2023 Volume 46 Issue 11 Pages 1517-1526
2023 Volume 46 Issue 11 Pages 1517-1526
Isoflavones and their derivatives possess neuroprotective activities against neurological disorders. Recently, the active compound SPA1413 (dehydroequol) derived from S-equol, an isoflavone-derived metabolite produced by human intestinal bacteria, was identified as a potent anti-amyloidogenic and neuroinflammatory candidate against Alzheimer’s disease. However, its detailed modes of action, associated signaling pathways, and comparison with potential isoflavone derivatives have not yet been studied. Hence, the current study aimed to identify signaling pathways associated with SPA1413 using lipopolysaccharides (LPS)-stimulated BV2 cells as the experimental model via biological assays, Western blotting, and quantitative (q)RT-PCR. The results indicate that the SPA1413 anti-neuroinflammatory effect arises due to suppression of the nitric oxide (NO), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and mitogen-activated protein kinase (MAPK) signaling networks, including those of p38 and c-Jun N-terminal kinase (JNK). Interestingly, SPA1413 inhibited IL-11 through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway. In addition, SPA1413 inhibited neuronal cell death by reducing LPS-activated microglia in neuronal N2a cells. Our findings suggest that SPA1413 may act as a strong anti-neuroinflammatory candidate by suppressing the MAPK and JAK/STAT signaling pathways.
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that weaken intellectual functions, such as memory and cognitive impairment, and frequently results in mood fluctuations, confusion, agitation, and delirium.1) Worldwide, the number of people with AD is expected to reach 87 million by 2050. The accumulation of neurofibrillary tangles (NFT) and amyloid-β (Aβ) plaque deposition in the aging brain are considered as pathological hallmarks of AD.2) Studies have shown that microglia are the primary cellular mediators of immunological inflammatory response to brain injury. Brain anatomy of patients with AD showed that both Aβ and NFT are encircled by activated microglia. The exact role of microglia in inflammatory responses in the brain remains unclear.
In clinical studies, patients with AD show an increased number of activated microglia and astrocytes, decreased neurotrophic factor production, and increased levels of pro-inflammatory cytokines.3) Depending on the environment, microglia can play a neuroprotective or neurotoxic role and are linked to several molecules and factors. These factors may enhance the Aβ disposition and ultimately increase neuroinflammation. Increasing research on connections among Aβ, microglia, and neuroinflammation highlights some potential targets. Further progression of AD can be prevented by controlling these targets with bioactive agents.
Microglia cells display a critical role in the neuronal homeostasis, but stimuli by lipopolysaccharides (LPS) as external inducer can lead cells to change toxic microglia M1 phenotype.4) The toxic microglia M1 phenotype provides a frontline defense against inflammatory diseases. The toxic microglia M1 phenotype activates an amoeboid morphology and secretes pro-inflammatory factors including interleukin-6 (IL-6), IL-1β, and tumor necrosis factor (TNF)-α, and increases production of nitric oxide (NO), cyclooxygenase 2 (COX-2), and related pro-inflammatory enzymes such as inducible nitric oxide synthase (iNOS).5,6) Moreover, these factors are interlinked via potential signaling networks such as mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and Janus kinase (JAK)/signal transducer and activator of trnascription 1 (STAT1) signaling. STAT1 may play an important role in mediating cellular transcriptional responses to inflammatory cytokines. JAKs are activated on cytokine stimulation and STAT phosphorylation. Based on these targets, we identified new bioactive agents that could be explored as natural resources for anti-neuroinflammation.
Natural products are rich sources of diverse active compounds. Previous studies have revealed the potential effects of many phytochemicals against several neurodegenerative diseases, including AD, in preclinical or clinical trials.7) In particular, soybean (Glycine max L.), which is a common ingredient for consumption in traditional Asian cuisines, is a major source of oil and protein in human and animal diets and contains pharmacologically active metabolites.8) In addition, it has been used as a natural drug to treat hormone-dependent/independent disorders.9) Soybean isoflavones are known to have neuroprotective and anti-inflammatory effects.10)
We had previously synthesized the active compound SPA1413 (dehydroequol) derived from S-equol with a potent inhibitory effect on amyloid-beta-induced 5xFAD (B6SJL) transgenic mice, as shown in Chart 1.11) However, the preventive effects of SPA1413 on neuroinflammation and the underlying mechanisms have not been evaluated in comparison with those of other identified isoflavones (i.e., daidzein, S-eqoul, and O-desmethylagolensin (O-DMA). We hypothesized that SPA1413 may have an anti-neuroinflammatory effect in activated microglial cells to restrain the pathogenesis of neurodegenerative diseases, such as AD, similar to other candidates (daidzein, O-DMA, and S-eqoul) in LPS-stimulated BV2 microglial cells. Therefore, the main aim of this study was to evaluate the potential mechanism of action of the active compound SPA1413 in the dysregulation of the JAK/STAT pathway, which is associated with neuroinflammation.

Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Hyclone (UT, U.S.A.), and fetal bovine serum (FBS) was purchased from Gibco (NY, U.S.A.). Penicillin/streptomycin was purchased from Lonza (Basel, Switzerland). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and IL-6 were bought from R&D Systems (MN, U.S.A.). Primary and secondary antibodies against iNOS, COX-2, extracellular signal regulated kinase (ERK), phospho(p)-ERK, c-Jun N-terminal kinase (JNK), p-JNK, p38, p-p38, and α-tubulin were purchased from Cell Signaling (MA, U.S.A.). The JAK inhibitor WP1066 was purchased from MedChem Express (NJ, U.S.A.). All other chemicals and reagents were purchased from Sigma-Aldrich (MO, U.S.A.). All soy isoflavone samples including SPA1413 compound were provided by Sookmyung Women's University (Seoul, Korea). All compounds and solvents were of HPLC grade.
Cell CultureBV-2 murine microglia cells (AcceGen, NJ, U.S.A., Catalog No. ABC-TC212S) and N2a murine neuroblastoma cells (ATCC, VA, U.S.A., Catalog No. ATCC-CCL-131) were used to study the effects of SPA1413 (dehydroequol). Cells were cultured in high-glucose DMEM (Hyclone) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Lonza). All cell lines were maintained at 37 °C in a humidified incubator containing 5% CO2.
Primary Microglia Cell CultureIsolated primary microglial cells provide robust data representing the in vivo environment for studying the underlying mechanisms of cellular inflammation and cell interactions of microglia in the central nervous system (CNS). The brain cortex was isolated from Institute of Cancer Research (ICR) P1 mice (OrientBio, Korea) and cultured for seven days. Subsequently, the microglia were isolated.12) Cells were cultured in high-glucose DMEM (Hyclone) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Lonza). All cell lines were maintained at 37 °C in a humidified incubator containing 5% CO2. The study protocol was approved by the Institutional Animal Care and Use Committee of the Gachon University (GIACUC-R2020022-1).
NO and Cell Viability MeasurementCell viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Catalog No. 88417; Sigma) assay.13) BV-2 cells were seeded on a 96-well plate (4 × 105 cells/mL) and then pre-treated with daidzein, O-DMA, S-equol, SPA1413 (0.5, 1, and 5 µM) or NG-methyl-L-arginine acetate salt (L-NMMA, 10 µM) for 30 min prior to treatment with 100 ng/mL LPS (Sigma, Catalog No. L2630) and incubated for one day. After incubation, the adherent cells in the plate were incubated with 0.5 mg/mL of MTT solution for 1 h, after which the MTT solution was removed. After removing the MTT solution, 200 µL of dimethyl sulfoxide (DMSO) was added to each well. The optical density (OD) was measured at 570 nm using a plate reader. For NO assay, 50 µL of conditioned medium (CM) was mixed with Griess reagent (0.1% N-1-napthylethylenediamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid). Sodium nitrite was used as a standard, and the absorbance was measured at 540 nm.
ELISA Kits AssayTreated or untreated CM from BV-2 cells was used to assess the levels of secreted mediators. BV2 cells were seeded (4 × 105 cells/mL) in 24-well plates and incubated for 24 h at 37 °C in 5% CO2. On the next day, cells were treated with daidzein, O-DMA, S-equol, or SPA1413 (5 µM) for 30 min prior to treatment with 100 ng/mL LPS and incubated for 1 d. Afterwards, CM was collected and stored at −80 °C. Competitive ELISA assay was performed thereafter to evaluate the secreted levels of IL-6 and TNF-α. The cytokine levels in the supernatants were quantified using an ELISA kit (R&D Systems, Minneapolis, MN, U.S.A.).
Western Blot AnalysisThe cells were harvested, washed with cold phosphate buffered saline (PBS), and lysed in lysis buffer (Pro-PREP, iNtRON). The lysates were centrifuged at 12000 rpm for 30 min. The supernatant was collected for protein estimation using the Bradford assay. Equal amounts of protein samples (30 µg) were subjected to sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) on 12% polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF) membranes (Sigma, Catalog No. 03010040001) using the Trans-Blot® Turbo™ Blotting System. The membrane was blocked with a 5% (v/v) skim milk solution in Tris-buffered saline containing 0.05% Tween 20 (TBST) and incubated with appropriately diluted primary antibody, namely iNOS, COX-2, ERK, p-ERK, JNK, p-JNK, p38, p-p38, or α-tubulin, overnight at 4 °C. After incubation and rinsing with TBST, the membrane was incubated with the second antibody conjugated with horseradish peroxidase (HRP) for 1 h at room temperature (r.t.). Membranes were visualized using the ChemiDoc XRS+ imaging system (Bio-Rad, Hercules, CA, U.S.A.).
Quantitative Real-Time PCR (qRT-PCR)Total RNA was extracted from cultured primary cortical cells using the TRIzol reagent (Invitrogen, CA, U.S.A.). In addition, 1 µg RNA was reverse transcribed to cDNA with the PrimeScript™ RT reagent Kit (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. The quantitative PCR (q-PCR) was accomplished with a SYBR® Premix Ex Taq (TaKaRa) on the Mx3005P qPCR system (Agilent Technology, CA. U.S.A.). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Each sample was run with the following primer pairs. IL-11: forward primer, 5′-AATTCCCAGCTGACGGAGATCACA-3′ and reverse primer, 5′-TCTACTCGAAGCCTTGTCAGCACA-3′; Janus kinase 2 (JAK2): forward primer, 5′-GCTACCAGATGGAAACTGTGCG-3′ and reverse primer, 5′-GCCTCTGTAATGTTGGTGAGATC-3′; Tyrosine kinase 2 (TYK2): forward primer, 5′-GCTTTCCTGCATGGTGTTTGCG-3′ and reverse primer, 5′-TGTCGCCGTAACCACCACATCCA-3′; Signal transducer and activator of transcription 1 (STAT1): forward primer, 5′-GCCTCTCATTGTCACCGAAGAAC-3′ and reverse primer, 5′-TGGCTGACGTTGGAGATCACCA-3′; STAT3: forward primer, 5′-AGGAGTCTAACAACGGCAGCCT-3′ and reverse primer, 5′-GTGGTACACCTCAGTCTCGAAG-3′; GAPDH: forward primer, 5′-TGCACCACCAACTGCTTAG-3′ and reverse primer, 5′-GGATGCAGAGAAGATGTTC-3′.
Microarray AnalysisTotal RNA was extracted from cultured primary microglial cells using TRIzol reagent (Invitrogen). Microarray analysis using the total RNA was performed at the Macrogen Company (Seoul, Korea). Microarray analysis was performed using the Agilent Mouse Genome (Agilent Technology) according to the manufacturer’s protocol.
Neurite Outgrowth AssayFor measuring the neurite outgrowth, we used BV2 conditioned medium treatment with N2a cells. BV2 cells were seeded in a 24-well plate at a density of 4 × 104 cells/well and treated with SPA1413, followed by induction with LPS (100 ng/mL). After that the conditioned medium of treated cells were transferred to N2a cells seed in a 24-well plate at a density of 1 × 104 cells/well. The treated SPA1413 in N2a cells were incubated during 24 h. N2a cell neurite lengths were measured and captured by using IncuCyte imaging system (Essen Instruments, Ann Arbor, MI, U.S.A.).14)
Statistical AnalysisResults of the statistical analyses are given as mean ± standard error of the mean (S.E.M.) and analyzed GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, U.S.A.). All results were characterized by a one-way ANOVA for multiple comparison or Student’s t-test. Neurite outgrowth data was statistically analyzed by a two-way ANOVA for multiple comparisons. p < 0.05 was considered statistically significant.
Daidzein, O-DMA, S-equol, and SPA1413 were screened for their anti-neuroinflammatory effects on NO production in LPS-stimulated BV-2 cells, as shown in Fig. 1. Among these metabolites, S-equol and SPA1413 significantly reduced NO production in a concentration-dependent manner (Fig. 1B). Interestingly, SPA1413, without any cytotoxicity, suppressed NO levels (IC50 = 5.20 µM) stronger than the positive control L-NMMA (IC50 = 15.12 µM) (Fig. 1A, Supplementary Table 1S). We also investigated the levels of pro-inflammatory cytokines IL-6 and TNF-α in CM treated with daidzein, O-DMA, S-equol, or SPA1413 at 5 µM concentration (Figs. 1C, D). The LPS-treated cells had significantly increased levels of IL-6 (p < 0.0001, ###) and TNF-α (p < 0.0001, ###) than those in the control group. Among other metabolites, SPA1413 significantly decreased the levels of IL-6 and TNF-α by 16.65 ± 1.27% (p < 0.0001, ***) and 61.65 ± 3.30% (p < 0.0001, ***), respectively. These findings suggest that SPA1413 ($$$p < 0.001) may be a potent anti-neuronal inflammatory substance among other metabolites.

(A) BV-2 cells were pre-treated with isoflavone compounds (0.5, 1, and 5 µM) for 30 min and then induced with LPS (100 ng/mL) for 24 h. Cytoprotective effects of isoflavone compounds in LPS-stimulated BV-2 cells were measured by MTT assay, (B) NO production was determined by measuring the level of NO in the conditioned medium via Griess assay, and (C) IL-6 and (D) TNF-α production in the conditioned medium of treated cells were assessed using an ELISA kit assay. Cell viability is expressed as a percentage of that of the control (ctrl) group (set as 100%). NO, IL-6, and TNF-α production are expressed as percentages of those of the LPS group (set as 100%). We took 10 µM L-NMMA as a positive control (PC). Results are the means of three independent experiments and the data are expressed as the mean ± S.E.M. ###p < 0.001 vs. control group, * p < 0.05 and *** p < 0.001 vs. LPS group, and $$p < 0.01 and $$$p < 0.001 vs. SPA1413 group by one-way ANOVA with Tukey’s post-hoc correction for multiple comparison or Student’s t-test.
Neuroinflammation is an important factor in AD, in which overactivated microglia accelerates the pathogenesis.11) Hence, we evaluated the anti-neuroinflammatory effects of daidzein, O-DMA, S-equol, and SPA1413 in LPS-stimulated primary microglia isolated from newborn pup mouse brains, which implicated the up-regulated inflammatory genes than those of BV2 cells15) (Fig. 2). In primary microglia cells, the treatment of SPA1413 mitigated LPS-induced cytotoxicity (cell viability, 92.25 ± 3.30%; NO production, 45.97 ± 2.41% [p = 0.0010, **]) by reducing NO production in comparison with that in the LPS group (cell viability, 67.61 ± 7.08%; NO production, 100 ± 2.89% [p = 0.0114, **]). These results showed that SPA1413 not only increased the cell survival but also suppressed the NO production as an anti-inflammatory agent. We can suggest that microglial activation attenuation by SPA1413 shall be examined by measuring protein levels of the related inflammatory agents.

(A) Primary microglia cells were pre-treated with isoflavone compounds (5 µM) for 30 min and then induced with LPS (100 ng/mL) for 24 h. Cytoprotective effect of isoflavone compounds in LPS-stimulated primary microglia cells were measured by MTT assay, (B) NO production was determined by measuring the level of NO in the conditioned medium via Griess assay. Cell viability is expressed as a percentage of that of the control (ctrl) group (set as 100%), and NO production is expressed as a percentage of that in the LPS group (set as 100%). We took 10 µM L-NMMA as a positive control (PC). Results are the means of three independent experiments and the data are expressed as the mean ± S.E.M. #p < 0.05 and ##p < 0.01 vs. control group, ** p < 0.01 vs. LPS group, and $p < 0.05 and $$p < 0.01 vs. SPA1413 group by one-way ANOVA with Tukey’s post-hoc correction for multiple comparison or Student’s t-test.
As the production of pro-inflammatory cytokines in microglia is regulated primarily by COX-2 and iNOS, Western blot analysis was performed to compare the effects of S-equol and SPA1413 on the expression of COX-2 and iNOS in BV-2 cells (Figs. 3A, B). SPA1413 at 5 µM concentration drastically suppressed the levels of COX-2 (p = 0.0049, **) and iNOS (p = 0.0062, **) in LPS-activated BV2 cells compared with those in the LPS group. These results indicate that SPA1413 ($$p < 0.01) inhibits NO production in LPS-activated BV2 cells by suppressing COX-2 and iNOS expression.16) Furthermore, we investigated the mechanism underlying the anti-inflammatory properties of SPA1413 by modulating MAPK pathways. BV-2 cells were treated with 5 µM concentrations of SPA1413 followed by LPS stimulation. All compounds significantly increased levels of phosphorylation of ERK (Fig. 3C) in a metabolized conformation-dependent manner. On the contrary, SPA1413 at 5 µM concentration significantly reduced the expression of phosphorylated JNK (p = 0.0063, **) and p38 (p = 0.0327, **) to 45 and 60%, respectively, of the corresponding OD values of the LPS-treated group (Figs. 3D, E). In addition, the inhibition of MAPK pathways, including those of JNK and p38 signaling, by SPA1413 showed higher potency than that by S-equol. Thus, the results of in vitro experiments indicate that SPA1413 regulates MAPK signaling to exert anti-inflammatory effects (Figs. 1–3).

(A, B) BV-2 cells were pre-treated with isoflavone compounds (5 µM) for 30 min and then induced with LPS (100 ng/mL) for 6 h. Protein expression and their densitometric analysis for COX-2 (A) and iNOS (B); α-Tubulin was used as loading control. (C–E) BV-2 cells were pre-treated with isoflavone compounds (5 µM) for 30 min and then induced with LPS (100 ng/mL) for 30 min. Protein expression and quantitative analysis for p-ERK/ERK (C), p-JNK/JNK (D), and p-p38/p38 (E). Results are the means of three independent experiments and the data are expressed as the mean ± S.E.M. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. control group, * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. LPS group, and $$p < 0.01 vs. SPA1413 group by one-way ANOVA with Tukey’s post-hoc correction for multiple comparison or Student’s t-test.
We evaluated gene expression using microarray analysis to identify specific inflammatory genes involved in S-equol and SPA141 activity. Microarray experiments to identify differentially expressed genes with significant differences in expression levels revealed that 739 genes were upregulated and 1545 genes were downregulated in the SPA1413-treated group compared with those in the LPS group. The expression levels of several genes, including five upregulated and five downregulated genes, are shown in Supplementary Table 2S. To validate the results of the microarray analysis, qRT-PCR was performed to measure specific gene expression in the designed model.
Among the candidate genes, the mRNA level of IL-11 was markedly high in LPS-stimulated BV2 cells, indicating that the function of the altered IL-11 gene (p = 0.0037) is primarily related to the maintenance of inflammatory activity in overactivated microglial cells. We further validated the mRNA levels of related major signaling proteins (i.e., JAK2, TYK2, STAT1, and STAT3) after activation LPS which affect to increase the mRNA of IL-11. As shown in Figs. 4B–E, the treatment of LPS significantly activated the JAK kinases including JAK2 (p = 0.0064, ##) and TYK2 (p = 0.0103, #) and increased the levels of STAT1 (p = 0.0031, ##) and STAT3 (p = 0.0028, ##) activated by JAK kinases. The results show that LPS strongly regulates the JAK/STAT pathway, which plays an important role in the pathogenesis of inflammatory diseases.

(A–E) BV-2 microglia cells were pre-treated with S-equol and SPA1413 at 1 and 5 µM concentrations for 30 min and then induced with LPS (100 ng/mL) for 24 h. IL-11, JAK2, TYK2, STAT1, and STAT3 levels were determined by q-PCR and were normalized with GAPDH mRNA. Results are the means of three independent experiments and the data are expressed as the mean ± S.E.M. #p < 0.05 and ##p < 0.01 vs. control group, * p < 0.05 and ** p < 0.01 vs. LPS group by one-way ANOVA with Tukey’s post-hoc correction for multiple comparison or Student’s t-test.
We evaluated the effect of S-equol and SPA1413 on the LPS-induced IL-11 and JAK/STAT pathways in the LPS group. At 5 µM concentration, the compounds significantly decreased mRNA levels of IL-11 and suppressed the JAK/STAT pathway (Fig. 4). SPA1413 completely inhibited LPS-induced IL-11 activation and the JAK/STAT pathway in a dose-dependent manner compared with S-equol. In particular, we further confirmed that SPA1413 at 5 µM concentration significantly decreased the mRNA levels of JAK2 (p = 0.0002, ***) and STAT3 (p = 0.0232, *) in the presence of WP1066 as a JAK/STAT inhibitor compared with those in the absence of WP1066 (Fig. 5). Our results suggest that SPA1413 plays a protective role in LPS-treated BV2 cells by regulating the JAK/STAT pathway.
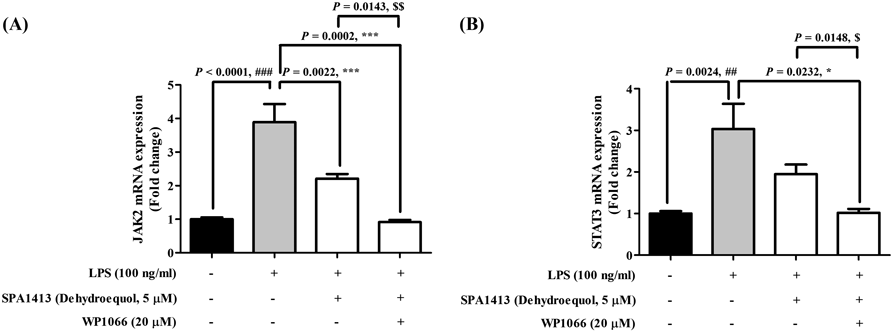
(A, B) BV-2 microglia cells were pre-treated with SPA1413 (5 µM) and/or WP1066 (20 µM, JAK inhibitor) for 30 min and then induced with LPS (100 ng/mL) for 24 h. JAK2 and STAT3 levels were determined by q-PCR and were normalized with GAPDH mRNA. Results are the means of three independent experiments and the data are expressed as the mean ± S.E.M. ##p < 0.01 and ###p < 0.001 vs. control group, * p < 0.05 and *** p < 0.001 vs. LPS group, and $p < 0.05 and $$p < 0.01 vs. SPA1413 group by one-way ANOVA with Tukey’s post-hoc correction for multiple comparison or Student’s t-test.
It was well-known that microglia, neuron, and astrocytes lives together to basic function of brain. To understand the role of activated microglia to neurons, BV2 cells were activated with LPS for 24 h followed by treating SPA1413 and the treated conditioned medium was transferred to neuron cells. This assay could be proof that activated microglia via inflammatory factors in medium is affect to neuronal death. For investigating the effect of SPA1413 on N2a cell lines by suppressing the activation of microglia BV2 cells were treated with SPA1413 with/without LPS (100 ng/mL). As shown in Fig. 6., the LPS treated group (p = 0.0468, #) decreased neurite outgrowth and length in compassion that of control group, but SPA1413 (p = 0.0475, *) showed increased neurite outgrowth and length in concentration-manner against LPS-induced microglia mediated toxicity to N2a cells. In the end points, the neurite length (7.39 ± 0.74–8.58 ± 0.49 mm/mm2, *) by treating SPA1413 was clearly recovered in dose-dependent manner, comparing with LPS-induced group (4.34 ± 0.12 mm/mm2, ##) (Fig. 6C). Moreover, the image of neurite outgrowth by treating SPA1413 clearly showed that inhibited the neuronal cell death by reducing microglial activation and inflammatory cytokines.

(A) N2a cell morphology with (or without) SPA1413 treatment. Neurite length was measured at regular intervals over a time span of 24 h, and an image of N2a cells was taken at the end point (scale bar = 50 µm). Purple color lines represent the neurite outgrowth. (B) Quantification of neurite length at various time points. (C) Quantification of neurite length at the endpoint. Results are the means of three independent experiments and the data are expressed as the mean ± S.E.M. #p < 0.05 and ##p < 0.01 vs. control group, * p < 0.05 vs. LPS group by two-way ANOVA with Tukey’s post-hoc correction for multiple comparison or Student’s t-test.
Natural products are rich sources of pharmacologically active compounds and are consumed on a daily basis because of their potential health benefits. Soybeans, an important source of phytonutrients, oil, protein, and food, are considered a powerhouse owing to their health benefits, particularly for people with diabetes, cancer, or neurodegenerative diseases. They contain various phytonutrients, such as phytosterols, saponins, isoflavones, flavonoids, and phenolic acids. Isoflavones are effective in relieving menopausal symptoms and preventing osteoporosis. Soy isoflavones show estrogen-like activity that affects brain functions such as neuronal growth and survival via ER-mediated pathways.17)
Genistein and daidzein are well-known to have practical anti-inflammatory effects, and soy-derived natural isoflavones are relatively small molecules that can cross the blood–brain barrier (BBB).18,19) Soy isoflavones, as potential anti-inflammatory agents, are being researched for the treatment of AD, as they mediate inflammatory responses.10) Recently, successfully synthesized the active compound SPA1413 (dehydroequol) derived from S-equol, which has a potent inhibitory effect on amyloid-beta-induced 5xFAD (B6SJL) transgenic mice, as shown in Chart 1.11)
However, further studies are needed to evaluate the preventive effect of SPA1413 on neuroinflammation and its underlying mechanisms compared to that of other identified isoflavones (i.e., daidzein, S-equol, and O-DMA). Our hypothesis was that SPA1413 may serve as a potential anti-neuroinflammatory agent in activated microglial cells to restrain the pathogenesis of neurodegenerative diseases such as AD. Therefore, the main aim of this study was to evaluate the anti-neuroinflammatory mechanism of SPA1413 in an AD model to identify a therapeutic strategy for neuroinflammation.
In the CNS, the main neuroinflammatory element is microglial activation, which provides a frontline defense against infection, inflammation, disease, or injury. During inflammation, astrocytes and activated microglia produce large amounts of NO in the inflamed brain. Moreover, microglial activation is a direct responder to pro-inflammatory cytokines such as TNF-α and IL-6 expression as these factors play an important role in brain degenerative and inflammatory processes. Targeting NO, TNF-α, and IL-6 that are induced by microglial over activation in inflamed brain can be a therapeutic strategy to treat neuroinflammation. Our experiments revealed that SPA1413 follows this pathway to mediate neuroinflammation by inhibiting microglial over activation associated with NO, TNF-α, and IL-6 production. By elucidating the detailed mode of action of SPA1413 in neuroinflammation, additional interesting factors and targets were identified. NO, TNF-α, and IL-6 that are subsequently released have been considered as important mediators of the inflammation process.
These pro-inflammatory cytokines are further characterized as responders that activate COX-2 and iNOS transcription. We showed that SPA1413 remarkably decreased iNOS and COX-2 expression, together with TNF-α and IL-6 secretion, in LPS-activated microglia in a concentration-dependent manner (Figs. 1–3), which supports the anti-neuroinflammatory role of SPA1413. The production of COX-2 and iNOS is mediated by several cell signaling networks, including the MAPK/ERK network. During microglial activated neuroinflammation in AD, MAPK and ERK are the focal regulators of inflammation, pro-survival signaling, and gene expression responsible for the trend towards increased p38 MAPK and JNK activation. Our experimental results showed that SPA1413 decreased the phosphorylation of p38 and JNK and increased the phosphorylation of ERK (Fig. 3). This result is in accordance with those of, who reported that MAPKs not only respond to LPS-mediated microglial activation but also promote neurogenesis factors (i.e., cell proliferation, differentiation, and survival) by short-term ERK activation.9) Among the compounds tested, SPA1413 showed the most potent inhibitory effect on neuroinflammation, implying that it can be used as an alternative therapeutic agent for CNS disorders.
In addition, we performed gene expression profiling of LPS-stimulated BV2 cells treated with (or without) SPA1413 using microarrays. The genes showing a difference in expression between that of the top five and bottom five have been newly identified in this study by microarray analysis. These genes have been speculated to play a role in degenerative brain disease, according to the findings from previous studies (Supplementary Table 1S, Supplementary Fig. 1S). In the SPA1413 group, Ttr, IL-11, Ang2, Ucp2, and Prdx6 were among the top five upregulated genes among the differentially expressed genes identified in this study. Conversely, they were included in the bottom five downregulated genes: Wnt6, CD74, Hpx, Sostdc1, and Chil1. These changes in gene expression suggest that SPA1413 may affect neuroinflammation, and the gene expression patterns between the SPA1413 and LPS groups showed slight differences. It also implied that SPA1413 have shown the multi-target mechanisms involved in neurodegenerative diseases including the neuroinflammation.
In the microarray and qRT-PCR results, SPA1413 treatment showed an increased mRNA levels of IL-11 signaling compared with the LPS treated group on BV2 cells (Figs. 4, 5, Supplementary Table 1S), but other gene is not shown correlation from qRT-PCT results (data not shown). A few reports suggested that IL-11 is a member of the cytokine family with well-established immunomodulatory and neuroprotective effects and may be considered a potential therapeutic target.20) However, several reports have implicated that IL-11 was related in the pathology of multiple inflammatory-related diseases, such as multiple sclerosis in the CNS.21) But, it is still unclear if IL-11 function is beneficial or not in inflammatory progress, and systematic explorations in vitro model is still necessary.
In this work, we found that SPA1413 dramatically suppressed the mRNA levels of IL-11 in LPS-stimulated BV2 cells by inhibiting mRNA expression of JAK2, TYK2, STAT1, and STAT3 (Fig. 4). Moreover, WP1066, a specific inhibitor of JAK/STAT, markedly reduced the mRNA levels of JAK2 and STAT3 upon co-treatment with SPA1413, validating that SPA1413 plays an important role in JAK/STAT signaling against neuroinflammation (Fig. 5). It indicated that IL-11 signaling has affected to inflammatory progression on LPS-stimulated microglia cells, and SPA1413 strongly reduced the IL-11 signaling via JAK/STAT pathway.
During the classical IL-11 signaling, interaction with the receptor complex activates JAKs and TYK2 in the cytoplasmic domain of GP130. This leads to the phosphorylation of cytoplasmic GP130 and activates JAK2 and STAT3 signaling.22) Activated JAK/STAT are translocated to the nucleus and upregulate genes related to inflammatory processes.21) Dysregulation of cytokines such as IL-11 may be a key feature in the induction of neuroinflammation and neurodegeneration. Moreover, the deregulation of STAT3-mediated IL-11-induced neuroinflammation is frequently associated with neurodegeneration. Our data suggest that the downregulation of JAK2/STAT3 by SPA1413 may suppress the detrimental effects of brain inflammation, thereby inhibiting astrogliogenesis and promoting neurogenesis. Further research should focus on precise mechanistic studies on the role of SPA1413 in brain inflammation-induced neuronal cell differentiation by targeting the JAK2/STAT3 pathway. Furthermore, we found that SPA1413 strongly had neuroprotective effect via regulating the activated microglia-induced neurotoxicity (Fig. 6). Thus, SPA1413 may be a potential candidate for treating neurodegenerative disorders, including AD.
In this study, we provide evidence for the anti-neuroinflammatory effects of the active compound SPA1413, derived from intestinal metabolites (specifically, S-equol). The experiment showed that SPA1413 not only reduces inflammatory activity but also diminishes the released levels of pro-inflammatory cytokines/mediators. As an advantage, SPA1413 can suppress the NO, IL-6, TNF-α, COX-2, iNOS, and MAPK signaling networks, including those of p38 and JNK. In particular, SPA1413 inhibits IL-11 activation through the JAK/STAT signaling pathway (Fig. 7). These results prove the potential benefits of SPA1413 and provide a new addition to the anti-neuroinflammatory candidate library. Further research needs to be conducted on SPA1413 and its anti-neuroinflammatory role using animal experimental models, focusing on LPS-induced neuroinflammation.

This research was supported by a Grant from the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-20221I1A4068917 and NRF-2023R1A2C2003366).
Da Hye Yoon: Conceptualization, Formal analysis, data curation, Methodology, Writing—Original Draft. Seong-Min Hong: Conceptualization, Formal analysis, data curation, methodology, writing, reviewing, and editing. Eun Ji Ko: Formal analysis, data curation, and methodology. Ra Ok Jeon: Conceptualization, Methodology. Sun Yeon Kim: Conceptualization, Supervision, Funding acquisition.
The authors declare no conflict of interest.
This article contains supplementary materials.