2022 Volume 45 Issue 5 Pages 561-568
2022 Volume 45 Issue 5 Pages 561-568
Ovarian cancer has long been considered the second-highest cancer threat to women’s reproductive system with high mortality. This is ascribed to the absence of highly efficient therapy and cancer metastasis. Accordingly, there is an urgent need for the development of new agents. Recently, Traditional Chinese medicine has gained extensive interest because of its safe use, validity, and distinct pharmacological effects. Polyphyllin E (PPE), as a major constituent in Rhizoma Paridis, is a promising cancer-fighting agent. However, the effect of PPE on ovarian cancers as well as associated latent mechanisms is still not completely understood. In this study, PPE was found to prohibit the proliferation of SK-OV-3 and OVCAR-3 ovarian cancer cells, causing marked cell death. Additionally, low-dose PPE could also inhibit motility and invasion of ovarian cancer cells. The mechanistic assessment revealed PPE-mediated matrix metalloproteinases, i.e., MMP2 and MMP9, inhibition via the AKT–nuclear factor kappa B (AKT/NF-κB) signaling pathway. Rescue experiments with transfection of AKT lentiviral particles remarkably reversed PPE inhibitory effects against ovarian cancer cells. In conclusion, PPE could inhibit proliferation of ovarian cancer cell migration and invasion by down-regulating the AKT/NF-κB pathway. Moreover, it has the potential to act as a novel agent for ovarian cancer treatment.
Ovarian cancer is one of the most lethal malignancies in women and is responsible for 5% of all the cancer deaths in women.1) There has been a stable decrease in ovarian cancer incidence after the mid-1970s.2) Given the difficulty in detection, a majority of patients (60%) can only be diagnosed until advanced stages (III–IV), greatly lowering their survival rate (≈46%).3) Unlike other epithelial cancer cells, ovarian cancer cells can disseminate directly to the peritoneum cavity due to the absence of anatomical barriers.4) Recent data also indicate that the majority of patients will relapse despite a satisfactory response to the initial treatment.5,6) Both chemotherapy and radiotherapy show restricted treatment effects since they merely apply to patients having localized ovarian cancers.7,8) Besides the above-mentioned issues, tumor metastasis further adds difficulties to the treatment.9) Therefore, further research is needed in the development of agents for the treatment and prevention of ovarian cancer migration and invasion.
Agents extracted from plants have gained more uses in drug discovery and development projects.10,11) Rhizoma Paridis, also known as Chonglou, is the root of Paris polyphylla, in the lily family. Modern pharmacological research suggests that Chonglou has a wide range of medicinal activities, including anticancer, immunoregulatory, and cardiovascular effects. As a class of saponins isolated from the Chonglou, polyphyllin is found to exert hemostatic, analgesic, bacteriostasis, inflammation regulation, immunoregulation, and particularly antitumor effects.12–16)
Polyphyllin E (PPE) is a primary steroidal saponin constituent of Chonglou. Its anti-proliferation characteristics have already been demonstrated in colorectal cancer cells.17,18) However, PPE-related anticancer activity and relevant latent mechanisms for ovarian cancers remain unknown yet. In this study, we investigated the sensitivity of ovarian cancer cells to PPE in vitro and its effect on cell migration and invasion. Furthermore, the molecular mechanisms behind these processes were also examined.
PPE was provided by Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China) with ≥98% purity. Cisplatin was purchased from Sigma-Aldrich (MO, U.S.A.). Cell counting kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies (Kumamoto, Japan). Click-iT® EdU Alexa Fluor® 594 Imaging Kit was purchased from ThermoFisher Scientific (MA, U.S.A.). The lentiviral particles expressed activated AKT protein (a double mutation S473D and T308D) were obtained from GenePharma (Shanghai, China). Antibodies for Matrix metalloproteinase-2 (MMP-2), MMP-9, AKT, p-AKT (S473), p-AKT (T308), c-Jun, inhibitor of kappaBα (IκBα), p-IκBα, p-IKKα/β nuclear factor kappa B (NF-κB)/P65, c-Fos, Lamin B1 and β-actin were obtained from Cell Signaling Technology (MA, U.S.A.). p-NF-κB/P65 (S536), Goat anti-Rabbit immunoglobulin G (IgG) (H + L) secondary antibody, and Goat anti-Mouse IgG (H + L) secondary antibody were the products of Abcam (Cambridge, U.K.). All other chemicals used were of reagent grade.
Cells and Cell TreatmentSK-OV-3 and OVCAR-3 ovarian cancer cells were provided by the Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences (Shanghai, China). OVCAR-3 cells were incubated with RPMI 1640 along with 10% fetal bovine serum (FBS, Gibco, CA, U.S.A.) and 1% penicillin–streptomycin (Invitrogen, CA, U.S.A.). SK-OV-3 cells were incubated with McCoy’s 5A containing 10% FBS (Gibco) and 1% penicillin–streptomycin (Invitrogen). OVCAR-3 stable cell line (OVCAR-3/AKT) that expressed AKT from lentiviral particles. In brief, we inoculated 5 × 104 OVCAR-3 cells in the 12-well plates. Following overnight cultivation, we introduced AKT and control lentiviral particles to specific wells and incubated with puromycin; 48 h later, cells showing successful transduction were selected. We successfully generated control OVCAR-3 stable cell line and AKT OVCAR-3 stable cell line. All cells were maintained at 37 °C with 5% CO2.
Assay for Cell ViabilityCCK-8 assay was used to measure cell viability. We positioned cells onto 96-well microtiter plates at 5 × 103 cells per well, followed by overnight incubation. Subsequently, cells were treated with varying PPE concentrations for 12, 24, and 48 h. Then, we added 10 µL CCK-8 into every well for extra 4 h incubation at 37 °C in the dark. Then, the absorbance (optical density (OD)) values at 450 nm were measured using a microplate reader (Thermo Fisher Scientific). The rate of cell viability was measured using the equation:
 |
We used the Click-iT® EdU imaging kit (Invitrogen) to measure ovarian cancer proliferation ability. SK-OV-3 and OVCAR-3 cells were inoculated on 6-well plates at 1 × 105/well density and incubated overnight at 37 °C. After PPE2 treatment, 10 µM EdU was added to the medium. After fasting, 4% formaldehyde was added, and cells were incubated in the dark with the Click-iT reaction cocktail. Corresponding nuclei were counterstained using 4′-6-diamidino-2-phenylindole (DAPI) (Beyotime, Shanghai, China) and observed under a fluorescence microscope (Olympus, Tokyo, Japan)
Wound-Healing AssayIn brief, SK-OV-3 and OVCAR-3 cells were added on six-well plates. After the cells grew to a monolayer with 90% confluence, a scratch was made using a P-200 pipette tip, then the shedding cells were removed by rinsing thrice. Then, the cells were cultivated with the culture medium (without serum), and PPE at diverse concentrations (0, 0.5, and 1.0 µM) for 24 and 48 h, and images were acquired using a phase-contrast microscopy system.
In Vitro Cell Invasion AssayThe Transwell plate approach was adopted to assess cell invasion. In brief, OVCAR-3 and SK-OV-3 cells were resuspended in a serum-free medium. Then, the cell suspension (500 µL, containing 5 × 105 cells/mL) was added to the Matrigel-coated Transwell chambers (polycarbonate membrane with the pore size of 8 µm). The bottom chambers were filled with the medium (1 mL, containing 10% FBS), and PPE was introduced to the top chamber at varying concentrations. Following 48 h cultivation, cells above membrane surface were removed using cotton swabs, while cells invading the lower membrane surface were fixed using 4% paraformaldehyde, followed by staining using 0.1% Cresyl Violet solution. Images were acquired using a phase-contrast microscopy system. The number of cells invading the bottom membrane surface was quantitatively measured.
Quantitative Real-Time PCR (qRT-PCR)The total cellular RNA was extracted from SK-OV-3 and OVCAR-3 cells under different treatments using 800 µL TRIzol reagent (Invitrogen). Then, 1 µg total RNA was used for reverse transcription via PrimeScript RT Master Mix kit (TaKaRa, Shiga, Japan) following the manufacturer’s instruction. Gene levels were quantified using SYBR Green PCR Master Mix (Toyobo, Osaka, Japan) on a LightCycler 480 (Roche, Basel, Switzerland). The gene expression was normalized relative to corresponding inner glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels. Primer sequences included: MMP-2: 5′-TGAGCTCCCGGAAAAGATTG-3′ (forward), 5′-TCAGCAGCCTAG CCAGTCG-3′ (reverse); MMP-9: 5′-TCCCTGGAGACCTGAGAACC-3′ (forward), 5′-CGGCAAGTCTTCCGAGTAGTT-3′ (reverse); GAPDH: 5′-GGAGCGAGATCCCTCCAAAAT-3′ (forward) 5′-GGCTGTTGTCATACTTCTCAT GG-3′ (reverse).
Western Blotting AssayWestern blotting assay was performed following standard protocol. Briefly, SK-OV-3 and OVCAR3 cells were treated with dimethyl sulfoxide (DMSO) (<0.1%) or different concentrations of PPE, then collected and lysed with radio immunoprecipitation assay (RIPA) lysis buffer (Amresco, PA, U.S.A.). The nuclear and cytoplasmic extraction was prepared using an NE-PER Nuclear Cytoplasmic Extraction Reagent kit (Thermo Fischer Scientific) supplemented with protease inhibitor cocktail according to the manufacturer’s instruction (Document Connect (thermofisher.cn)). The BCA protein detection kit (Beyotime) was used to measure corresponding protein content. Equivalent protein content in total cell lysates was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (BioRad Laboratories, CA, U.S.A.) and transferred onto polyvinylidene difluoride membrane (BioRad Laboratories) through wet transfer. Then, 5% skimmed milk in tris-buffered saline (containing 0.1% Tween 20, pH 7.6) was utilized to block the membrane under room temperature (r.t.) for 1 h, followed by overnight incubation with primary antibodies and 1 h incubation using appropriate secondary antibodies at r.t. The ECL system (CLINX, Shanghai, China) was used to expose protein bands. Band intensity was examined with Image lab (version 3.0, BioRad Laboratories).
Statistical AnalysisOverall trials were conducted at least three times. For statistical analysis, Prism software (GraphPad v6) was utilized. One-way ANOVA was used to analyze the statistical differences among three or more groups while unpaired Student’s t test was applied to analyze the statistical differences between two groups. Overall quantitative data were indicated by “mean ± standard deviation (S.D.).” Statistical significance was expressed as * p < 0.05, ** p < 0.01, *** p < 0.001.
For evaluating the anti-proliferative ability of PPE, SK-OV-3 and OVCAR-3 ovarian cancer cells were cultivated with PPE. According to the post-PPE treatment, CCK-8 assays conducted at varying concentrations and periods, PPE exhibited dose-and time-dependent inhibitory effects on ovarian cancer cells. The viability of SK-OV-3 and OVCAR-3 cells could be suppressed by PPE over time, and 24 h represented better treatment dose dependence (Figs. 1A, B). Cisplatin was used as a positive control as it is approved as a systemic treatment of ovarian cancer.19) The IC50 for 24 h PPE treatment of SK-OV-3 and OVCAR-3 cells were determined to be 7.462 ± 1.086 and 5.053 ± 0.5632 µM, respectively. In addition, EdU assay results showed that EdU-positive SK-OV-3 and OVCAR-3 cells received marked inhibition following treatment using 4 and 8 µM PPE for 24 h (Fig. 1C). Thus, the above findings showed the inhibitory effect of PPE on ovarian cancer cell viability.

OVCAR-3 (A) and SK-OV-3 (B) cell viability under treatment at varying PPE concentrations for 12, 24, and 48 h, with cisplatin as the positive reference. (C) The ratio of EdU-positive cells to SK-OV-3 and OVCAR-3 cells following 24 h of PPE treatment was visualized by EdU staining. Data are indicated by mean ± S.D. of three trials. Scale bar: 50 µm.
For evaluating the effect of PPE on ovarian cancer cell mobility, scratch-wound healing assay was conducted using low-dose PPE to avoid the interference of anti-proliferation on the results. During the study, low-dose PPE exhibited insignificant cytotoxicity against SK-OV-3 and OVCAR-3 cells (Figs. 2A, B). However, at 0.5 and 1.0 µM, PPE significantly inhibited wound field gap closure during the 24 h examination (Fig. 2C). Additional in vitro cell invasion assays were conducted to address the problem. Figure 2D shows that 0.5 and 1.0 µM PPE inhibited OVCAR-3 and SK-OV-3 cell invasion. The above findings prove the significant inhibitory effect of PPE against ovarian cancer cell migration and invasion.
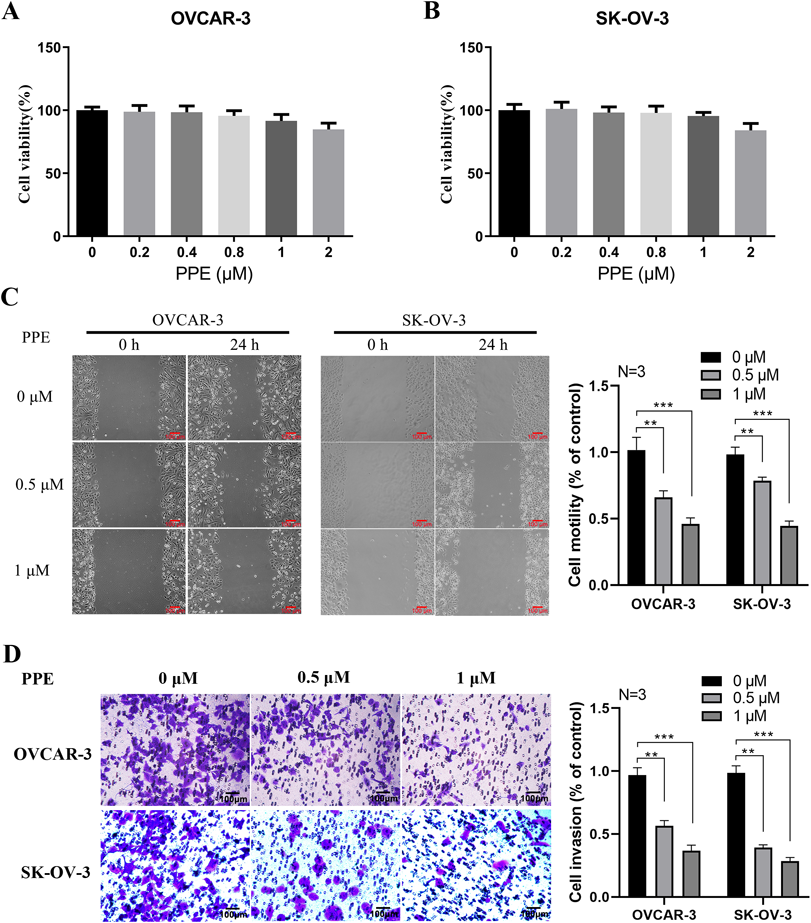
Quantification results of OVCAR-3 (A) and SK-OV-3 (B) cell viability through low-dose PPE treatment. (C) Wound healing assay shows lowered cellular motility of SK-OV-3 and OVCAR-3 cells following 24 h of treatment with PPE at 0, 0.5, and 1.0 µM. The group of 0 µM was used as the control group and the mean value was used for normalization. (D) The Matrigel-coated Boyden chamber was utilized to observe cell invasion. Typical photomicrograph of cells on the membrane was assessed by staining with 0.1% Cresyl Violet. The group of 0 µM was used as the control group and the mean value was used for normalization. Data are indicated as mean ± S.D. of three trials. ** p < 0.01, *** p < 0.001. Scale bars = 100 µm.
The correlation between MMP2/MMP9, ovarian cancer invasion, and metastasis has already been established.20) Thus, we further investigated the correlation between MMP2/MMP9 expression levels and PPE-inhibited invasion. The results indicate a significant downregulation of mRNA expression in MMP2/MMP9 in SK-OV-3 and OVCAR-3 cells under 0.5 and 1.0 µM PPE treatments (Fig. 3A). According to Fig. 3B, MMP2/MMP9 proteins showed equal dose-dependent inhibited expressions as that of their mRNA. Several transcriptional factors are engaged in regulation at the level of MMP2/MMP9, including NF-κB, c-Fos, and c-Jun.21,22) Therefore, we examined the mechanism through which PPE induced suppression of MMPs. We exposed SK-OV-3 and OVCAR-3 cells to 0.5 and 1.0 µM PPE for 24 h and their nuclear and cytosolic proteins were obtained. According to Figs. 3C and D, NF-κB protein levels in the nuclear fraction of these two cell lines were remarkably reduced, but their protein levels in cytosolic fraction increased when treated with PPE. Besides, the variation in c-Jun and c-Fos protein levels following PPE treatment was insignificant. For proving this, p-NF-κB, p-IKKα/β and p-IκBα were measured quantitatively in whole-cell lysates by Western blotting; the inhibitory effect of PPE on phosphorylation for NF-κB, IKKα/β and IκBα in SK-OV-3 and OVCAR-3 cells were remarkable, and the change in total NF-κB or IκBα protein levels was insignificant (Fig. 3E). Thus, PPE-suppressed NF-κB activity has some possible association with inhibited MMP2/MMP9 expression, which may be ultimately linked to PPE-suppressed ovarian cancer cell metastasis.

(A) MMP2/MMP9 mRNA expression was measured using RT-PCR when cells received PPE treatment at 0, 0.5, and 1.0 µM for 24 h. The group of 0 µM was used as the control group and the mean value was used for normalization. (B) WB assays and quantification results show reduced MMP2/MMP9 protein levels of cells under 24 h of PPE treatment at 0, 0.5, and 1.0 µM. (C, D) WB assays and quantification results show elevated NF-κB protein expression in cytosolic fractions and lowered expression in the nuclear fractions following 24 h of PPE treatment at 0, 0.5, and 1.0 µM. (E) WB assays and quantification results to show lowered NF-κB phosphorylation of cells receiving 24 h of PPE treatment at 0, 0.5, and 1.0 µM. Data are indicated by mean ± S.D. of three trials. * p < 0.05, ** p < 0.01, *** p < 0.001.
Earlier studies have shown that AKT phosphorylates IκB, which reversely degrades AKT via the ubiquitination pathway. This results in nuclear translocation and activation of NF-κB.23) Considering the inhibitory effect of PPE on NF-κB activity, we assessed the associated mechanism and found that 0.5 and 1.0 µM PPE inhibited AKT phosphorylation of SK-OV-3 and OVCAR-3 cells (Fig. 4A). Additionally, rescue research conducted with the OVCAR-3/AKT cell line established by lentiviral particles showed remarkably expression of activated AKT. For investigating the correlation of PPE with Akt-mediated ovarian cancer cell invasion and migration, we performed scratch-wound healing and in vitro cell invasion assays. As shown in Figs. 4B and C, the migratory and invasive ability of the cells in the AKT group was increased relative to that in the control following PPE exposure. Besides, the phosphorylated AKT–NF-κB, p-IKKα/β, p-IκBα and MMP2/MMP9 levels increased in OVCAR-3/AKT cells compared to those in control OVCAR-3 cells following PPE treatment (Fig. 4D). The above-mentioned findings prove a direct association of PPE with AKT–NF-κB-induced invasion and migration of ovarian cancer cells.

(A) Western blots and quantification results show lowered pAKT protein levels following 24 h of PPE treatment at 0, 0.5, and 1.0 µM of OVCAR-3 and SK-OV-3 cells. (B) Wound-healing assay showing the rescue of cellular motility following PPE treatment and activated AKT for 24 h. 0 µM of LV-NC was used as the control group and the mean value was used for normalization. (C) In vivo invasion assay proves that invasion following 24 h of PPE treatment was reversed through the AKT activation. 0 µM of LV-NC was used as the control group and the mean value was used for normalization. (D) WB assays and quantification results show that reduced NF-κB/AKT phosphorylation, and MMP2/MMP9 expressions, following PPE treatment, were rescued by activated AKT. Data are indicated by mean ± S.D. of three trials. * p < 0.05, ** p < 0.01, *** p < 0.001. Scale bars = 100 µm.
Rhizoma Paridis is a traditional herbal medicine with broad pharmacological effects, including anti-inflammation, anticancer, antimicrobial, cytotoxic, hemostatic, and antifungal effects.24,25) In 2020, over 320 chemical ingredients were detected in the genus Paris, including flavonoids, pentacyclic triterpenes, phytosterols, steroidal saponins, and insect hormones.26) Particularly, steroidal saponins constituted prime active components, which might be useful in cancer treatments.27–29) In vitro experiments have revealed that total saponins from Rhizoma Paridis inhibit the viability of HepG2 cells.30) For the individual monomeric saponin from Rhizoma Paridis, Polyphyllin I can activate c-Jun expression and the c-Jun N-terminal kinase (JNK) signaling pathway, elicit cell apoptosis via the mitochondrial-mediated Caspase activation pathway, and finally inhibit tumor growth and migration in vitro.31) Polyphyllin II can inhibit the liver cancer cell proliferation through the AKT–NF-κB pathway.32) Polyphyllin VII has been proved to suppress the growth of ovarian cancer cells by regulating the PP2A/AKT/DRP1 signaling axis.33) In addition, polyphyllin D increases ovarian cancer cell sensitivity to cisplatin-induced growth arrest12) and polyphyllin B could inhibit ovarian cancer cell-induced angiogenesis by modulating NF-κB signaling.34) While the effect of PPE on ovarian cancers remains unknown yet. In this study, high doses of PPE were found to markedly prohibited proliferation and induced death of SK-OV-3 and OVCAR-3 cells. Simultaneously, suppressed invasion and migration of SK-OV-3 and OVCAR-3 cells were observed during treatments with low-dose PPE. These findings prove the promising prospects of PPE in the treatment of ovarian cancer.
MMPs are members of the family of zinc-dependent endopeptidases that engage in protein degradation in the extracellular matrix. MMPs have been observed in cancer cells, and increased MMP levels are reported to be linked to tumor progression and invasiveness.35) Aberrant levels of MMP2/MMP9 have been detected in cancer cells and tissues closely related to metastasis of multiple cancers such as ovarian cancer.36,37) In our study, we found that PPE could suppress cell migration and invasion by the downregulation of MMP2/MMP9 expression. Inhibited MMP2/MMP9 activity has been strongly implicated in the AKT/NF-κB pathway.38–40) Given these previous findings, we attempted to characterize the role of the AKT/NF-κB signal pathway role in the aforementioned biological behaviors and assessed the therapeutic effect of PPE in vitro. In our results, the protein expression levels of total NF-κB and IκBα were not changed. In contrast, the phosphorylation status of both NF-κB and IκBα was altered by PPE. These findings indicated that PPE did not alter the expression of NF-κB and IκBα at transcriptional or translational levels. However, PPE could regulate the machinery that is involved in the phosphorylation status of both NF-κB and IκBα. For evaluating the effect of PPE on the AKT/NF-κB pathway, we used the rescue experiments. Our experimental findings confirmed former research results, indicating the dependence of PPE-suppressed MMP2/MMP9 on the AKT/NF-κB pathway.
To summarize, we found evidence supporting the ability of PPE to suppress cell death-induced proliferation in ovarian cancer cells. Furthermore, migration and invasion of ovarian cancer cells could be suppressed by downregulating the AKT/NF-κB signaling pathway, which reduced MMP-2 and MMP-9 expression following PPE treatment. Thus, PPE can be used as a promising agent to prevent migration and invasion of ovarian cancer and thus aid in its treatment.
This work was supported by the Natural Science Foundation of Nantong Science and Technology (No. JC2020007, to Yinglei Liu; No. MS12020005, to Haifeng Qiao); The Natural Science Foundation of Jiangsu Science and Technology (No. Z2021078, to Yinglei Liu).
The authors declare no conflict of interest.