2023 Volume 46 Issue 1 Pages 86-94
2023 Volume 46 Issue 1 Pages 86-94
From our previous observation that the anesthetic effects of phenobarbital potentiate in rats with a decreased cerebral protein expression of the potassium chloride cotransporter KCC2 (SLC12A5), an in vivo study was conducted to clarify whether the pharmacological effect of phenobarbital alters by stimulating the cerebral tropomyosin receptor kinase B (TrkB) that is known to down-regulate the KCC2 protein expression. The stimulation was performed in rats with repetitious intraperitoneal administration of a TrkB agonist, namely 7,8-dihydroxyflavone (DHF). After that, the rats underwent an intraventricular infusion of phenobarbital using a dwelled cannula, and the onset time of the phenobarbital-induced general anesthesia was determined. In addition, their brain tissues were excised and cerebral cortices were collected. Then, subcellular fractions were prepared and the cerebral expression of various proteins involving the anesthetic effects of phenobarbital was examined. It was demonstrated that phenobarbital induced general anesthesia about 2 times faster in rats receiving the DHF treatment than in control rats, and that the phenobarbital amount in the brain tissue at the onset time of anesthesia was lower in rats with the treatment. Western blotting showed that the cerebral protein expression of KCC2 decreases, and the phosphorylation of the TrkB protein increases with the DHF treatment. These observations indicate that the anesthetic effects of phenobarbital potentiate with the TrkB stimulation and the resultant decrease in the cerebral KCC2 protein expression. The results also suggest that the TrkB protein and its phosphorylation status may be a key modulator of the pharmacological efficacy of phenobarbital.
Understanding and recognizing factors affecting the pharmacokinetics and pharmacodynamics of therapeutic compounds are essential to provide effective pharmacotherapy, particularly for patients with severe conditions. Numerous researchers have investigated those factors, and their findings have shown that impaired renal function is among those factors. The impairment influences the pharmacokinetics of various therapeutic compounds,1) some parts of which are accounted for by uremic toxins, such as hippuric acid and indoxyl sulfate, that are released during the impairment.2,3) Since these toxins are associated with serum albumin, competing with therapeutic compounds for their binding sites, the serum unbound fraction of the compounds increases, leading to a change in their total body clearance, volume of distribution, and pharmacokinetics.4–6) In addition to those effects on the unbound fraction, the renal impairments can decrease the hepatic drug metabolizing activity of therapeutic compounds,1,7) to which proinflammatory mediators released from damaged tissues are reported to largely contribute.8,9) The mediators, including interleukin-6 and tumor necrosis factor-α, activate the nuclear factor-kappaB and interfere with the heterodimerization of nuclear receptors.10–12) Since the heterodimerization is an essential step for transactivation of genes of the hepatic drug metabolizing enzymes, the interference results in suppression of their protein expression and drug metabolizing activity.13,14)
Considering the influences on the compound's pharmacokinetics due to the renal impairments, it was assumed that the impairments also affect the pharmacological efficacy of therapeutic compounds by modulating protein expression and cellular signal transaction in the target tissues. In our previous study with intraventricular phenobarbital infusion, it was observed that phenobarbital induces general anesthesia with a smaller dose in rats when they are suffering from acute renal failure, showing that the pharmacological efficacy of phenobarbital is affected by impaired renal function.15,16) These observations indicate that it is necessary to investigate and understand the mechanisms underlying the altered pharmacological efficacy of therapeutic compounds, especially in patients with severe diseases, because current optimization and individualization methods in pharmacotherapy do not consider alteration of the pharmacological efficacy of the compounds. Regarding the underlying mechanisms for the increased pharmacological efficacy of phenobarbital, it was also observed that the cerebral protein expression of the potassium chloride cotransporter KCC2 (SLC12A5) decreases, but there were no detectable alterations for the cerebral expression of the phenobarbital target site, or the γ-aminobutyric acid A (GABAA) receptor, in rats with acute renal failure.16) Since KCC2 is an electrolyte transporter, mainly mediating chloride effluence in the nerve cells,17,18) it is speculated that a decreased expression of the KCC2 protein may lead to altered electrolyte handling in the nerve cells, causing a suppression of the nerval action potential and transmission.
This study aimed to understand the extent that KCC2 and its cellular electrolyte handling contribute to the pharmacological efficacy of phenobarbital. To examine this, the anesthetic effect of phenobarbital was evaluated in rats with and without 7,8-dihydroxyflavone (DHF) pretreatment (Fig. 1). DHF, also known as tropoflavin, is a natural product found in the weed tridax daisy and some other plants, and it is currently under examination as a potential drug candidate for depression, anxiety, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases.19–21) DHF acts as an agonist toward the tropomyosin receptor kinase B (TrkB), a high affinity receptor of the brain-derived neurotrophic factor (BDNF), promoting the TrkB phosphorylation and causing a down-regulation of the cellular expression of the KCC2 protein.19,22,23) Therefore, it is likely that the expression of the KCC2 protein in nerve cells is suppressed in rats receiving the DHF treatment, and this probably alters the pharmacological effect of phenobarbital. Thus, we examined the anesthetic effect of phenobarbital in rats receiving DHF treatment to clarify whether altered sensitivity of the target tissue is one of the key factors that modulates the pharmacological efficacy of phenobarbital.

Phenobarbital was obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). DHF was obtained from Tokyo Chemical Industry (Tokyo, Japan). Chrysin, or 5,8-dihydroxyflavone, a structural isomer of DHF, was purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.). The purified rabbit polyclonal antibody against rat KCC2 (Catalogue No. 07-431) and that against rat NKCC1 (SLC12A2, sodium, potassium, chloride cotransporter 1) (Catalogue No. AB3560P) were purchased from Millipore (Burlington, MA, U.S.A.). In addition, the purified rabbit polyclonal antibody against human TrkB protein (Catalogue No. BS3668), which has a cross-reactivity with rats, bought from Bioworld Technology (Bloomington, MN, U.S.A.). The rabbit polyclonal antibody against phosphorylated TrkB (Catalogue No. GTX50335) was obtained from GeneTex (Irvine, CA, U.S.A.). According to the product information, this antibody is raised against the peptide sequence around the phosphorylation site of tyrosine 515 prepared from human TrkB protein,24) and this has a cross-reactivity with the corresponding sequence of rat TrkB embracing the phosphorylated tyrosine. The phosphorylation of tyrosine 515 was shown to be a crucial step for the TrkB-mediating down-regulation of the cellular expression of the KCC2 protein.25,26) The protein molecular weight maker (Precision Plus Protein™ Standards) was purchased from Bio-Rad Laboratories (Hercules, CA, U.S.A.). The primer pairs used in the PCR were obtained from a custom primer synthesis service (Thermo Fisher Scientific, Waltham, MA, U.S.A.). Their sequences are listed in Table 1. All other reagents used in this study were analytical grade.
| mRNA | Forward primer | Reverse primer | Location | Amplicon size | Reference |
|---|---|---|---|---|---|
| Kcc2 | 5′-cca tgg ctt tga tgt ctg tgc caa-3′ | 5′-act cat cac agg tgg cat tga gga-3′ | 1003–1115 | 113bp | Farmer et al.49) |
| Nkcc1 | 5′-ggc cat cgc tga ctt cgt cat agg-3′ | 5′-gca agg tca ccc gag atg ttc gc-3′ | 1432–1635 | 204 bp | Laube et al.50) |
| Actb | 5′-gag tac aac ctc ctt gca gct c-3′ | 5′-ttg tag aaa gtg tgg tgc caa a-3′ | 25–356 | 332 bp | Nejsum et al.51) |
Male Wistar rats at 6 or 7 weeks old (195–325 g) were obtained from Jackson Laboratory Japan (Yokohama, Japan). The 6-week-old rats underwent the DHF treatment for a week, as described below, and the 7-week-old rats were handled as the control group. In total, 11 rats were used in the DHF treatment group, and 8 rats were employed in the control group. The time line of the animal experiment in this study is provided as Fig. 2. Each rat received the cannula insertion surgery as described below. For the rats with DHF treatment, the surgery was performed on the last day of the treatment. For the control rats, the surgery was carried out following a 24-h acclimatization after the animal delivery. All rats were caged in the institutional laboratory animal facility. In the cage room, the temperature and relative humidity were maintained at 20–25 °C and 40–50%, respectively, with a 12-h light cycle. The rats were allowed to freely eat laboratory diet and drink water. All animal experiments were conducted in accordance with the guidelines for animal experimentation of Okayama University. The study plan, experiment schemes, and animal handlings were approved by the institutional animal ethics committee (OKU-2018321/OKU-2021384).

The horizontal line shows the time line of the experiment, in which the day of the rat being supplied is designated as Day 0 and it is expressed with dot on the horizontal line. The days on which the DHF treatment was performed are indicated with gray arrows, where DHF was intraperitoneally administered at a dose of 5 mg/kg/d. The day on which the cannula insertion surgery was conducted is shown with white arrow, and on the next day of the surgery that is denoted with closed arrow at the end of the horizontal line, the evaluation of the phenobarbital anesthetic effect and the subsequent processes for the brain tissue excision were executed. The excised brain tissues were stored at −20 °C until used as specimens in the studies with Western blotting, real-time PCR, and the determination of the tissue amount of phenobarbital. See text for more details. The 6-week and 7-week-old rats were used in the DHF treatment and control groups, respectively.
As for the DHF treatment, the 6-week-old rats were subjected to a peritoneal injection of DHF solution at a dose of 5 mg/kg once a day for 7 d. The dosage regimen of the DHF treatment was settled by referring to the report, in which DHF was shown to induce the TrkB activation in rats.27) The DHF solution was prepared by dissolving DHF in dimethyl sulfoxide (DMSO), which was then mixed with isotonic sodium phosphate solution (pH 7.6) to obtain 30% DMSO solution containing DHF at a concentration of 5 mg/mL for the injection. In supplemental experiments, three rats were subjected to chrysin treatment, which was prepared in the same manner as the DHF treatment, employing a DMSO solution containing chrysin at a concentration of 5 mg/mL.
The cannula insertion surgery was performed as reported previously.16) Following anesthetization with sodium pentobarbital (80 mg/kg, intraperitoneally, each rat was fixed to a stereotaxic instrument (Narishige, Tokyo, Japan) and its skull surface was disinfected with 10% povidone iodine solution. Then, a hole of 1 mm in diameter was drilled in the skull to access the right lateral ventricle at the brain atlas coordinates of 0.0 mm anterior and 1.5 mm lateral from the bregma, with a close attention not to compromise the brain tissue.28) After that, a guide cannula (AG-8, Eicom, Kyoto, Japan) was inserted into the ventricle through the hole in a delicate manner, and the tip of the cannula was placed in the ventricle, 3.2 mm ventral from the skull surface.28) The guide cannula was then fixed on the skull surface with anchor bolts (AN-8, Eicom) and dental cement, followed by temporary plugging with a dummy cannula (AD-8, Eicom) and cap nut (AC-8, Eicom). At the end of the surgery, the skull surface and the surgical region were sterilized with povidone iodine solution and 0.1% gentamicin sulfate ointment.
Evaluation of the Pharmacological Effect of Phenobarbital in RatsThe pharmacological effect of phenobarbital in rats was evaluated based on the amount of phenobarbital required to induce general anesthesia. In this study, we regarded the phenobarbital-induced anesthesia as the general state of lacking of response to and perception of external noxious stimuli,29,30) and therefore, we evaluated it without discriminating the anesthetic forms, such as analgesia, unconsciousness, and lack of response to external stimulation. The evaluation was carried out according to the previously reported method with a slight modification,16) where the phenobarbital solution was prepared at a concentration of 70 mg/mL and it was gently infused into the right lateral ventricle. After a 24-h equilibrium period following the surgery, the rat in full consciousness was transferred to an animal cage equipped with the cannula swivel (TSC2-21, Sugiyamagen, Tokyo, Japan). The swivel had two polyethylene tubes (0.5 mm i.d. × 0.8 mm o.d., KN392/SP-31, Natsume Seisakusho, Tokyo, Japan). One tube was connected to the 24-gauge needle of a tuberculin syringe filled with the phenobarbital solution, and the other was equipped at its end with a 27-gauge needle that is to be connected with the guide cannula at the skull of the rat. The syringe was then set on an infusion pump (SPE-1, As One, Osaka, Japan). After purging the air in the lumen of the tubes and in the swivel, the tip of the 27-gauge needle at the end of the polyethylene tube was firmly attached to the unplugged guide cannula. Then, the infusion pump was operated to introduce the phenobarbital solution into the rat’s ventricle until anesthesia was induced. The solution was introduced at a flow rate of 2.6 µL/min.16,31)
The onset time of anesthesia was determined using the tail-pinch method, as previously reported,16) in which lack of response to noxious stimuli was employed as an indication of the state of general anesthesia.29,30) Briefly, the rat was subjected to the nociceptive stimulation by pinching the tail. The first stimulation was made at a time point set in a preliminary study. The stimulation was repeated every 1 min while the rat was reacting to the stimulation. Otherwise, an additional stimulation was made 30 s later to confirm that the rat did not react to the previous stimulation. The confirmation was repeated three times at intervals of 30 s. The onset time of anesthesia was determined as the first time point when the rat showed no reaction to the stimulation. The onset time was used to calculate the amount of phenobarbital required to induce anesthesia.
After the onset time was determined, each rat was immediately sacrificed by exsanguination, and the brain was perfused with ice-cold isotonic sodium chloride solution to wash out the remaining blood. After that, the brain tissue was excised, and it was gently rinsed with ice-cold isotonic sodium chloride solution and stored at −20 °C until used in the studies described later.
Semi-quantitative Evaluation of Cerebral Cortical Protein Amounts with Western BlottingEach excised brain tissue was sliced lengthwise, and the cortex was manually sectioned to be collected with ophthalmologic tenotomy scissors. Each excised brain tissue was processed separately from the brain tissues of other rats. Parts of these collected cortices were stored until evaluation of the mRNA expression described later.
For the evaluation of protein expression by Western blotting, the cortices from 11 control rats and those from 8 rats receiving the DHF treatment were collected, respectively. They were processed with the commercial kit for the subcellular protein fractionation (Catalogue No. 87790, Thermo Fisher Scientific) by referring to the manufacturer’s instructions, and the fraction containing the membrane proteins was obtained as the specimen for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Each specimen was mixed and boiled with the commercially available 2-mercaptoethanol/30% glycerol solution, a reagent of β-Mercaptoethanol Sample Treatment for Tris SDS (Cosmo-Bio, Tokyo, Japan), and the processed specimen was then applied to an SDS-polyacrylamide (10%) gel with a protein amount of 5 µg/lane. SDS-PAGE was then conducted in a conventional manner. The specimens from control rats and those from rats receiving the DHF treatment were handled at the same time under the same conditions, so that the undermentioned densitometric evaluations regarding the visualized migration pattern of the proteins were evenly performed in the control and DHF treatment groups. The protein concentration of each specimen was determined with a protein quantification kit (Proteostain PQ01, Dojindo Laboratories, Mashiki, Kumamoto, Japan), in which any interference coming from surfactants in the specimens was suppressed by 300–1000 times dilution.
Following a 180-min electrophoresis, the migrated proteins were transferred to a nitrocellulose membrane, and the membrane was subjected to overnight blocking with skim milk. It was then incubated with the anti-KCC2, anti-NKCC1, anti-TrkB, and anti-phosphorylated TrkB antibodies. These antibodies were diluted to 1 : 1000–1 : 2000 for use. The migration pattern was chromogenically visualized using the commercial immunostaining kit (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, U.S.A.) and 3,3′-diaminobenzidine solution. The visualized patterns were analyzed in a semi-quantitative manner based on their densitometry values.16) In the analysis for the KCC2 protein, a 130–140 kDa protein visualized with anti-KCC2 antibody was used,32) while in the immunostaining with anti-NKCC1 antibody, the migration pattern of the protein having a larger molecular weight of about 140 kDa was employed in the analysis.32) The immunostainings with anti-TrkB and anti-phosphorylated TrkB antibodies both mainly visualized an 80–90 kDa protein.33–35) The densitometry values used in the analysis were normalized solely based on the protein amount of the specimen.
Semi-quantitative Evaluation of Cerebral Cortical mRNA Expression by Real-Time PCRThe sliced cortices collected from each rat were processed with the commercial total RNA extraction kit (FastGene RNA Basic Kit, NIPPON Genetics, Tokyo, Japan) according to the manufacturer’s instructions, in which five sliced cortices from control rats and five from rats receiving the DHF treatment were individually processed.
To evaluate mRNA expression, the RNA solution obtained with the extraction kit was subjected to reverse transcription (RT). The RT reaction was performed using the commercial RT reaction kit containing a primer mixture of random and oligo(dT) primers (ReverTra Ace qPCR RT Kit, Toyobo, Osaka, Japan). Subsequently, the obtained reaction mixture was submitted to PCR to evaluate the cerebral mRNA expression of Kcc2 and Nkcc1, for which the commercially available SYBR green PCR kit (THUNDEBIRD SYBR qPCR Mix, Toyobo) and real-time PCR instrument (StepOnePlus real-time PCR system, Thermo Fisher Scientific) were utilized. As for the evaluation of mRNA expression,36) the fluorescence was measured in the extension step of the PCR’s 3-step amplification cycle to determine the threshold cycle number at which the fluorescence began to increase in a log-linear manner. The threshold cycle number was also determined for the mRNA of β-actin protein (Actb), and the mRNA expressions of Kcc2 and Nkcc1 were evaluated relative to that of Actb. The evaluation values for their mRNA expressions were expressed with their common logarithms.36)
Determination of Cerebral Cortical Amounts of PhenobarbitalThe cerebral cortical amount of phenobarbital was determined by employing HPLC equipped with an octadecyl-silica column (InertSustain C18, GL Science, Tokyo, Japan). Briefly, the sliced cortices were minced, and they were homogenized with 3 volumes of isotonic phosphate buffer (pH 7.4). The homogenate was then mixed with 3 volumes of methanol and vigorously agitated, followed by incubation for 5 min at room temperature. After that, the mixture was centrifuged at 12000 × g, 4 °C for 10 min, and the supernatant was collected. The supernatant was subjected to filtration with a nylon membrane syringe filter, 0.22 µm pore size, and the filtered specimen was submitted to HPLC. The elution of phenobarbital was carried out at a flow rate of 1.0 mL/min with 50% methanol prepared based on 10 mM sodium phosphate buffer (pH 6.0). Phenobarbital was spectrophotometrically detected at a wavelength of 215 nm.
Data AnalysisThe anesthetic effects of phenobarbital were presented by showing the time-dependent decrease of the number of non-anesthetized rats using a Kaplan–Meier plot.16) The intergroup difference of the Kaplan–Meier curve was examined using the log-rank test, and p < 0.05 was considered significant. The data obtained in other experiments were summarized based on the assumption that they are not normally distributed. That is, data were expressed using a box-and-whisker plot, in which the first and third quartiles were indicated at the bottom and top of the box, respectively, and the median was shown as a bar in the box. The differences between the groups were evaluated using the Mann–Whitney U-test, with p < 0.05 being significant.
Firstly, the pharmacological effects of phenobarbital were evaluated in rats. It was shown that phenobarbital induced anesthesia with a shorter infusion time in rats receiving the DHF treatment compared with control rats. Specifically, more than half of the rats fell into anesthesia with the intraventricular phenobarbital infusion at 100 min in rats with the DHF treatment, while it took about 210 min in control rats (Fig. 3). The median value of the phenobarbital dose required to induce anesthesia was 14.2 mg in rats receiving the DHF treatment, and it was 31.1 mg in control rats (Fig. 4A). In addition, when the phenobarbital amounts in the brain tissues were determined, the amounts in rats receiving the DHF treatment were noticeably lower than those in control rats (Fig. 4B). The anesthetic effects of phenobarbital were additionally examined in 3 rats receiving chrysin instead of DHF treatment. As a result, the doses of phenobarbital required to induce anesthesia in those rats were in the range of 30.6–35.5 mg, similar to the doses required in control rats. These findings indicate that the anesthetic effects of phenobarbital potentiate with the DHF treatment.

The time–courses of the decrease in numbers of rats that remained conscious against the intraventricular infusion of phenobarbital are presented using a Kaplan–Meier plot, in which the time-courses for 11 control rats and 8 rats receiving the DHF treatment are indicated with lines in grey and black, respectively. For both time-courses, the upper and lower bounds of the 95% confidence intervals are shown with dotted lines. The time-course for control rats and that for rats receiving the DHF treatment are significantly different from each other (p < 0.05).

In panel A, the phenobarbital doses required to induce anesthesia were compared between control rats and in rats receiving the DHF treatment. Data from 8–11 experiments are presented using a box-and-whisker plot. In panel B, the phenobarbital amounts in the brain tissues determined at the onset time of anesthesia are shown using a box-and-whisker plot, in which 3 control rats and in 3 rats receiving the DHF treatment were employed. In both panels, the significant difference between the groups was verified using the Mann–Whitney U-test. * p < 0.05, significantly different from the value in control rats.
The cerebral protein expressions of the electrolyte transporters, KCC2 and NKCC1, were semi-quantitatively examined by Western blotting, employing the specimens prepared from the cortices of the excised brain tissues. The protein expression of KCC2 decreased by about 15% in rats receiving the DHF treatment compared with that in control rats (Fig. 5A), whereas the differences in the expression of NKCC1 were not detected. (Fig. 5B).

The expressions of the target proteins were evaluated by Western blotting in octuplicate, and the obtained values were expressed using a box-and-whisker plot. For both panels, the two representative Western blotting results are also presented above the corresponding plots, along with the molecular weight indices being shown on their right side. The significant difference between the groups was assessed using the Mann–Whitney U-test. * p < 0.05, significantly different from the value in control rats.
In addition to protein expression, the mRNA expressions of Kcc2 and Nkcc1 were examined. The mRNA expression of those two transporters appeared to increase with the DHF treatment (Figs. 6A, B), although their expression levels in rats receiving the DHF treatment exhibited a larger variance than those in control rats.
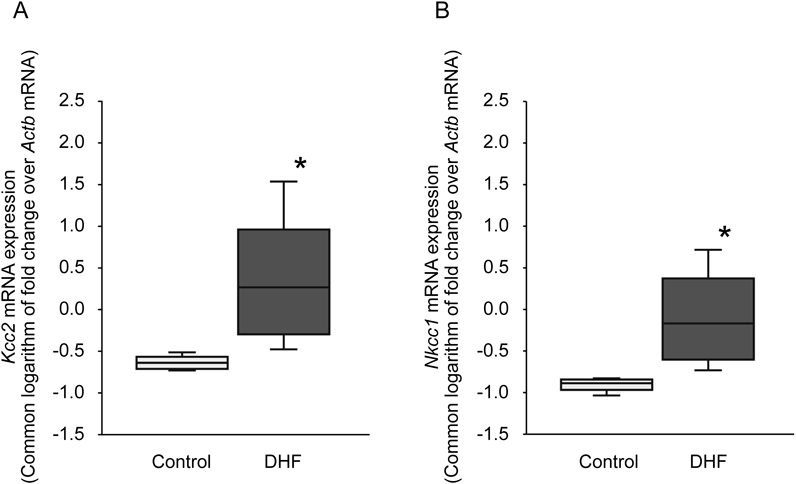
The mRNA expressions of those electrolyte transporters were evaluated as relative value to that of Actb, and the obtained values are expressed using a common logarithmic scale to be presented using a box-and-whisker plot. The significant difference between the groups was assessed by the Mann–Whitney U-test. * p < 0.05, significantly different from the value in control rats.
Since it is considered that DHF acts as an agonist of the TrkB protein to promote the phosphorylation that leads to down-regulation of the KCC2 protein expression, the amount of phosphorylated TrkB protein was evaluated by Western blotting, along with the TrkB protein expression. As a result, the expression of the TrkB protein was shown to decrease with the DHF treatment (Fig. 7A), while the phosphorylation of the TrkB protein was detected more in rats receiving the DHF treatment (Fig. 7B). These results suggest that more TrkB proteins are phosphorylated with the DHF treatment.

The expression and amount of the target proteins were evaluated by Western blotting with 20 replicates, and the obtained values are expressed using a box-and-whisker plot, in which outliers are indicated with circles. For both panels, the two representative Western blotting results are also presented above the corresponding plots, along with the molecular weight indices being shown on their right side. The significant difference between the groups was assessed using the Mann–Whitney U-test. * p < 0.05, significantly different from the value in control rats.
To provide effective pharmacotherapy, it is necessary to understand the factors affecting the pharmacological efficacy of therapeutic compounds, and the altered sensitivity of their target tissues may be considered as one of the factors. As mentioned earlier, it was shown in our previous study that phenobarbital induces anesthesia with a lower dose in rats suffering from renal failure,16) and a decrease in the cerebral protein expression of the KCC2 was also observed. Although these findings suggest that an altered expression of the KCC2 protein is the potentiation of the phenobarbital anesthesia, it is possible that the altered protein expression and potentiation of the phenobarbital effect are two independent phenomena, having no pharmacological link to each other.
In this study, we examined to what extent the KCC2 protein is involved in the altered pharmacological effect of phenobarbital using an indirect approach. Since the protein expression of KCC2 is known to be subject to the TrkB-mediated down-regulation, we set up the experiments employing DHF (Fig. 1), a flavonoid derivative that acts as an agonist with association to the ligand binding site of the TrkB protein. According to the literature, the ligand association to the TrkB protein induces the dimerization and autophosphorylation of the TrkB protein,22,23) triggering several cellular signaling pathways, including the pathway involving Src homologous and collagen (Shc) protein and the pathway involving phospholipase Cγ.37,38) These two TrkB-originated signaling pathways regulate KCC2 expression in a collaborative manner. That is, while the expression of the KCC2 protein is facilitated when the signaling pathway of Shc is solely activated with a selective abolishment of phospholipase Cγ, it is suppressed when those two signaling pathways are concurrently activated.25) In rats receiving the DHF treatment, it is likely that the TrkB protein is stimulated, activating the cellular signaling pathways to the down-regulation of the KCC2 protein expression.
When the anesthetic effects of phenobarbital were evaluated, it was shown that general anesthesia was induced about two times faster in rats receiving the DHF treatment (Fig. 3), in which the dose required for the anesthesia was about half of the control value (Fig. 4A), and the amounts of phenobarbital in the brain tissues determined at the onset time of the anesthesia were noticeably lower than those in control rats (Fig. 4B). These findings reflect the fact that the anesthetic effects of phenobarbital potentiate with the DHF treatment stimulating the TrkB protein. Furthermore, the cerebral expression of the KCC2 protein decreased in rats receiving the DHF treatment (Fig. 5A), whereas the expression of the NKCC1 protein was negligibly affected (Fig. 5B). An alteration related to the DHF treatment was also recognized for the mRNA expression of those proteins (Figs. 6A, B), implying that an alteration in the gene transcription process underlies the altered protein expression with the DHF treatment. Then, we assessed whether the DHF treatment influences the phosphorylation status of the TrkB protein, an essential step for regulation of the KCC2 protein expression. In the assessment, it was shown that the protein expression of the TrkB decreased in rats receiving the DHF treatment (Fig. 7A). The decrease in the protein expression of the TrkB seems to reflect the fact that DHF is associated with the TrkB protein as the agonist. It is reported that the TrkB protein undergoes the down-regulation following the ligand association.39,40) While the protein expression of the TrkB decreased, the amount of the phosphorylated TrkB protein slightly increased with the DHF treatment (Fig. 7B), suggesting that the TrkB protein phosphorylation was facilitated in rats receiving the DHF treatment, being consistent with the reported findings.22,23,25) As a limitation of this study, it was not evaluated to what degree the TrkB protein was cleft in the preparation process of the specimens, and this is likely to infiltrate inaccuracy into the measurement of the TrkB protein expression.41) In addition, it should be noted that the observed alteration of the protein expression of the KCC2 and NKCC1 in rats receiving the DHF treatment appears not to be consistent with the alteration of their mRNA expression (Figs. 5, 6). The mechanism underlying this discrepancy remains to be clarified in this study, although a regulation process with microRNA is reported to be involved in their protein expressions.42,43) On the other hand, it seems not to be probable that phenobarbital has some effects on the cellular expression of the TrkB protein and its phosphorylation status, since it was reported that the GABA-induced activation of the GABAA receptor, the target site of phenobarbital, causes little alteration in the TrkB-mediated cellular signaling pathway in mature neurons.44) The findings obtained in this study indicate that the stimulated TrkB protein induces a down-regulation of the KCC2 protein expression, and this probably causes an increase in the cerebral sensitivity to phenobarbital, leading to the potentiation of the anesthetic effects of phenobarbital.
As for a mechanism involving the increase in the cerebral sensitivity, it probably arises from an altered regulation of the action potential in the nerve cells. As shown in Western blotting, the DHF treatments resulted in the decreased expression of the KCC2 proteins (Fig. 5A), while the NKCC1 protein was not significantly influenced (Fig. 5B). Since the KCC2 and NKCC1 proteins play a key role in regulating and maintaining the cellular chloride levels by mediating cellular chloride efflux and influx,17) such an uneven alteration of the protein expression of those transporters is likely to cause an increase of cellular chloride levels in nerve cells.18,45) It was reported that when the cellular chloride concentration is maintained at a higher level, the nerve impulse is more potently suppressed, probably due to the fact that cellular membrane polarization inclines to hyperpolarization.46,47) Additionally, the anesthetic effect of phenobarbital primarily comes from the fact that phenobarbital opens chloride channels incorporated in the GABAA receptors and that a consequent facilitation of chloride entry into the nerve cells increases the cellular chloride levels in the nerve cells.48) Therefore, the potentiation of the anesthetic effect of phenobarbital with DHF treatment is accounted for by the fact that the stimulated TrkB protein down-regulates the cerebral KCC2 protein expression, which results in the cellular chloride concentration being maintained a higher level, and intensifies the suppressive regulation of the membrane action potential in the nerve cells. This appears to be consistent with the reported findings that an altered function or altered cerebral expression of the KCC2 protein is a major factor to determine the susceptibility to convulsive agent.16,18,45,48)
Overall, we demonstrated that the anesthetic effect of phenobarbital potentiates in rats receiving the DHF treatment, in which a decrease in the cerebral expression of the KCC2 protein due to the stimulated TrkB protein appears to be largely involved. It was also indicated that the pharmacological efficacy of therapeutic compounds varies when the sensitivity toward these compounds is affected in their target tissues, and that evaluating the altered sensitivity of the target tissues may be necessary for providing adequate pharmacotherapy, especially in patients suffering from serious diseases.
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Sciences (19K07220).
The authors declare no conflict of interest.