2022 Volume 45 Issue 5 Pages 659-663
2022 Volume 45 Issue 5 Pages 659-663
Previously we showed that the water-soluble fraction of sorghum extract (SE) improves adipogenesis in 3-isobutyl-1-methylxanthine (IBMX)/dexamethasone/insulin (MDI)/thiazolidinedione (TZD)-induced 3T3-L1 preadipocytes but downregulates genes related to peroxisome proliferator-activated receptor γ (PPARγ) and adipogenesis in both MDI- and MDI/TZD-induced 3T3-L1 adipocytes. In this study, we showed that SE treatment altered the accumulation of stained lipids in 3T3-L1 adipocytes induced by MDI/troglitazone (Tro). Immunoblot analyses indicated that SE treatment reduced adipocyte protein 2 (aP2) expression and induced peroxisome proliferator-activated receptor α (PPARα) protein expression in the presence of Tro in 3T3-L1 adipocytes. MDI/Tro treatment, but not MDI treatment, of 3T3-L1 cells induced PPARγ phosphorylation at Ser273. SE downregulated PPARγ expression in MDI-induced 3T3-L1 adipocytes and did not affect its phosphorylation at Ser273 in MDI- and MDI/Tro-induced 3T3-L1 adipocytes. Therefore, SE likely promotes adipogenesis and lipid metabolism in 3T3-L1 preadipocytes by cooperating with Tro independent of PPARγ Ser273 phosphorylation.
Peroxisome proliferator-activated receptor γ (PPARγ) is most strongly expressed in adipose tissues and is required for preadipocytes to differentiate into mature adipocytes.1) Previously, we reported a water-soluble fraction of sorghum powder (SE) that increased 3-isobutyl-1-methylxanthine (IBMX)/dexamethasone/insulin (MDI)/thiazolidinedione (TZD)-induced lipid accumulation in 3T3-L1 adipocytes, whereas MDI did not induce lipid accumulation.2) Sorghum extracts have also been reported to have antidiabetic activity through improvement of insulin sensitivity in mouse models.3–6) In addition, sorghum contain a lot of polyphenol and phenolic compounds, which modulate adipogenesis by interacting with PPARγ.7) We previously demonstrated that SE inhibits adipogenic genes in MDI- and MDI/TZD-treated 3T3-L1 cells, and it enhances insulin sensitivity by cooperating with the TZDs of PPARγ agonists.2) The expression of the transcription factors PPARγ and CCAAT/enhancer binding protein α (C/EBPα), and of the adipogenesis-related genes adipocyte protein 2 (aP2) and adiponectin, were reduced in SE-treated 3T3-L1 adipocytes exposed to MDI or MDI/troglitazone (Tro).2)
PPARγ is reportedly phosphorylated at Ser273 by CDK5 in adipose tissues, and has been linked to insulin resistance through the dysregulation of a subset of genes.8,9) Ser273-phosphorylated PPARγ does not alter adipogenic activity but deregulates genes that have altered expression in patients with obesity and diabetes, such as adiponectin, aP2, and resistin.8) To understand the mechanisms involved in the upregulation of adipogenesis by SE in Tro-induced 3T3-L1 adipocytes, we evaluated the effect of SE on PPARγ Ser273 phosphorylation during 3T3-L1 preadipocyte differentiation.
Sorghum extract (SE) was prepared from sorghum powder as described previously.2) The concentration of SE was assumed to be the same as the polyphenol concentration of the SE solution.
Cell Culture and Drug TreatmentCell culture is performed as indicated previously.2) The 3T3-L1 cells were cultured in Dulbecco’s modified Eagle’s medium containing low glucose (DMEM; Wako, Osaka, Japan) with 10% bovine calf serum, 100 U/mL penicillin/100 µg/mL streptomycin in 24-well plates at 37 °C in 5% CO2 and 95% air incubator. After confluence, the cells were treated with an adipocyte induction medium in DMEM containing 10% fetal bovine serum (FBS) for 3 d. The medium was changed to an insulin solution in DMEM with 10% FBS for the following 4 d. From days 0 to 7 of culture, the cells were treated with 5 µg/mL of SE in the presence of Tro or dimethyl sulfoxide (DMSO) and the culture medium was changed every 2 d. Cells were fixed with 10% formaldehyde in phosphate buffered saline (PBS) and stained with Oil Red O solution (Cayman Chemical, Ann Arbor, MI, U.S.A.).
Oil Red O Staining3T3-L1 adipocytes which were cultured for 7 d in 24-well cell culture plates, were washed with PBS and fixed in formalin at room temperature. After washing with water, the dried wells were stained with Oil red O solution, and then the stained cells were washed with water and dried. Lipid droplets in cells were viewed using a Keyence BZ-X800 microscope.
Quantitative Real-Time PCRThe conditions for RT-PCR reactions and data analysis were performed as described previously.2) Primer sequences used for real-time PCR is as follows, PPARα: 5′-AGAGCCCCATCTGTCCTCTC-3′ and 5′-ACTGGTAGTCTGCAAAACCAAA-3′, β-actin: 5′-CAGCCTTCCTTCTTGGGTATGG-3′ and 5′-CTGTGTTGGCATAGAGGTCTTTACG-3′.
Immunoblot Analysis 3T3-L1 cells were cultured in 24-well plates and washed twice with PBS. Then, cell lysis buffer (180 µL; 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1% NP-40) containing 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, U.S.A.) was added to each well. The lysates (6–10 µg protein) were subjected to sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis and the proteins were immunoblotted as described previously.3) Anti-PPARγ (#2435) and anti-β-actin (#4967) antibodies, as well as horseradish peroxidase (HRP)-conjugated secondary donkey anti-rabbit immunoglobulin G (IgG) (#7074) and HRP-conjugated secondary rabbit anti-mouse IgG (#7076) antibodies, were purchased from Cell Signaling Technology Co., Ltd. (Danvers, MA, U.S.A.). Anti-PPARα (15540-1-AP), anti-aP2/FABP4 (67167-1-Ig) and PPM1A (12961-1-AP) antibodies were purchased from Proteintech (Rosemont, IL, U.S.A.). Anti-phosphorylated (Ser273)-PPARγ antibody (bs-4888R-TR) was purchased from Bioss (Woburn, MA, U.S.A.).
Statistical AnalysisResults are shown as the mean ± standard deviation of at least three replicates. Differences between two groups were evaluated using Student’s t-test. A p-value of less than 0.05 were considered statistically significant.
We previously showed that sorghum (Sorghum bicolor (L.) Moench) extract (water-soluble fraction) improves 3T3-L1 preadipocyte differentiation in the presence but not in the absence of TZDs. 3T3-L1 adipocytes were cultured until day 7 as described in the materials and methods, then the cells were fixed, stained with Oil Red O and observed using a BZ-X800 microscope. In MDI/Tro-treated 3T3L1 adipocytes, some lipid droplets were microscopically clustered in SE-treated adipocytes but not in SE-untreated adipocytes (Figs. 1A, B).

The differentiation of post-confluent 3T3-L1 cells was induced in the absence (SE−) or presence of 5 µg/mL of SE (SE+). On day 7, cells (control or treated with Tro, MDI, and MDI/Tro) were fixed and stained with Oil Red O to visualize lipid droplets. (A) Representative images of stained 3T3-L1 adipocytes were observed under a BZ-X800 microscope (×20). Scale bar:1000 µm. (B) Higher magnification of attained 3T3-L1 adipocytes (MDI and MDI/Tro) (×100) Scale. bar: 200 µm.
A previous report showed that SE treatment inhibited adipogenic genes in MDI- and MDI/Tro-treated 3T3-L1 adipocytes.2) To investigate the effect of SE on protein levels, cell lysate treated with SE was subjected to immunoblot analysis. Figure 2 shows significant reduction of aP2 protein expression in 3T3-L1 adipocytes treated with SE. We then examined whether SE affects PPARα expression, a lipid metabolic activator, in 3T3-L1 adipocytes, and observed significant induction of PPARα mRNA and protein expression in 3T3-L1 adipocytes treated with MDI/Tro (Figs. 3A, B).
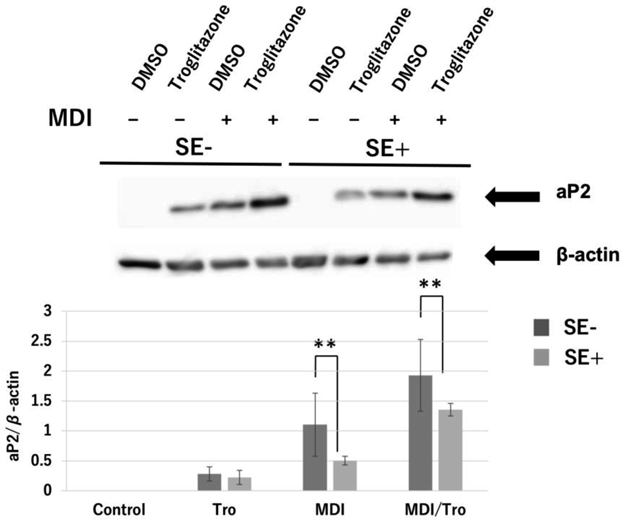
The differentiation of post-confluent 3T3-L1 cells was induced with SE in the presence of DMSO or Tro (5 µM). Cell lysates were prepared on day 6 and aP2 protein expression was analyzed by Western blot (upper panel). In addition, aP2 protein levels normalized to β-actin protein levels (lower panel). Immunoblots are representative of three independent experiments with similar results. In each group, n = 3; ** p < 0.01.

The differentiation of post-confluent 3T3-L1 cells was induced with SE in the presence of DMSO or Tro (5 µM). (A) Total RNA was isolated on day 6 and real-time PCR was performed and each expression level was normalized to β-actin. (B) Cell lysates were prepared on day 6, and PPARα protein expression was analyzed by Western blot (upper panel). In addition, PPARα protein levels were normalized to β-actin protein level (lower panel). Immunoblots are representative of three independent experiments with similar results. In each group, n = 3, * p < 0.05.
Next, we investigated whether Ser273 phosphorylation of PPARγ is associated with the effect of SE on 3T3-L1 preadipocyte differentiation, given that Ser273 phosphorylation of PPARγ is involved in insulin sensitivity. SE treatment suppressed the increase in PPARγ protein levels in MDI-treated 3T3-L1 adipocytes, as reported previously2) (Figs. 4A, B), and very high levels of Ser273 phosphorylation were detected in the PPARγ1 subtype (Figs. 4A, B). The relative level of PPARγ Ser273 phosphorylation was upregulated in MDI/Tro-treated 3T3-L1 adipocytes, but SE did not change the induction level (Figs. 4C, D). PPM1A, a phosphatase that directly dephosphorylates Ser273 of PPARγ, was upregulated in MDI- or MDI/Tro-induced 3T3-L1 adipocytes compared to control 3T3-L1 preadipocytes, and its expression level was unchanged by SE treatment (Fig. 5).

The differentiation of post-confluent 3T3-L1 cells was induced with SE in the presence of DMSO or Tro (5 µM). (A) Cell lysates were prepared on day 6. PPARγ and phosphorylated (Ser273) PPARγ protein expression was analyzed by Western blot. Immunoblot data are representative of three independent experiments with similar results. *indicates P-PPAR γ2 bands. PPARγ protein levels were normalized to β-actin levels (B) and phosphorylated (Ser273) PPARγ protein levels were normalized to PPARγ1 (C) or PPARγ2 (D) levels. In each group, n = 3, * p < 0.05.

The differentiation of post-confluent 3T3-L1 cells was induced with SE in the presence of DMSO or Tro (5 µM). Cell lysates were prepared on day 6, and PPM1A protein expression was analyzed by Western blot (left panel). Representative immunoblot data are shown. PPM1A protein levels were normalized to β-actin levels (right panel). In each group, n = 3, * p < 0.05 and ** p < 0.01.
Previously, we reported that SE treatment increased lipid accumulation but inhibited adipogenic genes in MDI-treated 3T3-L1 adipocytes in the presence of TZDs.2) Several studies have reported that the phosphorylation of Ser273 of PPARγ is related to obesity-induced development of insulin resistance.8–10) PPM1A is a phosphatase that directly dephosphorylates Ser273 of PPARγ, activating the protein.11,12) In the present study, the relative level of Ser273-phosphorylated PPARγ in MDI/Tro-induced cells was much higher than that of MDI-treated cells. SE treatment did not affect S273 phosphorylation (Fig. 4). The phosphorylated form of PPARγ was not suppressed, although PPARγ protein expression was downregulated in MDI-treated 3T3-L1 adipocyte in the presence of Tro. Tro treatment appears to inhibit the unphosphorylated form of PPARγ in MDI-induced 3T3-L1 adipocytes, with resulting in downregulation of PPARγ protein expression. We hypothesize that PPARγ protein expression is downregulated and that the remaining PPARγ may be phosphorylated in 3T3-L1 adipocytes in the presence of Tro. Khim et al. showed that PPM1A protein dephosphorylates PPARγ at Ser273 to control genes involved in diabetes.9) As shown in Fig. 5, the protein phosphatase PPM1A is upregulated in MDI/Tro-induced 3T3-L1 adipocytes compared with control preadipocytes. The upregulation of PPARγ phosphorylation at Ser273 in MDI/Tro-treated cells appears to be independent of PPM1A activity. This is the first report of the upregulation of PPARγ Ser273 phosphorylation that does not occur in parallel with the downregulation of PPM1A activity. Given that the upregulation of PPM1A expression, but not of PPARγ phosphorylation at Ser273, in MDI/Tro-induced 3T3-L1 adipocyte is associated with insulin sensitivity, further study is needed to resolve these contradictory findings. In addition, SE treatment did not change the PPM1A protein level in MDI/Tro-induced cells.
SE enhanced MDI/Tro-induced adipogenesis and inhibited adipogenic genes, but upregulated lipid metabolism proteins such as PPARα in 3T3-L1 adipocytes. Several compounds reportedly activate PPARα and PPARγ as PPARα/γ dual agonists in 3T3-L1 adipocytes.11,12) Another compound decreased lipid accumulation by upregulating PPARα in 3T3-L1 adipocytes.13–15) Component from Scutellaria baicalensis was reported to suppresses adipogenesis by upregulating PPARα and downregulating PPARγ in 3T3-L1 cells.16) In contrast, SE enhances adipogenesis by downregulating PPARγ and upregulating PPARα. PPARα is a master regulator of fatty acid oxidation, resulting in reduction of triglyceride in liver cells. It is unknown how PPARα-targeted factors are associated with 3T3-L1 adipocytes treated with SE at this point. Some lipid metabolic proteins upregulated by SE in cells treated with TZDs may help improve insulin sensitivity independent of the PPARγ Ser273 phosphorylation status. Sorghum (Sorghum bicolor (L.) Moench) is rich in phytochemicals such as tannins, phenolic acids, anthocyanins, phytosterols, and policosanols.2–7) Most of polyphenolic compounds included in SE may be involved in 3T3-L1 adipocytes differentiation cooperatively. Further analysis is required to elucidate the molecular mechanisms of the interaction between SE and TZDs in metabolic diseases and diabetes.
We thank Ms. Mei Nagata, Ms. Ikumi Aizu, Ms. Yuko Oba, and Ms. Marina Tonosaki for helping with this research project. We thank Professor Shin Sato for preparing the sorghum powder. This work was supported by an Education Research Project of Aomori University.
The authors declare no conflict of interest.