2022 年 45 巻 7 号 p. 904-909
2022 年 45 巻 7 号 p. 904-909
Brigatinib and gilteritinib are oral tyrosine kinase inhibitors (TKIs). We aimed to develop a simple and sensitive indirect competitive enzyme-linked immunosorbent assay (ELISA) to quantify brigatinib and gilteritinib in various biological matrices. Antiserum against these TKIs was obtained from mice by using 3-methoxy-4-(-4-(4-methylpiperazin-1-yl) piperidin-1-yl) aniline as a hapten, which has a common substructure with these TKIs. The generated antibody was used to develop an indirect competitive ELISA for these TKIs in human serum. The lower limit of quantification of brigatinib and gilteritinib in human serum was 6.2 and 6.8 ng/mL, respectively. The developed ELISA was used to examine the pharmacokinetics of these TKIs after oral administration in mice and rats. This ELISA is expected to be a valuable tool in pharmacokinetic studies of these TKIs.
The development of tyrosine kinase inhibitors (TKIs) has flourished in recent years and has led to improved outcomes in cancer drug therapy. Although TKIs offer high therapeutic efficacy, they may cause unique adverse reactions not seen with conventional cytotoxic anticancer drugs, which stop many patients from completing treatment. Consequently, there is a need to determine the optimal blood concentration range that maximizes the effects of each TKI. Optimal blood concentration ranges have been established for several TKIs, and therapeutic drug monitoring (TDM) has been shown to improve their efficacy and safety.1–3) A simple analytical method to measure the blood concentrations of TKIs must be developed in order to determine their optimal blood concentration ranges and conduct TDM. Recently, we developed enzyme-linked immunosorbent assays (ELISAs) for several TKIs that were simple, sensitive, specific, and useful for pharmacokinetic studies of the target drugs.4–9)
Brigatinib is an oral inhibitor of anaplastic lymphoma kinase in non-small cell lung cancer and it can overcome resistance to crizotinib.10) Gilteritinib is an oral inhibitor of FMS-like tyrosine kinase 3 (FLT3) that is used as an anti-tumor agent in the treatment of FLT3 mutation-positive acute myeloid leukemia.11) To our knowledge, there is no relevant published report of an ELISA to detect brigatinib and gilteritinib in human blood. Therefore, we aimed to develop a simple, sensitive, and specific ELISA to quantify these TKIs in human serum.
In this study, we successfully developed the first indirect competitive ELISA for brigatinib and gilteritinib using a polyclonal antibody against a common substructure of these TKIs and report the technique.
Brigatinib was purchased from Haoyuan Chemexpress Co., Ltd. (Shanghai, China). Gilteritinib was purchased from Advanced Chemblocks, Inc. (Burlingame, CA, U.S.A.). 3-Methoxy-4-(4-(4-methylpiperazin-1-yl) piperidin-1-yl) aniline (MPA) and 1-methyl-4-(piperidin-4-yl) piperazine were purchased from Ambeed, Inc. (Arlington Heights, IL, U.S.A.). 1-Methyl-4-[1-(4-piperidyl)-4-piperidyl] piperazine (MPPP) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). 3,3′,5,5′-Tetramethylbenzidine (TMB) was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). 1-(1-Methyl-4-piperidyl) piperazine, bovine serum albumin (BSA) and keyhole limpet hemocyanin (KLH) were purchased from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan). Histofine Simple Stain MAX-PO (M) was obtained from Nichirei Bioscience (Tokyo, Japan). Normal human serum (liquid Nescole-NⓇ) was purchased from Alfresa Pharma Co., Ltd. (Osaka, Japan).
Preparation of Hapten-Protein ConjugatesThe hapten was attached to BSA and used as an immunogen, relatively coupled with KLH as a coating antigen. They were prepared with the common substructure (MPA) of brigatinib and gilteritinib (Figs. 1, 2). Briefly, MPA (5 mg, 16.4 µmol) in 100 µL dimethylformamide was acidified by the addition of 100 µL of 1 M HCl and then diazotized with NaNO2 (2.27 mg, 32.8 µmol) in 100 µL distilled water for 10 min at 0 °C. The resulting solution was added with 10 mg BSA or KLH in 1 mL of 0.1 M borate buffer (pH 9.5), incubated for 1 h at room temperature. The reaction solution was dialyzed with H2O, and the dialyzed conjugate was lyophilized and used as an immunogen or coating antigen. The degree of coupling of diazotized MPA with BSA was determined by amino acid analysis after hydrolysis with 6 M HCl for 24 h at 110 °C.12) Approximately 13 mol of the hapten was found to be coupled with one mole of BSA on the basis of the decrease in moles of histidine and tyrosine. The amounts of proteins were determined by Lowry’s method.13)

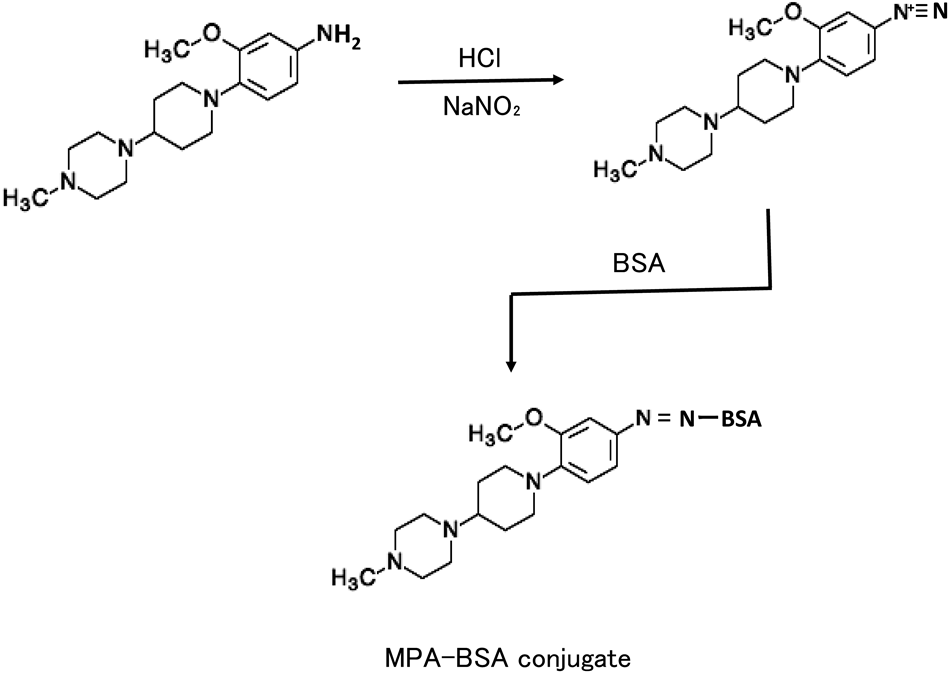
Five-week-old female BALB/c mice were injected intraperitoneally with 0.1 mg MPA–BSA conjugate emulsified with incomplete Freund’s adjuvant. The mice received three injections of the conjugate (0.05 mg) alone at 2-week intervals. Seven days after the last injection, serum was collected from the mice. By performing the indirect non-competitive ELISA method, each antiserum was tested for the presence of detectable levels of antibody. Mixed sera from five mice were centrifuged at 1048 × g for 10 min at 4 °C, heated for 30 min at 55 °C. The obtained antiserum was used directly as an anti-TKIs antibody for ELISA. The experimental protocol was approved by the Sojo University Ethics Review Committee for Animal Experimentation (2021-L-006).
Indirect Competitive ELISAIndirect competitive ELISA was performed according to a previous method.14) Briefly, microtiter plate wells were coated by adding 100 µL MPA-KLH (1 µg/mL) in 10 mM Tris–HCl buffer (pH 8.5) containing 10 mM NaCl and 10 mM NaN3 (Buffer A), and allowed to react at 37 °C for 1 h. The wells were washed twice with phosphate-buffered saline containing 0.1% BSA (PBS-BSA) and incubated with 100 µL of Buffer A containing 1% skim milk for 30 min at 37 °C. The MPA-KLH-coated wells were filled with 50 µL of either TKI-treated samples or PBS-BSA as a control, followed by the addition of 50 µL of the anti-serum (diluted 1 : 10000 in PBS-BSA). The wells were incubated at 37 °C for 2 h and washed 3 times with PBS-BSA, followed by incubation with Histofine Simple Stain MAX-PO (M) (1 : 500 in PBS-BSA) at 37 °C for 60 min. The wells were washed 3 times with PBS-BSA and the activity of the enzyme was measured by adding 100 µL of 0.42 mM TMB in 0.05 M acetate-citric acid buffer (pH 5.5) containing 0.01% hydrogen peroxide, and incubating the wells for 30 min at 37 °C. Then, 100 µL of 2.0 M sulfuric acid was added to each well, and the color intensity was measured using a spectrophotometer at 450 nm with a microplate reader.
Quantification of Brigatinib in Mouse Serum by ELISAThree 7-week-old male Slc:ICR mice weighing 30–40 g were used in this experiment. Brigatinib was dissolved in 25 mM sodium citrate buffer (pH 4.5) to yield a concentration of 2.5 mg/mL. An oral dose of 25 mg/kg brigatinib was administered using a gavage needle at 5 mL/kg to mice after an overnight fast (12 h). Serum samples were collected from the tail vein at 0.5, 1, 2, 4, and 6 h post-administration. The serum samples were diluted 10-fold with PBS-BSA in order to obtain brigatinib concentrations suitable for measurement by the ELISA.
Quantification of Gilteritinib in Rat Serum and Tissues by ELISAThree male Wistar rats weighing 160–180 g were used in this experiment. Gilteritinib was dissolved in 25 mM sodium citrate buffer (pH 4.5) to yield a concentration of 1 mg/mL. An oral dose of 5 mg/kg were administered using a gavage needle at 5 mL/kg to the rats after an overnight fast (12 h). Blood, liver, and kidney samples were removed at 4 h post-administration. Each tissue (approximately 50 mg) was weighed and homogenized in 1 mL PBS using a disposable tissue homogenizer (BioMasher II; Nippi, Inc., Tokyo, Japan). The homogenate was centrifuged (20000 × g, 4 °C, 10 min) and the supernatant was used for the ELISA. The obtained serum and homogenized samples were analyzed directly by ELISA without dilution.
HPLC for GilteritinibHPLC for gilteritinib was performed according to a previous study.15)
Brigatinib and gilteritinib share a common substructure (Fig. 1). Based on insights gained from our past success in developing a method for producing antibodies against small-molecule drugs, we believed that this common structural component of the TKIs might be large enough to be used as a hapten.4–9) In addition, this common structural site is altered in the major metabolites of brigatinib16) and gilteritinib.17) Therefore, to obtain an antibody specific for both TKIs, MPA, the common substructure of both TKIs, was used as a hapten. MPA has an aromatic amino group; therefore, we coupled a diazonium compound to the tyrosine and histidine residues on the carrier protein (Fig. 2). The generated MPA-BSA conjugate (immunogen) induced the formation of specific antibodies in each of the mice immunized. Furthermore, to avoid the influence of antibodies to the carrier protein BSA, KLH was used as the carrier protein for the coating antigen.
ELISA for Brigatinib and GilteritinibFigure 3 shows the calibration curves for brigatinib and gilteritinib as obtained using human serum. The calibration range was 0.2–1,250 ng/mL for brigatinib and gilteritinib. The lower limit of detection for brigatinib and gilteritinib was determined to be 2.8 and 2.9 ng/mL, respectively, by interpolation at 3 standard deviations (S.Ds.) above the mean background signal. The lower limit of quantification for brigatinib and gilteritinib was determined to be 6.2 and 6.8 ng/mL, respectively, by interpolation at 10 S.Ds. above the mean background signal. The intra-assay precision of brigatinib and gilteritinib in the low, medium, and high assay ranges (4 levels, n = 5 each) showed coefficients of variation ranging from 5.2 to 8.5 and from 5.0 to 9.6%, respectively (Table 1). The inter-assay precision of brigatinib and gilteritinib in the low, medium, and high assay ranges (4 levels, n = 5 each) showed coefficients of variation ranging from 2.4 to 9.0 and from 2.0 to 9.7%, respectively (Table 1). Spike-in recoveries of brigatinib and gilteritinib in the low, medium, and high assay ranges (4 levels, n = 5 each) showed values from 95.7 to 109.1 and from 93.8 to 107.0%, respectively (Table 1). The ELISA fulfilled the acceptance criteria for all addressed validation parameters of the current bioanalytical guidelines of the U.S. Food and Drug Administration.18) In addition, the calibration curves of brigatinib and gilteritinib in the serum system were similar to those in the buffer system.

The curves show the amount (%) of bound enzyme activity (B) for various doses of brigatinib and gilteritinib as a ratio of that bound using Histofine Simple Stain MAX-PO (M) alone (B0). Brigatinib: ●; Gilteritinib: 〇. Each point represents the mean ± S.D. of three replicates.
| Brigatinib | ||||
|---|---|---|---|---|
| Assay | Added (ng/mL) | Estimated (ng/mL) | Recovery (%) | C.V. (%) |
| Intra assay | 10 | 9.57 ± 0.7 | 95.7 | 7.5 |
| 50 | 48.4 ± 2.5 | 96.8 | 5.2 | |
| 250 | 251.3 ± 21.4 | 100.5 | 8.5 | |
| 1250 | 1200.9 ± 65.9 | 96.0 | 5.5 | |
| Inter assay | 10 | 10.9 ± 1.0 | 109.1 | 9.0 |
| 50 | 52.1 ± 3.1 | 104.2 | 6.0 | |
| 250 | 250.0 ± 14.9 | 100.0 | 6.0 | |
| 1250 | 1252.4 ± 29.6 | 100.2 | 2.4 | |
| Gilteritinib | ||||
| Assay | Added (ng/mL) | Estimated (ng/mL) | Recovery (%) | C.V. (%) |
| Intra assay | 10 | 10.7 ± 0.5 | 107.0 | 5.0 |
| 50 | 46.9 ± 2.9 | 93.8 | 6.2 | |
| 250 | 248.4 ± 13.5 | 99.3 | 5.4 | |
| 1250 | 1300.0 ± 124.6 | 104.0 | 9.6 | |
| Inter assay | 10 | 10.0 ± 0.8 | 100.5 | 8.0 |
| 50 | 49.4 ± 4.8 | 98.2 | 9.7 | |
| 250 | 252.0 ± 4.8 | 100.1 | 2.0 | |
| 1250 | 1301.8 ± 86.1 | 104.1 | 6.6 | |
Values represent mean ± S.D. (n = 5).
During long-term therapy, the plasma levels of brigatinib17) and gilteritinib19) are 1000–2100 and 400–700 ng/mL, respectively; therefore, this ELISA seems to have adequate sensitivity to quantify both TKIs in pharmacokinetic studies.
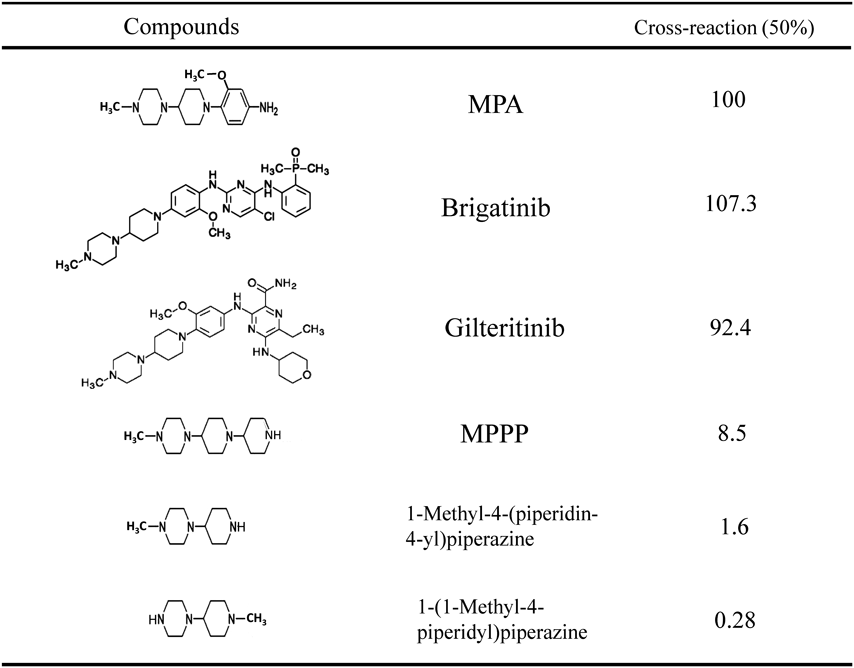
Antibody Cross-ReactivityAntibody cross-reactivity was determined by the displacement of the bound anti-MPA antibody with analogous compounds. The cross-reactivity values were set as the ratio of each compound to MPA at the concentration needed for 50% inhibition of anti-MPA antibody binding to the coating antigen. The anti-MPA antibody showed 100% cross-reactivity with MPA as the hapten antigen, 107.3% with brigatinib, 92.4% with gilteritinib, 8.5% with MPPP, 1.6% with 1-methyl-4-(piperidin-4-yl) piperazine, and 0.28% with 1-(1-methyl-4-piperidyl) piperazine (Table 2). These cross-reactivity results suggested that the anti-MPA antibody recognized the part of the MPA structure from the N-methylpiperazin moiety to part of the methoxyaniline moiety (not including the methoxy group). The antibody appeared to have a particularly strong ability to recognize the N-methylpiperazin moiety. The major metabolite of brigatinib in human plasma is N-desmethyl brigatinib (M36:AP26123)16) (Fig. 4). The plasma concentration of N-desmethyl brigatinib was found to be only 3.5% of the plasma concentration of the overall parent exposure.16) The major metabolites of gilteritinib in human plasma include M17 (formed via N-dealkylation and oxidation) and M16 and M10 (both formed via N-dealkylation) (Fig. 4). None of the three metabolites exceeded 10% of the overall parent exposure.17,19) The major metabolites of these TKIs have alterations in the structural components strongly recognized by the anti-MPA antibody. Judging from the specificity of the anti-MPA antibody, it is suggested that it shows little cross-reactivity with these major metabolites. Namely, this ELISA may have sufficient specificity to enable the quantification of brigatinib and gilteritinib for pharmacokinetic studies.
 |

As a demonstration of the potential of the developed ELISA, preliminary pharmacokinetic studies of brigatinib and gilteritinib in animals were performed (Fig. 5, Table 3). Brigatinib was rapidly absorbed, reached a peak serum concentration of 1900 ng/mL at 1.1 h after oral administration, and then decreased slowly in mice. The kinetics of brigatinib in mouse serum were almost the same as those reported by Sparidans et al. measured by means of LC-tandem mass spectrometry.20)

Three mice weighing 30–40 g were administered 25 mg/kg brigatinib orally. At each interval, serum was collected and the levels of brigatinib were measured by ELISA. Each point represents the mean ± S.D. (n = 3).
| Tissue | Gilteritinib concentration |
|---|---|
| Serum | 13.5 ± 4.14 ng/mL |
| Kidney | 2066 ± 340 ng/g |
| Liver | 3633 ± 450 ng/g |
Each value is the mean± S.D. obtained from 3 rats.
The ELISA was used to measure gilteritinib concentrations in various serum, liver, and kidney samples from male rats (Table 3). The highest concentration of gilteritinib was found in the liver (2896 ng/g tissue), followed by 1658 and 7.97 ng/g in the kidney and serum, respectively. To our knowledge, these results on the distribution of gilteritinib in rat tissues are the first to be reported. This ELISA enabled the simple measurement of the concentrations of these TKIs in serum and tissues without pretreatment of the samples. These results suggest that this ELISA is useful for studying the kinetics of brigatinib and gilteritinib in animals.
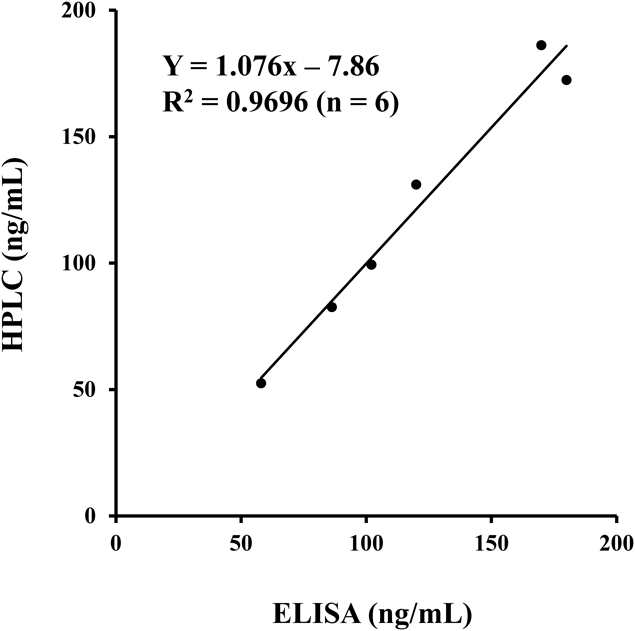
The developed ELISA was compared with an existing HPLC method15) for gilteritinib quantification. Samples from the kinetic study of gilteritinib in rat liver and kidney were used (Fig. 6). A good correlation was found between the values of both methods. The equation Y = 1.076X−7.86 was derived, with Y as the concentration determined by HPLC analysis and X as the concentration determined by ELISA. A correlation coefficient of 0.9696 was obtained (n = 6). This finding also supports the high specificity of the developed ELISA.
An ELISA for the quantification of two TKIs, brigatinib and gilteritinib, was developed and validated for three matrices: human serum, mouse serum, and rat tissues. To our knowledge, this is the first ELISA for these TKIs. This assay can be used for TDM, pharmacokinetic studies, and preclinical mouse and rat studies.
The authors declare no conflict of interest.