2022 Volume 45 Issue 8 Pages 1203-1207
2022 Volume 45 Issue 8 Pages 1203-1207
The opioid system in the central nervous system regulates depressive-like behavior in animals. Opioid receptors and their endogenous ligands have been focused on as novel therapeutic targets for depression. We synthesized dermorphin (DRM)-dynorphin (DYN) analogs (DRM-DYN001–004) using the message-address concept concerning interactions with opioid receptors. It has previously been reported that DRM-DYN001, 003, and 004 have shown high affinities for μ- and κ-opioid receptors, whereas all analogs had a lower affinity for the δ-opioid receptor than for other receptors using a receptor binding assay. However, it remains unknown whether these analogs show antidepressant-like effects in mice. We examined the effects of DRM-DYN analogs on the duration of immobile behavior in a tail suspension test. Intracerebroventricular administration of DRM-DYN001 in mice shortened the duration of immobile behavior, but did not affect locomotion. The DRM-DYN001-induced antidepressant-like effect was inhibited by co-administration of naloxone (non-selective opioid receptor antagonist), naloxonazine (selective μ1-opioid receptor antagonist), or nor-BNI (κ-opioid receptor antagonist), but not naltrindole (δ-opioid receptor antagonist). These data suggest that DRM-DYN001 exerts an antidepressant-like effect via activation of the central μ1- and κ-opioid receptors in mice and may represent a new lead peptide for further investigation for the development of novel therapeutic approaches for depression.
Approximately 30% of patients with depression do not improve with existing antidepressants.1) Recently, there has been a focus on opioid receptors and their endogenous ligands as novel therapeutic targets for depression.2) The antidepressant-like effects of δ-opioid receptor agonists is well documented. δ-opioid receptor agonists exhibit antidepressant-like effects,3) and δ-opioid receptor-knockout mice show depression-like behavior.4) Similarly, μ-opioid receptor agonists exhibit antidepressant-like effects,5) and the antidepressant effects of venlafaxine, a serotonin norepinephrine reuptake inhibitor, are eliminated in mice due to μ-opioid receptor knockout.6) For κ-opioid receptors, their receptor agonists induce depressive-like and antidepressive-like behaviors,5,7,8) whereas their receptor antagonists (e.g., nor-binaltorphimine [nor-BNI]) induce antidepressant-like effects.5) Thus, the opioid system in the central nervous system regulates depression-like behavior in animals. One study reported that a combination of μ- and κ-opioid receptors attenuated drug abuse,9) while the effects on motivation were unclear.
Dynorphin A (DYN) is an endogenous opioid peptide with a high affinity for the κ-opioid receptor. Based on Schwyzer’s classical message-address concept,10) the C-terminal sequence has been suggested to regulate receptor affinity and biological activity. The N-terminal “message” sequence of dermorphin (DRM, Tyr-D-Ala-Phe), isolated from the skin of the frog Phyllomedusa sauvagii, contributes to high opioid receptor affinity and resistance to enzymatic degradation. Through combining these two sequences, DRM-DYN hybrid analogs with relatively high binding to the κ-opioid receptor and metabolic stability have been successfully created.11) However, it remains unknown whether these analogs have an antidepressant-like effects in rodents.
This study aimed to examine the effects of DRM-DYN analogs on the duration of immobile behavior and to evaluate the resulting antidepressant-like effects of these mechanisms.
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals from Tohoku Medical and Pharmaceutical University (Approval Nos. 18047-cn and 19022-cn) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize suffering and to reduce the number of animals used.
AnimalsMale ddY mice (weight, 28–32 g; Japan SLC, Shizuoka, Japan) were used in all experiments (n = 285). The mice were housed in cages with free access to food and water under controlled temperature (22 ± 2 °C) and humidity (55 ± 5%) conditions on a 12-h light–dark cycle (lights on: 07 : 00 to 19:00).
DrugsOne of our colleagues synthesized the DRM-DYN analogs, DRM-DYN001– DRM-DYN004. The amino acid sequences of the analogs are shown in Table 1. The DRM-DYN analogs and nor-BNI (Sigma-Aldrich, St. Louis, MO, U.S.A.) were dissolved in Ringer’s solution and administered at a volume of 5 µL intracerebroventricularly using a 50 µL Hamilton microsyringe attached to a disposable 27-G needle under inhalatory diethyl ether anesthesia. Naloxone (Sigma-Aldrich), naloxonazine (Sigma-Aldrich), and naltrindole (Sigma-Aldrich) were dissolved in saline and intraperitoneally administered at a dose of 0.1 mL/10 g of body weight. The doses of nor-BNI, naloxone, naloxonazine, and naltrindole used were based on previous studies.12–14)
| Peptides | Amino acid sequence |
|---|---|
| DRM-DYN 001 | H-Tyr-D-Ala-Phe-Leu-Arg-Arg-Ile-Arg-NH2 [DRM (1-3)-DYN (5-9)-NH2] |
| DRM-DYN 002 | H-Tyr-D-Ala-Phe-Leu-Arg-Arg-Ile-NH2 [DRM (1-3)-DYN (5-8)-NH2] |
| DRM-DYN 003 | H-Tyr-D-Ala-Phe-Leu-Arg-Arg-NH2 [DRM (1-3)-DYN (5-7)-NH2] |
| DRM-DYN 004 | H-Tyr-D-Ala-Phe-Leu-Arg-Arg-Arg-NH2 [DRM (1-3)-DYN (5-7)-Arg-NH2] |
The tail suspension test was performed as previously described,3) to evaluate the antidepressant-like effects of the DRM-DYN analogs. Mice were suspended with their tails taped, such that they were 30 cm above the floor. An investigator blinded to the treatment assignments observed the immobility time for 10 min.
Locomotor ActivityLocomotor activity was determined using SUPERMEX software (Muromachi Kikai Co., Ltd., Tokyo, Japan). The details of the apparatus have been previously described.3) Locomotor activity was measured for 120 min during the light phase, between 11:00 a.m. and 15:00 p.m. Each mouse was placed in an activity box of SUPERMEX for 15 min for adaptation prior to injection with either vehicle or DRM-DYN001.
Statistical AnalysisThe experimental results are presented as mean ± standard error of the mean (S.E.M.). Significant differences were determined using one- or two-way ANOVA, followed by a Tukey–Kramer test for multiple group comparisons. The criterion for a significant difference was set at p < 0.05.
To evaluate whether DRM-DYN analogs have antidepressant-like effects in mice, we observed changes in immobility time in the tail suspension test after treatment with these analogs. Treatment with DRM-DYN001 significantly shortened the duration of immobility behavior compared to that in the vehicle group (p = 0.0148, Fig. 1A), while the other analogs did not show a statistical difference in immobility time compared to that in the control group, suggesting that DRM-DYN001 has potential antidepressant-like effects.

Vehicle or DRM-DYN analogs were administered (i.c.v.) 30 min before the tail suspension test. The bars represent the mean ± S.E.M. One-way ANOVA: (F [2, 30] = 4.853, p = 0.0149, Fig. 1A; F [2, 34] = 0.1183, p = 0.8888, Fig. 1B; F [3, 38] = 2.21, p = 0.1027, Fig. 1C; F [2, 26] = 0.4434, p = 0.6466, Fig. 1D). Numbers in square brackets indicate the number of animals in each group. * p < 0.05, compared with the vehicle group. Abbreviations: DRM-DYN, dermorphin-dynorphin; i.c.v, intracerebroventricularly; S.E.M., standard error of the mean.
To exclude the possibility that DRM-DYN001 administration alters locomotion, which in turn could affect the duration of immobility behavior in the tail suspension test, we measured locomotor activity in mice using SUPERMEX. There were no changes in locomotor activity for 120 min in mice treated with DRM-DYN001 compared with mice in the vehicle group (Fig. 2).
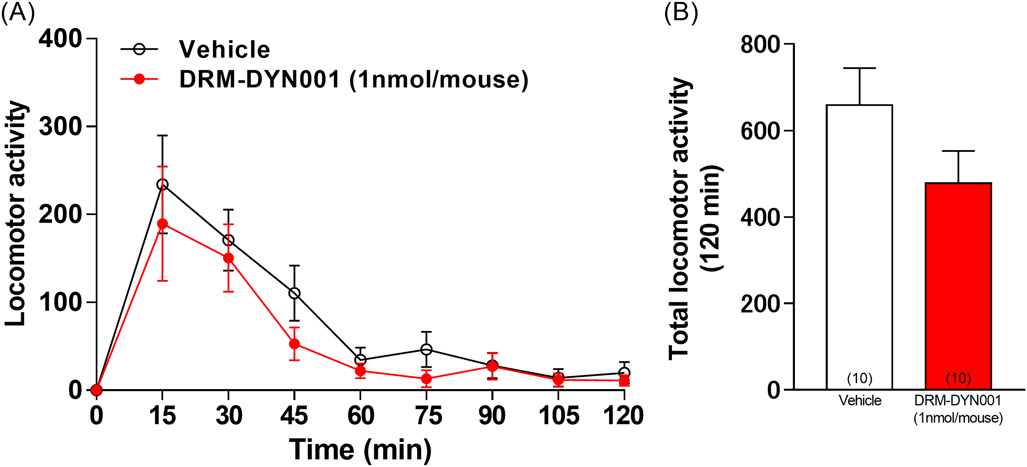
Mice were injected with vehicle or DRM-DYN001, and locomotor activity was recorded for 120 min. The bars represent the mean ± S.E.M. Two-way ANOVA: (time × treatment: F [8, 144] = 0.3014, p = 0.9644; time: F [8, 144] = 15.44, p < 0.0001; treatment: F [1, 18] = 2.483, p = 0.1325, Fig. 2A). Student’s t-test: (t = 1.576, df = 18, p = 0.1325, Fig. 2B). Numbers in square brackets indicate the number of animals in each group.
To determine whether the opioid receptors, μ, δ, and κ, were involved in the DRM-DYN001-induced antidepressant-like effects in mice, we observed changes in immobility behavior at the time of co-administration of DRM-DYN001 and each opioid receptor antagonist. The antidepressant-like effect was attenuated by treatment with the non-selective opioid receptor antagonist naloxone (p = 0.0016, Fig. 3B), selective μ1-opioid receptor antagonist naloxonazine (p = 0.0126, Fig. 3C), and κ-opioid receptor antagonist nor-BNI (p = 0.0435, Fig. 3D); however, the δ-opioid receptor antagonist naltrindole did not affect immobility behavior in mice.
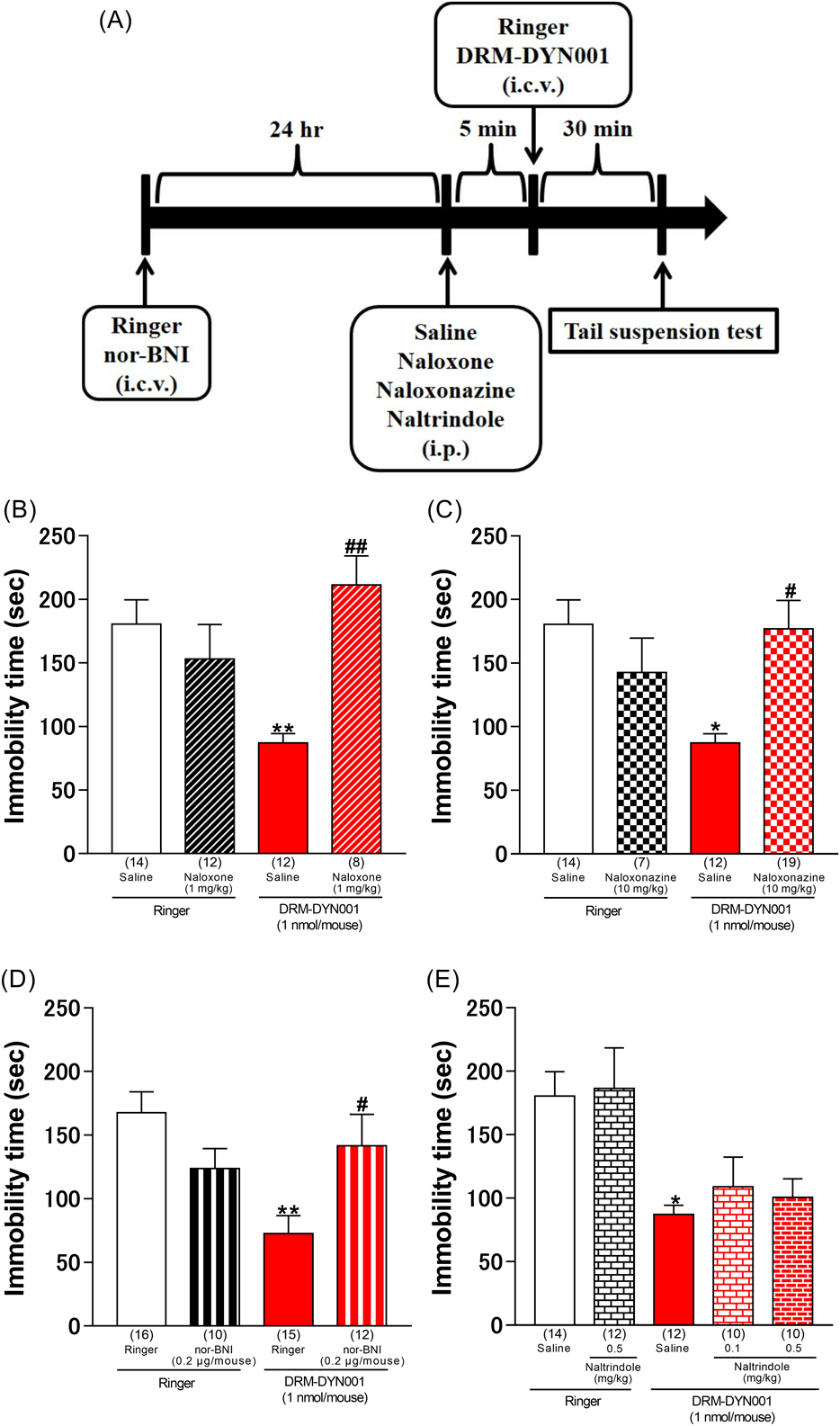
A: The time course of the experimental protocol. The bars represent the mean ± S.E.M. Two-way ANOVA: (peptide × inhibitor: F [1, 42] = 13.28, p = 0.0007, peptide: F [1, 42] = 5.428, p = 0.0247, inhibitor: F [1, 42] = 0.7257, p = 0.3991, Fig. 3B); peptide × inhibitor: F [1, 48] = 8.067, p = 0.0066, peptide: F [1, 48] = 1.314, p = 0.2574, inhibitor: F [1, 48] = 1.719, p = 0.1961, Fig. 3C; peptide × inhibitor: F [1, 49] = 9.484, p = 0.0034, peptide: F [1, 49] = 0.4703, p = 0.4961, inhibitor: F [1, 49] = 4.482, p = 0.0393, Fig. 3D). One-way ANOVA: F [4, 53] = 5.143, p = 0.0014, Fig. 3E). Numbers in square brackets indicate the number of animals in each group. * p < 0.05, ** p < 0.01, compared with the vehicle only group; # p < 0.05, ## p < 0.01, compared with the DRM-DYN001 only group.
In this study, we showed that DRM-DYN001 exhibited an antidepressant-like effect through stimulation of the μ1- and κ-opioid receptors.
Opioid receptors, such as μ, δ, and κ, and their endogenous ligands play an important role in the modulation of neural pathways associated with regulation of the psychiatric state.5) In our previous study, we reported that DRM-DYN001, 003, and 004 had higher affinities for μ- and κ-opioid receptors than for δ-opioid receptors using a receptor-binding assay.11) We observed changes in immobility time after treatment with DRM-DYN analogs in the tail suspension test. In this study, DRM-DYN001 shortened the duration of immobile behavior in mice in the tail suspension test (Fig. 1A), but did not affect locomotion compared with those in the vehicle-treated group (Fig. 2). We hypothesized that DRM-DYN001 had an antidepressant-like effect in mice through the μ- and/or κ-opioid receptors.
To determine which opioid receptor subtypes were associated with the DRM-DYN001-induced antidepressant-like effects in mice, we observed changes in immobility behavior when DRM-DYN001 was co-administered with opioid receptor antagonists. We found that the DRM-DYN001-induced antidepressant effect was inhibited due to the co-administration of naloxone (non-selective opioid receptor antagonist), naloxonazine (selective μ1-opioid receptor antagonist), or nor-BNI (κ-opioid receptor antagonist), but not naltrindole (δ-opioid receptor antagonist) (Fig. 3). Based on these results, we suggest that the stimulation of μ1- and κ-opioid receptors may be involved in the DRM-DYN001-induced antidepressant effect.
In general, κ-opioid receptor antagonists produce antidepressant effects through inhibiting dopamine (DA) release in the nucleus accumbens.5,15) It has been reported that low doses of salvinorin A, a κ-opioid receptor agonist, show antidepressant effects through increasing DA release in the nucleus accumbens,8,16) whereas high doses induce depression-like behavior with decreasing DA release in the same brain region.17) Moreover, μ/κ-opioid receptor agonists, such as levorphanol, morphine, and N-ethyl substituted aminothiazolomorphinan ATPM-ET, have shown antidepressant effects in mouse tail suspension tests.7,18) Stimulation of μ-opioid receptors induces an increase in extracellular DA in the nucleus accumbens,19) and suppresses the responsiveness of the hypothalamo-pituitary-adrenal (HPA) axis,20) which results in antidepressant effects. Ascorbic acid-induced antidepressant effects have been associated with the activation of μ1-opioid receptors.12) Based on these findings, we hypothesized that activation of μ- and/or κ-opioid receptors was involved in the antidepressant effect of DRM-DYN001. Our study findings indicated that DRM-DYN001-induced antidepressant-like effects were eliminated with co-administration of μ1- or κ-opioid receptor antagonists. We therefore hypothesized that DRM-DYN001-induced antidepressant-like effects may be associated with activation of the dopaminergic system in the nucleus accumbens or suppression of the responsiveness of the HPA axis via stimulation of μ1- and κ-opioid receptors. Nevertheless, additional experiments concerning this hypothesis are needed.
In conclusion, our findings indicated that intracerebroventricular administration of DRM-DYN001 shortened the immobility time in mice via activation of μ1- and κ-opioid receptors, suggesting the antidepressant therapeutic potential of μ1- and κ-opioid receptor agonists. DRM-DYN001 represents a new lead peptide for further investigation for the development of novel therapeutic approaches for depression.
The authors would like to thank Dr. Takayo Odaira and Dr. Wakana Sakuma, Ms. Sena Hayasaka, Ms. Yuko Oshima, and Mr. Kazuaki Shoji of Tohoku Medical and Pharmaceutical University for their technical assistance. This study was supported in part by JSPS KAKENHI (Grant Nos. JP18K06687 and JP21K15351).
The authors declare no conflict of interest.