2022 Volume 45 Issue 5 Pages 664-667
2022 Volume 45 Issue 5 Pages 664-667
Hepatic stellate cells (HSCs) play a significant role in the development of chronic liver diseases. Hepatic damage activates HSCs and results in hepatic fibrosis. The functions of activated HSCs require an increase in the cytosolic Ca2+ concentration ([Ca2+]cyt). However, the regulatory mechanisms underlying Ca2+ signaling in activated HSCs remain largely unknown. In the present study, functional analyses of Ca2+-sensing receptors (CaSRs) were performed using activated human HSCs, LX-2. Expression analyses revealed that CaSR proteins were expressed in α-smooth muscle actin-positive LX-2 cells. Extracellular Ca2+ restoration (from 0 to 2.2 mM) increased [Ca2+]cyt in these cells. The extracellular Ca2+-induced increase in [Ca2+]cyt was reduced by the CaSR antagonists, NPS2143 and Calhex 231. Furthermore, the growth of LX-2 cells was blocked by NPS2143 and Calhex 231 in concentration-dependent manners (IC50 = 6.0 and 9.5 μM, respectively). LX-2 cell proliferation was also attenuated by NPS2143 and Calhex 231. In conclusion, CaSRs are functionally expressed in activated HSCs and regulate Ca2+ signaling and cell proliferation. The present results provide insights into the molecular mechanisms underlying hepatic fibrosis and will contribute to the development of potential therapeutic targets.
Hepatic stellate cells (HSCs) are a key player in the progression of hepatic fibrosis. Under physiological conditions, these cells maintain a “quiescent” phenotype and store retinoid droplets in the cytoplasm for liver regeneration.1,2) Once HSCs are “activated” by liver injury, they transdifferentiate from quiescent pericytes into proliferative and fibrogenic myofibroblasts. Activated HSCs secrete extracellular matrix (ECM) components, proteases, and cytokines. They then promote hepatic fibrosis, leading to cirrhosis with major clinical complications, such as portal hypertension. Despite extensive efforts, therapeutic drugs specific to the treatment of hepatic fibrosis have not yet been developed.3)
The pathogenic mechanisms underlying hepatic fibrosis involve an elevated cytosolic Ca2+ concentration ([Ca2+]cyt) in activated HSCs.4,5) [Ca2+]cyt is mainly controlled by receptors (e.g., G protein-coupled and tyrosine kinase receptors) and ion channels (e.g., Ca2+ and K+ channels) in the plasma membrane. An increase in [Ca2+]cyt promotes HSC proliferation, fibrogenesis, and contractility, which ultimately leads to hepatic fibrosis. Therefore, it is important to elucidate the regulatory mechanisms of Ca2+ signaling in HSCs under both physiological and pathological conditions.
The present study investigated the pathophysiological role of Ca2+-sensing receptors (CaSRs), which belong to class C of the G protein-coupled receptor family,6) in HSCs. Immunocytochemical staining, [Ca2+]cyt measurement, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test, and bromodeoxyuridine (BrdU) incorporation assay were performed on activated human HSCs, LX-2, to examine the functional expression of CaSRs. We found that the expression of CaSRs regulates Ca2+ signaling and proliferation in activated HSCs.
LX-2 cells (Sigma-Aldrich, St. Louis, U.S.A.) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, U.S.A.), 100 U/mL penicillin G (Wako Pure Chemical Corporation), and 100 µg/mL streptomycin (Wako Pure Chemical Corporation) in a 5% CO2 incubator set to 37 °C.
Immunocytochemical StainingImmunocytochemical staining was performed using anti-CaSR (1 : 100; MA1-934, Invitrogen), Alexa Fluor 488-labeled immunoglobulin G (IgG) (1 : 1000; A11001, Invitrogen), anti-α-smooth muscle actin (α-SMA, 1 : 50; #19245, Cell Signaling Technology, Danvers, MA, U.S.A.), and Alexa Fluor 647-labeled IgG (1 : 1000; A21244, Invitrogen) antibodies, as previously reported.7) Nuclei were labeled with Hoechst 33342 (1 : 1000; H3570, Invitrogen). Immunocytochemical images were obtained using the confocal laser scanning microscopy A1R system (Nikon, Tokyo, Japan).
[Ca2+]cyt MeasurementsLX-2 cells were loaded with the Ca2+-sensitive fluorescence indicator, 10 μM fluo-4 acetoxymethyl ester (fluo-4/AM; Invitrogen) at room temperature for 30 min. [Ca2+]cyt images were obtained every 5 s by the confocal laser scanning microscopy A1R system. Standard N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES)-buffered solution containing 137 mM NaCl, 5.9 mM KCl, 2.2 mM CaCl2, 1.2 mM MgCl2, 14 mM glucose, and 10 mM HEPES (pH 7.4 with NaOH) was used as the extracellular solution. Ca2+-free HEPES-buffered solution was prepared by replacing 2.2 mM CaCl2 with 1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA).
MTT and BrdU Incorporation AssaysLX-2 cells (5 × 103 and 2 × 103 cells/well for the MTT and BrdU incorporation assays, respectively) were cultured at 37 °C. The MTT and BrdU assays were performed using the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) and the Cell Proliferation enzyme-linked immunosorbent assay (ELISA), BrdU (colorimetric) kit (Roche Diagnostics, Mannheim, Germany), respectively, as previously reported.8)
Statistical AnalysisPooled data are shown as the mean ± standard error (S.E.). The significance of differences among groups was assessed by Scheffé’s test after ANOVA using BellCurve for Excel software (version 3.22; Social Survey Research Information, Tokyo, Japan).
Since LX-2 cells express α-SMA, a marker of activated HSCs,9–11) they are commonly employed in experiments as the activated phenotype of human HSCs.12,13) The activated phenotype of LX-2 cells was confirmed in the present study by the expression of α-SMA (Fig. 1). The expression of CaSR proteins was detected in α-SMA-positive LX-2 cells by immunocytochemistry.

The expression of CaSR proteins in the human HSC line, LX-2, was examined by immunocytochemical staining. Representative immunocytochemical staining of CaSR proteins (green) in LX-2 cells. Nuclei and α-SMA were co-stained with Hoechst 33342 (blue) and an anti-α-SMA antibody (red), respectively. Similar results were obtained from 117 cells in three independent experiments.
To establish whether CaSRs function in the regulation of Ca2+ signaling, changes in [Ca2+]cyt were assessed in fluo-4/AM-loaded LX-2 cells. [Ca2+]cyt increased following extracellular Ca2+ restoration, which was induced by increasing the Ca2+ concentration in the extracellular solution from 0 to 2.2 mM (Fig. 2). The extracellular Ca2+-induced increases in [Ca2+]cyt are mediated by CaSRs, as previously reported.14) Therefore, the effects of the CaSR antagonists, NPS2143 and Calhex 231, on Ca2+ signaling were investigated in LX-2 cells. Pretreatments with 10 μM NPS2143 and Calhex 231 attenuated extracellular Ca2+-induced increases in [Ca2+]cyt.

The effects of CaSR antagonists on CaSR-mediated increases in [Ca2+]cyt were examined in fluo-4/AM-loaded LX-2 cells. CaSR-mediated increases in [Ca2+]cyt were induced by extracellular Ca2+ restoration (increase from 0 to 2.2 mM Ca2+ solution). (A) Representative time-course of [Ca2+]cyt changes in the absence and presence of the CaSR antagonists, 10 μM NPS2143 and Calhex 231, in LX-2 cells. (B) Summarized data of [Ca2+]cyt changes in the absence (control, n = 34 cells) and presence of NPS2143 (n = 31 cells) or Calhex 231 (n = 46 cells) in LX-2 cells. The peak amplitude of the second [Ca2+]cyt increase was normalized to that of the first response. ** p < 0.01 vs. control.
The MTT and BrdU incorporation assays were performed to examine the potential contribution of CaSRs to LX-2 cell proliferation. NPS2143 and Calhex 231 both inhibited cell growth in concentration-dependent manners (IC50 = 6.0 and 9.5 μM, respectively; Figs. 3A and B). BrdU assay revealed significant reductions in LX-2 cell proliferation by 3 μM NPS2143 and 5 μM Calhex 231 (Fig. 3C).
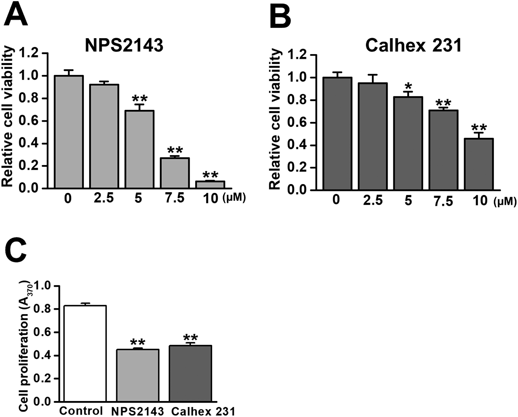
MTT and BrdU incorporation assays were performed to assess the effects of CaSR antagonists on LX-2 cell proliferation. (A) The concentration-dependent inhibitory effects of NPS2143 on LX-2 cell growth after a 48-h treatment (IC50 = 6.0 μM, n = 4 experiments). (B) The inhibitory effects of Calhex 231 on LX-2 cell growth after a 48-h treatment (IC50 = 9.5 μM, n = 4 experiments). (C) Cell proliferation was decreased by the treatment with 3 μM NPS2143 and 5 μM Calhex231 for 48 h (n = 4 experiments). * p < 0.05, ** p < 0.01 vs. 0 μM or control.
Activated HSCs are involved in the regulation of proliferation, fibrogenesis, and ECM deposition, which ultimately result in hepatic fibrosis.1,2) These pathological events are promoted by increased [Ca2+]cyt that is mediated by Ca2+-related receptors and ion channels.4,5) We found that CaSRs were expressed in activated human HSCs and were responsible for the regulation of Ca2+ signaling and cell proliferation. To the best of our knowledge, this is the first study to demonstrate the functional expression of CaSRs in HSCs; however, a previous study showed that CaSRs were expressed in hepatocytes and regulated bile flow.15)
CaSRs are distributed in the parathyroid glands, kidney, bone, skin, gut, brain, and vasculature.6) CaSRs detect and respond to changes in Ca2+ concentration in the extracellular space and regulate its homeostasis under physiological conditions. Abnormal CaSR functions are associated with hyperparathyroidism, neurological disorders (Alzheimer’s disease, ischemic brain injury, and epilepsy), cardiovascular diseases (myocardial infarction, pulmonary arterial hypertension, and chronic kidney disease), asthma, and cancer.16) In addition, the activity of CaSRs has been implicated in the development of chronic liver diseases.
The activation of CaSRs stimulates two Ca2+ influx pathways: store-operated Ca2+ entry (SOCE) and receptor-operated Ca2+ entry (ROCE). The stimulation of CaSRs coupled to the Gq protein activates phospholipase C and produces inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DG). IP3 facilitates Ca2+ release through IP3 receptors on the endoplasmic reticulum, and the store depletion induces SOCE though store-operated Ca2+ channels. On the other hand, DG promotes ROCE via the activation of receptor-operated Ca2+ channels.17) The activation of CaSRs has also been shown to stimulate the mitogen-activated protein kinase and Akt cascades, which respond to increased [Ca2+]cyt, resulting in cellular proliferation and differentiation.6,18) The proliferation assay revealed that the activity of CaSRs plays a role in HSC proliferation. Similar signal transduction following the activation of CaSRs may be involved in HSC proliferation.
Platelet-derived growth factor (PDGF) is an essential molecule for the progression of hepatic fibrosis.5) It induces the activation of HSCs and facilitates the proliferation of activated HSCs.1,10) The expression of CaSRs is up-regulated by PDGF, leading to cell proliferation and subsequent vascular remodeling.19) Therefore, PDGF may play a role in the transcriptional regulation of protein expression when HSCs are activated. Moreover, CaSRs have been shown to promote the proliferation of and ECM secretion in cardiac fibroblasts.20) Our preliminary experiments revealed that CaSR antagonists down-regulated the expression of ECM genes in LX-2 cells (unpublished observations), suggesting a role for CaSRs in the mechanisms underlying hepatic fibrosis.
In summary, the present results revealed the expression of CaSRs in activated human HSCs. CaSRs are involved in the regulation of Ca2+ signaling and proliferation of activated HSCs. Since HSCs are a key player in hepatic fibrosis, molecular assessments of Ca2+ signaling will provide important information for elucidating the mechanisms underlying hepatic fibrosis. In addition, CaSRs expressed in HSCs have potential as a therapeutic target for hepatic fibrosis.
The present study was supported by Grants-in-Aid for Scientific Research (C) (16K08278 and 19K07125 to H. Yamamura), Grant-in-Aid for Scientific Research (B) (19H03381 to Y. Suzuki), and Grant-in-Aid for Promotion of Joint International Research (Fostering Joint International Research (B)) (18KK0218 to H. Yamamura and Y. Suzuki) from the Japan Society for the Promotion of Science. We acknowledge the assistance of the Research Equipment Sharing Center at Nagoya City University.
The authors declare no conflict of interest.