 5′-CCTTCCTTCTTGGGTATGGAATC-3′.
The reverse primer: 5′-TGCTAGGAGCCAGAGCAGTAATC-3′.
Other primers were obtained from Qiagen (Valencia, CA, U.S.A.).
The mRNA expressions of TNF-α and interleukin (IL)-1β were normalized to that of β-actin from the same sample of cDNA.
Data AnalysisThe degree of proximal tubular injury was graded as described above, and changes in histopathological scores were assessed using Mann–Whitney’s U-test. Data are represented as the mean ± standard error of mean (S.E.M.). Statistical significance was determined Dunnett’s test or Student’s t-test.
Figure 1 illustrates time-dependent changes in BUN and Scr after the cisplatin infusion. Increases in BUN and Scr levels was not evident until 48 h after the 20 mg/kg of cisplatin infusion. However, an obvious elevation in both parameters was showed at 72 h after the infusion, and statistically significant differences were observed compared with the untreated group (p < 0.01). It was observed that these values did not change following the 10 mg/kg of cisplatin infusion. Therefore, 20 mg/kg of cisplatin was used to evaluate the effect of SMTP-7, and its efficacy was assessed at 72 h after the cisplatin infusion.
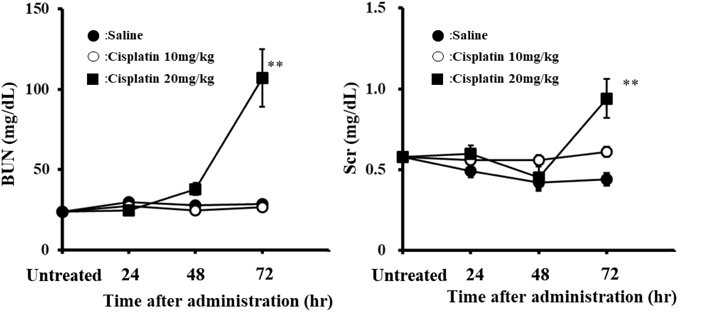
10 or 20 mg/kg of cisplatin was intravenously infused for 15 min. Each point represents the mean ± standard error (S.E.) of 7–10 animals. ** p < 0.01 (compared with untreated group).
Figure 2 shows the results of the SMTP-7 administration at different time points. In the negative control group, the levels of BUN and Scr were 27.5–29.9 and 0.41–0.58 mg/dL, respectively. Cisplatin caused a rise in the levels of BUN and Scr (BUN, 65.5–86.2 mg/dL; Scr, 0.82–1.11 mg/dL); moreover, these values differed from those of the negative control groups (p < 0.01). When 10 mg/kg of SMTP-7 was administered 24 h after the cisplatin treatment, the increase in BUN and Scr levels was mitigated. There were statistical differences between the control and SMTP-7-treated group (BUN, p < 0.05; Scr, p < 0.01). However, SMTP-7 scarcely affected the levels of BUN and Scr at other time periods of the administration, and no significant differences were observed. Increases in BUN and Scr levels were also attenuated by the repeated administration of SMTP-7. However, the effect of the repeated dosing was nearly equal to the single administration at 24 h after the cisplatin infusion (44.5 and 52.8 mg/dL for BUN; 0.59 and 0.80 mg/dL for Scr) (Fig. 3). Consequently, drug effects were evaluated by the treatment at 24 h after the cisplatin infusion in the subsequent experiments examining the dose–response relationship of SMTP-7 on cisplatin-induced nephrotoxicity. SMTP-7 dose-dependently inhibited the elevation of BUN and Scr levels in comparison with positive control groups (p < 0.05 at 0.1 mg/kg and p < 0.01 at 1 and 10 mg/kg for BUN; p < 0.05 at 1 mg/kg and p < 0.01 at 10 mg/kg for Scr) (Fig. 4).

AKI was induced by an intravenous infusion of cisplatin (20 mg/kg). 10 mg/kg of SMTP-7 was also intravenously infused for 15 min just before the cisplatin infusion (immediate) or 24 and 48 h after the cisplatin dosing. BUN and Scr were measured 72 h after the cisplatin infusion. Each column represents the mean ± S.E. of 6–7 animals. ** p < 0.01 (compared with negative control group). # p < 0.05, ## p < 0.01 (compared with control group).

AKI was induced by an intravenous infusion of cisplatin (20 mg/kg). SMTP-7 (10 mg/kg) was also intravenously infused for 15 min as a single dose (at 24 h after the cisplatin infusion) or repeated doses (24 and 48 h after the cisplatin infusion). BUN and Scr were measured 72 h after the cisplatin infusion. Each column represents the mean ± S.E. of 6 animals. ** p < 0.01 compared to negative control group). # p < 0.05, ## p < 0.01 (compared with control group).

AKI was induced by an intravenous infusion of cisplatin (20 mg/kg). SMTP-7 was also intravenously infused for 15 min at 24 h after the cisplatin infusion. BUN and Scr were measured 72 h after the cisplatin infusion. Each column represents the mean ± S.E. of 7–8 animals. ** p < 0.01 (compared with negative group). # p < 0.05, ## p < 0.01 (compared with control group).
The negative control mice showed essentially no abnormality in the renal tissue histology. The cisplatin infusion induced proximal tubular necrosis and degeneration, tubular dilatation (hyaline casts), and regenerated tubules. These changes were limited to a part of the renal tissue in the SMTP-7-treated group. (Fig. 5). The histopathological scores are shown in Table 1. The mean scores of the negative control, positive control, and SMTP-7 groups were 0, 2.8, and 1.6, respectively, for necrosis and degeneration, and 0, 0.6, and 0.1, respectively, for tubular dilatation. The difference between the negative and positive controls were significant (p < 0.01 for necrosis and degeneration and p < 0.05 for tubular dilatation). SMTP-7 minimized these changes, but the difference between the positive control was not statistically significant.

AKI was induced by an intravenous infusion of cisplatin (20 mg/kg). SMTP-7 was also intravenously infused for 15 min at 24 h after the cisplatin infusion. Renal tissue was isolated at 72 h after the cisplatin infusion. Arrows represent location of each histological damage.
| Histopathological findings | Group | Negative | Control | SMTP-7 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 7 | 8 | 7 | ||||||||||||||||
| Score | 0 | 1 | 2 | 3 | 4 | Mean | 0 | 1 | 2 | 3 | 4 | Mean | 0 | 1 | 2 | 3 | 4 | Mean | |
| Proximal tubule | |||||||||||||||||||
| Necrosis degeneration | 7 | 0.0 | 1 | 1 | 5 | 1 | 2.8** | 2 | 2 | 1 | 1 | 1 | 1.6 | ||||||
| Tubular dilatation (Hyaline casts) | 7 | 0.0 | 3 | 5 | 0.6* | 6 | 1 | 0.1 | |||||||||||
| Regenerated tubules | 6 | 1 | 0.1 | 6 | 2 | 0.3 | 7 | 0.0 | |||||||||||
Score: 0, no remarkable changes; 1, minimal (<10%); 2, slight (10–20%); 3, moderate (20–50%); 4, severe (>50%), where the percentage represents the extent of the damage. * p < 0.05, ** p < 0.01, statistically significant differences from the control group (Mann–Whitney’s U-test).
Changes in the mRNA expression of TNF-α and IL-1β following the cisplatin administration are shown in Fig. 6A. Real time RT-PCR indicated statistically significant up-regulation of TNF-α mRNA expression at 72 h after the cisplatin infusion. Moreover, a positive correlation was found between TNF-α mRNA expression and BUN or Scr, with correlation coefficients of 0.82 and 0.62, respectively (Fig. 6B). However, there was no significant up-regulation of IL-1β mRNA expression (Fig. 6A).

A) Changes in TNF-α and IL-1β mRNA expression level in cisplatin-treated mice. AKI was induced by an intravenous infusion of cisplatin (20 mg/kg). The mRNA levels were measured at 72 h after the cisplatin infusion. Each column represents the mean ± S.E. of 7–8 animals. ** p < 0.01 (compared with untreated group). B) Correlation of TNF-α mRNA expression and BUN or Scr in cisplatin-treated mice.
Subsequently, we investigated the time-course changes in TNF-α mRNA expression after the cisplatin and SMTP-7 administration (Fig. 7). TNF-α mRNA expression level up-regulated at 48 h after the cisplatin infusion, and this change sustained until 72 h. When SMTP-7 (10 mg/kg) was administered at 24 h after the cisplatin infusion, expression level of TNF-α mRNA reduced, and meaningful differences (p < 0.05) were observed compared with control group at 72 h.

AKI was induced by an intravenous infusion of cisplatin (20 mg/kg). SMTP-7 was also intravenously infused for 15 min at 24 h after the cisplatin infusion. Each point represents the mean ± S.E. of 8–10 animals. * p < 0.05 (compared with negative control group). # p < 0.05 (compared with control group).
The present study found that SMTP-7, a compound under clinical evaluation for ischemic stroke, attenuated the effects of cisplatin-induced AKI. In the present study, 20 mg/kg of cisplatin induced an increase in the levels of BUN and Scr at 72 h after infusion. Moreover, histopathological examination revealed remarkable proximal tubular necrosis and degeneration, and tubular dilatation. These changes are consistent with the previous studies describing that both blood biochemical parameters and histochemical changes have been associated with cisplatin-induced nephrotoxicity at 72 h following a injection of cisplatin in mice.19) In addition, an animal study also demonstrates an acute tubular necrosis with azotemia after a cisplatin injection.20) These changes were attenuated by administration of SMTP-7.
Recently, the importance of inflammation in cisplatin-induced AKI has grown in addition to its direct toxicity. It has been reported that various proinflammatory cytokines play an important role in the development of cisplatin-induced AKI.5) TNF-α mRNA is up-regulated in the kidney, and TNF-α levels in serum and urine are increased by a cisplatin treatment. Moreover, IL-1β mRNA expression also increases in cisplatin-induced AKI.21) As shown in Fig. 6, a significant up-regulation of TNF-α mRNA was observed in this study, and TNF-α mRNA expression correlated very well with BUN or Scr levels. However, there were no considerable changes in the expression of IL-1β mRNA in this study. TNF-α has a potential role in the pathogenesis of cisplatin-induced AKI because the blockade of TNF-α reduces the production of other proinflammatory cytokines22) and attenuates cisplatin-induced AKI.23) On the one hand, it has been reported that the administration of IL-1 receptor antagonist did not attenuate cisplatin-induced AKI.24) Although whether cytokines other than TNF-α contribute to cisplatin-induced AKI remains to be determined.25) TNF-α may be a key upstream regulator, and the functional role of other cytokines may be different from that of TNF-α.
Interestingly, SMTP-7 was effective when it was administered at 24 h after the cisplatin administration. There was no enhancement in the therapeutic efficacy by the repeated administration of SMTP-7. Cisplatin induces the formation of reactive oxygen species (ROS), although it is not the direct cause of cell death.26) ROS have stimulatory roles in the nuclear factor-kappaB (NF-κB) signaling pathway,27) leading to the transcription of proinflammatory cytokines such as TNF-α.28) Previously, we reported that up-regulation of TNF-α mRNA was observed at 48 h, but an increase in BUN was not evident until 72 h after a cisplatin injection.29) It has been reported that ROS production was fully elevated within 48 h of a cisplatin injection.30) On the basis of these reports, we theorized that ROS-dependent up-regulation of TNF-α mRNA occurred at 48 h after cisplatin injection, consequently increasing the levels of BUN and Scr. Furthermore, this up-regulation and subsequent increase in BUN and Scr were inhibited by SMTP-7 administration at 24 h. In the case of infusion, SMTP-7 has a short biological half-life. Therefore, the concomitant administration with cisplatin is not effective. In addition, up-regulation of TNF-α mRNA already occur at 48 h after cisplatin infusion and sustainable up-regulation seem necessary to development of AKI. Thus, the effect also becomes weaker in the case of post administration at 48 h. Prior administration just before up-regulation of TNF-α mRNA is important in order to attenuate cisplatin-induced AKI.
In this study, the reason behind the inhibition of the TNF-α mRNA up-regulation by SMTP-7 is unclear. Matsumoto et al. reported that soluble epoxide hydrolase (sEH) is anti-inflammatory target of SMTP-7.31) sEH is an enzyme that converts eoxyeicosatrienoic acids (EETs) to dihydroxyeicosatrienoic acids. EETs exhibit an anti-inflammatory effect by decreasing production of proinflammatory cytokines via inhibiting NF-κB signaling pathway, moreover EETs and its analogs reduce cisplatin-induced AKI, and sEH inhibition provides a renoprotective effect.32,33) Hence, the potential activity of SMTP-7 against cisplatin-induced AKI may attribute the inhibition of sEH, leading to an increase in EETs, the suppression of NF-κB signaling pathway, and the reducing of proinflammatory cytokine production.
Although it is reported that various kinds of natural or chemical compounds prevents cisplatin-induced AKI,34) these effects are mostly based on antioxidant activity. As previously mentioned, SMTP-7 inhibits sEH activity. sEH has two hydrolase activity. One is epoxide hydrolase activity in C-terminal and the other is phosphatase activity in N-terminal. Epoxide hydrolase activity is associated with conversion of EET and phosphatase activity involves nitric oxide synthase (NOS) dysfunction.35) Nitric oxide has a role in maintenance of renal function and NOS inhibition aggravates cisplatin-induced AKI.36) Therefore, renoprotective effect of SMTP-7 may be also attribute to inhibition of phosphatase activity, because SMTP-7 has inhibitory effect against both activity.31) This mechanism is novelty of SMTP-7 compared with other compounds.
We did not examine anticancer activity of SMTP-7 or possible effect on anticancer activity of cisplatin. Biological half-life of cisplatin is short (within 30 min of intravenous (i.v.)).37) Therefore, we speculated that SMTP-7 did not interact with cisplatin in the case of post (at 24 or 48 h after cisplatin infusion) administration. However, these points are a limitation of the present study and it needs to clarify that whether SMTP-7 does not inhibit anticancer activity in the future.
In summary, we showed that SMTP-7 has a renoprotective effect against cisplatin administration, possibly based on the suppression of the production of proinflammatory cytokines such as TNF-α. We believe this novel compound can be a potential candidate drug reducing the side effect of cisplatin chemotherapy.
We thank to TMS Co., Ltd. for the generous supplying of SMTP-7.
The authors other than KH declare that they have no conflict of interest. KH is a director and a stake holder of TMS Co., Ltd.