Abstract
Biologic medications have dramatically improved the treatment outcomes of immunological inflammatory diseases, but their immunosuppressive effects put patients at risk for tuberculosis (TB). We investigated the risk factors for developing TB in patients treated for latent tuberculosis infection (LTBI) who also had experience of using biologic medications. At Keio University Hospital, we retrospectively investigated patients treated with anti-mycobacterial drugs before or concurrently with biologic medications from January 2012 to August 2020. Patients in the ‘follow-on cases group’ who had a positive TB screening test after initiating biologic medications and subsequently started LTBI treatment were excluded. We researched and compared the patient characteristics for TB and non-TB patient groups. Of the 146 eligible patients, 5 (3.4%) developed TB. The incidence rate was 600/100000 person-years. There were no significant differences between TB and non-TB patient groups in the history of TB, interferon-gamma release assay (IGRA), duration of biologic medication therapy, LTBI treatment periods, concomitant use of calcineurin inhibitors or anti-rheumatic drugs. The percentage of patients who received prednisolone at a dose of ≥15 mg for more than 1 month was higher in those who developed TB than in those who did not (40.0 vs. 7.1%, p = 0.054); however, this difference was not statistically significant. Regular monitoring of TB is necessary for long-term concomitant use of high prednisolone doses during and after the administration of biologic medications.
INTRODUCTION
Latent tuberculosis infection (LTBI) is defined as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically active tuberculosis (TB) manifestation.1) Risk factors contributing to the development of active TB include human immunodeficiency virus (HIV) infection, diabetes mellitus, renal failure, history of TB, corticosteroids, and biologic medications,2–7) etc. Several TB outbreaks have recently been reported as a result of biologic medications use.8–11) The establishment of screening methods and prophylactic administration of antimycobacterial drugs have led to a reduction of TB development.12) Consequently, anti-mycobacterial prophylactic treatment (LTBI treatment) was recommended for patients receiving biologic medications who were potentially at high risk of TB reactivation. Several studies found that the incidence of TB after LTBI treatment ranged from 0.1 to 0.64%,13–16) indicating that LTBI treatment reduced the incidence of TB when biologic medications were used. LTBI treatment, on the other hand, has no effect on the incidence of TB when using biologic medications17) and only partially or completely prevents TB reactivation. The incidence of TB after LTBI treatment has been reported to be 22.2 or 2.9%.18,19)
There have been no reports on factors associated with TB incidence in biologic medication-treated patients receiving LTBI prophylactic treatment. As a result, this study investigated biologic medication-treated patients who had a history of antimycobacterial treatment, and retrospectively examined risk factors for the development of TB in LTBI patients.
PATIENTS AND METHODS
EthicsThis study was approved by the Keio University School of Medicine Ethics Committee (Approval No. 20140032).
PatientsThe nested case-control study was performed on patients who entered Keio University Hospital. Patients treated with at least one antimycobacterial agent and one biologic medication each from January 1, 2012, to August 31, 2020, at Keio University Hospital were surveyed. Detailed methods are provided as supplementary materials.
Data ExtractionPatients’ data were extracted from the medical records. Detailed methods are provided as supplementary material.
Statistical AnalysisIBM® SPSS® Statistics version 26 was used to perform the Mann–Whitney U-test and Fisher's exact test. A p-value of less than 0.05 was considered a statistically significant difference. Detailed methods are provided as supplementary material.
RESULTS
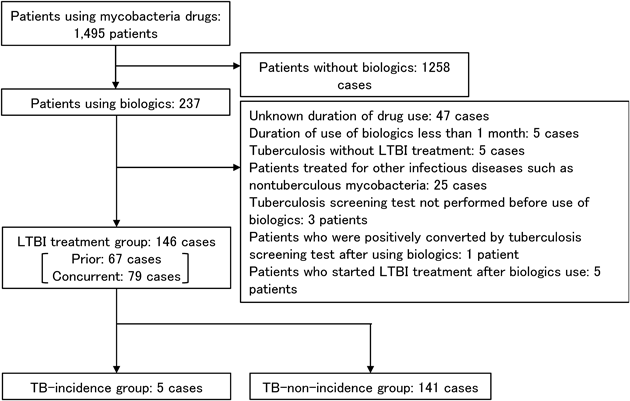
Search ResultsTwo hundred and thirty seven (237) patients treated with one or more biologic medications in combination with antimycobacterial agents were extracted from the 1495 patients treated with antimycobacterial agents (Fig. 1). A total of 91 patients were excluded from the study, while 146 patients were included. The 146 patients were divided into 67 “prior case” patients and 79 “concurrent case” patients, in whom the risk of developing TB was determined prior to the start of the biological agent, and LTBI treatment was initiated as a precaution.
More than half of the 146 patients treated with LTBI, 77 (52.7%), were given tumor necrosis factor (TNF)-alpha inhibitors, 44 (30.1%) were given interleukin (IL)-6 inhibitors, and 23 (15.8%) were given T-cell selective co-stimulation modulators (Table 1).
Table 1. Biologic Medications Used by LTBI Patients
| N | TNF-alpha inhibitors | IL-6 inhibitors | T cell selective co-stimulation modulators | Other | Sum |
|---|
| Prior cases | 38 | 18 | 9 | 2 | 67 |
| Concurrent cases*1 | 37 | 26 | 14 | 2 | 79 |
| Sum | 75 | 44 | 23 | 4 | 146 |
*1Co-administration was performed when LTBI treatment was initiated within 5 d before and after the start date of the biologic.
After starting LTBI treatment, 5 of the 146 patients developed active TB, for an incidence rate of 600.18 cases per 100000 person-years. In the prophylactic arm, isoniazid (INH) monotherapy was used to treat LTBI in 145 patients (99.3%) (Table 2). INH monotherapy was used to treat all 5 patients who developed active TB (Table 2). The percentages of interferon-gamma release assay (IGRA)-positive or indeterminate in patients who developed active TB and those who did not develop TB, were 100% (5 patients) and 60.3% (85 patients), respectively (Table 2).
Table 2. Patient Characteristics
| All patients (n = 146) | Prophylactic group (n = 146) | p-Value |
|---|
| TB-incidence group (n = 5) | TB-non-incidence group (n = 141) |
|---|
| Sex, male § n (%) | 39 (26.7) | 1 (20) | 38 (27) | 1.000 |
| Median age † (IQR) | 64.5 (53–74) | 61.0 (55.0–74.0) | 65.0 (53.0–74.0) | 0.755 |
| Median height † (cm), (IQR) | 158.0 (150.5–165.0) | 155.0 (150.0–163.5) | 158.0 (151.0–165.0) | 0.488 |
| Median weight † (kg), (IQR) | 56.6 (50.0–63.0) | 55.1 (54.1–58.0) | 56.6 (50.0–63.0) | 0.790 |
| Median BMI†, (IQR) | 22.2 (20.3–24.5) | 22.2 (21.3–25.5) | 22.2 (20.3–24.4) | 0.492 |
| IGRA§ (positive or indeterminate) | 90 (61.6) | 5 (100) | 85 (60.3) | 0.157 |
| Rheumatoid arthritis § | 108 (74.0) | 3 (60.0) | 105 (74.5) | 0.605 |
| Inflammatory bowel disease § | 17 (11.6) | 1 (20.0) | 16 (11.3) | 0.466 |
| Complications § (respiratory diseases) | 41 (28.1) | 1 (20.0) | 40 (28.4) | 1.000 |
| Median observation periods † (months) (IQR) | 76 (41.25–96.75) | 14 (9–23) | 77 (43–97) | 0.024 |
| Number of types of biologics used † | 1 (1–2) | 1 (1–1) | 1 (1–2) | 0.546 |
| Median duration of biologics use † (months) | 61.5 (24.25-86.0) | 21.0 (12.0–60.0) | 64 (27.0–89.0) | 0.110 |
| LTBI treatment period † (months) | 10 (8.0–19.0) | 9.0 (6.0–10.0) | 10.0 (8.0–19.0) | 0.209 |
| Timing of LTBI treatment initiation (prior/concurrent) | 67/79 | 3/2 | 64/77 | — |
| LTBI treatment regimen | — | — | — | — |
| INH treatment | 145 | 5 | 140 | — |
| RFP treatment | 1 | 0 | 1 | — |
BMI body mass index, IGRA interferon gamma release assay, INH isoniazid, RFP rifampicin. Mann–Whitney’s U-test (†) and Fisher's exact test (§) were used for statistical analysis. Results were expressed as median (1st–3rd quartiles) or % (n).
In the TB-incidence group (Fig. 2a), the total treatment duration of biologic medications varied greatly, with the shortest time being 4 months and the longest time being 72 months.
In the TB-incidence group, the shortest treatment period was 3 months, and the longest treatment period was 12 months (Fig. 2b). In contrast, the shortest and longest treatment periods in the TB-free group were 1 and 126 months, respectively (Fig. 2b).
Evaluation of Risk Factors for TBThe factors associated with the development of TB in patients who developed active TB (5 patients) and those who did not develop TB (141 patients) were investigated (Table 3). With a p-value of 0.054, patients who received prednisolone (PSL) ≥15 mg for at least 1 month developed TB at a rate of 7.1% in the TB-non-incidence group compared to 40% in the TB-incidence group, indicating a higher tendency in the TB-incidence group (Table 3).
Table 3. Risk Factors Contributing to the Development of Tuberculosis
| Prophylactic group (n = 146) | TB-incidence group (n = 5) | TB-non-incidence group (n = 141) | p-Value |
|---|
| Smoking history § | 2 (40.0) | 51 (36.2) | 1.000 |
| History of tuberculosis § | 1 (20.0) | 35 (24.8) | 1.000 |
| HIV/AIDS § | 0 (0) | 0 (0) | — |
| Diabetes § | 0 (0) | 16 (11.3) | 1.000 |
| Renal function † (eGFR [mL/min/1.73 m2]) | 64.0 (59.0–98.0) | 76.0 (61.8–91.0) | 0.965 |
| Surgical history § Organ transplantation, hemodialysis, gastrectomy | 0 (0) | 0 (0) | — |
| PSL ≥ 15 mg Dosing for more than one month §, * | 2 (40.0) | 10 (7.1) | 0.054 |
| CNI §, ※ | 0 (0) | 20 (14.2) | 1.000 |
| MTX §, ※ | 3 (60.0) | 79 (56.0) | 1.000 |
| SASP, 5-ASA §, ※ | 1 (20.0) | 30 (21.3) | 1.000 |
| AZA, 6-MP §, ※ | 1 (20.0) | 11 (7.8) | 0.353 |
| BUC, MZB, IGU, LEF §, ※ | 0 (0) | 20 (14.2) | 1.000 |
HIV human immunodeficiency virus, AIDS acquired immune deficiency syndrome, eGFR estimated glomerular filtration rate, PSL predonisolone, CNI calcineurin inhibitor, MTX methotrexate, SASP salicylazosulfapyridine, 5-ASA 5-aminosalicylic acid, AZA azathioprine, 6-MP mercaptopurine, BUC bucillamine, MZB mizoribine, IGU iguratimod, LEF leflunomide. Mann–Whitney’s U-test (†) and Fisher’s exact test (§) were used for statistical analysis. Results were expressed as median (1st–3rd quartiles) or % (n). * Drugs used in combination during the biologics administration period were tabulated.
Four of the five patients who developed TB after starting LTBI treatment were female, with one being male (Table 4). Despite the fact that only one patient had a history of TB, all patients tested positive for IGRA on the TB screening test. The biologic medications TNF-alpha inhibitors infliximab (IFX) and adalimumab (ADA) were used, as well as IL-6 inhibitor tocilizumab (TCZ) and T-cell selective co-stimulation modulator abatacept (ABT). The biologic medications used for primary disease treatment were free of bias. Methotrexate (MTX), PSL ≥15 mg, and mercaptopurine (6-MP) were used as concomitant immunosuppressive agents. Four patients had been on LTBI treatment for more than 6 months, and one patient had developed TB during LTBI treatment and was switched to a 4-drug combination treatment after only 3 months. Two of the three cases with susceptibility testing results were resistant to INH.
Table 4. Details of Tuberculosis after LTBI Treatment
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|
| Sex | Woman | Woman | Woman | Man | Woman |
|---|
| Age | 30 | 61 | 55 | 74 | 76 |
|---|
| Smoking history | Never | Never | Quit
(14.4 pack-years) | Quit
(43 pack-years) | Never |
| History of tuberculosis | None | None | None | None | Yes (high school student, no treatment) |
| Screening | QFT positive | T-SPOT positive | T-SPOT positive | QFT positive / nodule shadow | QFT positive |
| Primary disease | Crohn’s disease | Incomplete Behcet’s disease | Rheumatoid arthritis Takayasu arteritis | Rheumatoid arthritis | Rheumatoid arthritis |
| Other comorbidities | None | None | Nephrotic syndrome | None | None |
| Biological medications·Period of use | ADA
72 months | IFX
12 months | TCZ
3 months | TCZ
52 months | ABT
21 months |
| Concomitant drugs | 6-MP
PSL ≥ 15 mg | Without | MTX
PSL ≥ 15 mg | MTX | MTX |
| Timing of TB development (month after initial biologic use) | 23 months | 14 months | 9 months | 2 moths | 99 months
(8.25 years) |
| LTBI Treatment period*1 | −4wk–35wk
(Total: 9 months) | −33wk–22wk
(Total: 12 months) | −89wk–−41wk
(Total: 10 months) | 0wk–12wk*2
(Total: 3 months) | 0wk–29wk
(Total: 6 months) |
| Mycobacterium tuberculosis susceptibility | No exam | INH susceptible | INH resistant | No exam | INH resistant |
ADA adalimumab, IFX infliximab, TCZ tocilizumab, ABT abatacept, 6-MP mercaptopurine, MTX methotrexate, PSL predonisolone, INH isoniazid. *1 The start date of the biologic was set to “0.” *2 Tuberculosis developed during LTBI treatment and changed to four-drug combination treatment.
DISCUSSION
After starting LTBI treatment, 5 out of 146 patients (3.4%) developed TB, for a TB incidence rate of 600/100000 person-years. This incidence rate is higher than in other studies,13–16) but about the same as in the study in the United States.19) This study’s higher incidence of TB after initiating LTBI treatment could attributed to the different TB screening tests and LTBI treatment regimens used. The tuberculin test was used for screening in the Turkish and Spanish studies.13,14) The U.S. study, on the other hand, and this study both used IGRA.19) Because IGRA has a higher specificity than the tuberculin skin test, its use may have resulted in a population at increased risk of TB. Taking into account the potential impact of treatment regimen differences, trials reporting low rates of incidence after LTBI treatment used 9-month INH monotherapy, 4-month rifampicin (RFP) monotherapy, or 3-month INH in combination with RFP.13–16) Trials that reported high rates of TB, on the other hand, included patients treated with INH monotherapy for 6 months.18,19) Similarly, the current study included cases that had been treated with INH for less than 9 months, and none of them had received two-drug combination therapy. As a result, variations in the LTBI treatment regimen used may have influenced the results.
We examined the risk factors for TB development after initiating LTBI treatment that were identified in the guidelines and articles (Table 3). According to the report, PSL at 15 mg or higher per day for 2 to 4 weeks increases the risk of developing TB.20) We investigated PSL as a concomitant immunosuppressive drug, focusing on the dose and duration of use. Concomitant use of PSL at a daily dose of 15 mg or more for a month or longer tended to be higher in the TB-incidence group compared to the TB-non-incidence group when each factor was examined. However, the difference was not statistically significant (p = 0.054).
According to Jick et al., the odds ratio for TB development is 7.7 when the daily dose of PSL alone is 15 mg or higher.21) Also, according to Brassard et al., despite the lack of a detailed dosage description, the use of PSL was significantly associated with the occurrence of TB.22) In addition, when biologic medications and PSL are combined, 10 or 5 mg or more of PSL per day is reported to be a significant risk factor.5,7) Since there have been no reports examining risk factors after initiation of LTBI treatment, the findings of this study may be significant, indicating that concomitant PSL of 15 mg or more per day for more than 1 month may be a risk factor for developing TB even after LTBI treatment initiation.
The biologic medications TNF inhibitors, IL-6 inhibitors, and T cell-selective co-stimulation modulators were used in patients who developed TB (Table 4). For each biologic medication, we analyzed the risk of TB development. As a result, no significant risk factors for any of the biologic mediations were identified (Supplementary Table S1). According to Tubach et al., the standardized incidence ratio for TB varies greatly by drug: 18.6 for IFX, 29.3 for ADA, and 1.8 for ETN.7) On the other hand, TCZ and ABT are thought to have a low risk of developing TB.23) The incidence of TB in post-marketing surveillance of all rheumatoid arthritis cases was 0.28% for IFX, 0.05% for TCZ, and 0.03% for ABT.24) TNF inhibitors have been linked to a higher risk of developing TB than other biologic medications.23) The current study found no bias toward any specific biologic medications in TB development following LTBI treatment initiation.
Cases 1 and 3 used PSL at 15 mg or higher per day for more than one month, which may have increased the risk of developing TB. Borekci et al. reported that Behcet's disease was associated with the development of active TB,13) and as the primary disease, Behcet’s disease could have influenced the development of TB in case 2. Although the clinical symptoms ruled out active TB and the patient was diagnosed with LTBI, the LTBI screening test was positive for IGRA, and the CT scan revealed a nodular shadow, which could have been a risk factor for the development of TB in case 4. Case 5 had a history of TB and was listed as untreated in the medical record. Since no findings on TB screening were suspicious of active TB at the start of biologic medication therapy, the untreated status had no direct influence on TB development. Mycobacterium TB susceptibility test revealed INH resistance in cases 3 and 5. INH resistance could be due to Mycobacterium TB being originally INH-resistant, or it could have developed INH-resistant during LTBI treatment. The bioburden was insufficient at the start of LTBI treatment for TB PCR to confirm the presence of drug-resistant bacteria. Hence, drug resistance is unknown until the patient develops active TB.
Furthermore, the status of compliance was not investigated this time. It has been reported that as the duration of drug use increases from 3 to 6 months, to 12 months, the compliance rate decreases to 86.8, 78.1, and 65.7%, respectively.25) As a result of the longer duration of treatment (6 or 9 months) compared to other treatment regimens, INH monotherapy may have a lower adherence rate. In light of this, the emergence of resistant strains may have an impact on the incidence of TB after LTBI treatment is initiated. Overseas, two-drug combination therapy based on rifamycin antibiotics is recommended with a shorter treatment period26); therefore, two-drug combination therapy may also be an option to reduce TB incidence after initiating LTBI treatment.
One significant limitation of this study is that it was a retrospective study conducted at a single institution, with a small number of patients. Therefore, we cannot rule out the possibility that risk factors influencing the development of TB after initiating LTBI treatment were missed. In addition, since adherence to LTBI treatment was not assessed in the study, adherence during LTBI treatment could have influenced TB development. Future multicenter studies are required because this retrospective study was conducted at Keio University Hospital up until 2012.
This study found that high PSL doses for more than one month were a risk factor for developing TB in LTBI and biologic medication-treated patients, with rates comparable to other countries with a high TB incidence. Therefore, even if LTBI treatment was used, attention should be paid to breakthrough TB development when using a high PSL dose in combination with a biological agent for an extended period of time.
Author Contributions
Conceptualization: Osamu Iketani, Yuki Enoki, Shunsuke Uno, Sho Uchida, Ho Namkoong, Yoshifumi Uwamino, Naoki Hasegawa, Kazuaki Matsumoto; Data curation: Marina Itagaki, Osamu Iketani; Formal analysis: Marina Itagaki, Osamu Iketani, Yuki Enoki, Kazuaki Matsumoto; Investigation: Marina Itagaki, Osamu Iketani, Yuki Enoki, Kazuaki Matsumoto; Methodology: Osamu Iketani, Shunsuke Uno, Sho Uchida, Ho Namkoong, Yoshifumi Uwamino, Naoki Hasegawa, Kazuaki Matsumoto; Project administration: Marina Itagaki, Osamu Iketani, Yuki Enoki, Victor Tuan Giam Chuang, Kazuaki Taguchi, Shunsuke Uno, Sho Uchida, Ho Namkoong, Yoshifumi Uwamino, Naoki Hasegawa, Kazuaki Matsumoto; Resources, Supervision: Osamu Iketani, Yuki Enoki, Victor Tuan Giam Chuang, Kazuaki Taguchi, Shunsuke Uno, Sho Uchida, Ho Namkoong, Yoshifumi Uwamino, Naoki Hasegawa, Kazuaki Matsumoto; Validation: Marina Itagaki, Yuki Enoki, Victor Tuan Giam Chuang, Kazuaki Matsumoto; Visualization: Marina Itagaki, Osamu Iketani, Yuki Enoki, Victor Tuan Giam Chuang, Kazuaki Matsumoto; Writing—original draft: Marina Itagaki, Osamu Iketani, Yuki Enoki, Kazuaki Matsumoto; Writing—review and editing: Yuki Enoki, Victor Tuan Giam Chuang, Kazuaki Matsumoto.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med., 372, 2127–2135 (2015).
- 2) Landry J, Menzies D. Preventive chemotherapy. Where has it got us? Where to go next? Int. J. Tuberc. Lung Dis., 12, 1352–1364 (2008).
- 3) Ai JW, Zhang Y, Zhang W. Zika virus outbreak: ‘a perfect storm’. Emerg. Microbes Infect., 5, e21 (2016).
- 4) Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, McFarland B, Austin D, Radcliffe L, Suhler EB, Choi D, Rosenbaum JT, Herrinton LJ. Mycobacterial diseases and antitumour necrosis factor therapy in the USA. Ann. Rheum. Dis., 72, 37–42 (2013).
- 5) Lim CH, Chen HH, Chen YH, Chen DY, Huang WN, Tsai JJ, Hsieh TY, Hsieh CW, Hung WT, Lin CT, Lai KL, Tang KT, Tseng CW, Chen YM. The risk of tuberculosis disease in rheumatoid arthritis patients on biologics and targeted therapy: A 15-year real world experience in Taiwan. PLOS ONE, 12, e0178035 (2017).
- 6) Yonekura CL, Oliveira RDR, Titton DC, et al. Incidence of tuberculosis among patients with rheumatoid arthritis using TNF blockers in Brazil: data from the Brazilian Registry of Biological Therapies in Rheumatic Diseases (Registro Brasileiro de Monitoração de Terapias Biológicas - BiobadaBrasil). Rev. Bras. Reumatol. Eng. Ed, 57 (Suppl. 2), 477–483 (2017).
- 7) Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, Pallot-Prades B, Pouplin S, Sacchi A, Chichemanian RM, Bretagne S, Emilie D, Lemann M, Lorthololary O, Mariette X. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French research axed on tolerance of biotherapies registry. Arthritis Rheum., 60, 1884–1894 (2009).
- 8) Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum., 50, 372–379 (2004).
- 9) Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Cöster L, Geborek P, Jacobsson LT, Lindblad S, Lysholm J, Rantapää-Dahlqvist S, Saxne T, Romanus V, Klareskog L, Feltelius N. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum., 52, 1986–1992 (2005).
- 10) Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med., 345, 1098–1104 (2001).
- 11) Gómez-Reino JJ, Carmona L, Rodríguez Valverde V, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum., 48, 2122–2127 (2003).
- 12) Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, Kamatani N, Harigai M, Ryu J, Inoue K, Kondo H, Inokuma S, Ochi T, Koike T. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann. Rheum. Dis., 67, 189–194 (2008).
- 13) Borekci S, Atahan E, Demir Yilmaz D, Mazıcan N, Duman B, Ozguler Y, Musellim B, Hamuryudan V, Ongen G. Factors affecting the tuberculosis risk in patients receiving anti-tumor necrosis factor-α treatment. Respiration, 90, 191–198 (2015).
- 14) Gómez-Reino JJ, Carmona L, Descalzo MÁ. Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum., 57, 756–761 (2007).
- 15) Lee J, Kim E, Jang EJ, Lee CH, Lee EY, Im JP, Han SK, Yim JJ. Efficacy of treatment for latent tuberculosis in patients undergoing treatment with a tumor necrosis factor antagonist. Ann. Am. Thorac. Soc., 14, 690–697 (2017).
- 16) Liao TL, Lin CH, Chen YM, Chang CL, Chen HH, Chen DY. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLOS ONE, 11, 1–14 (2016).
- 17) Kang J, Jeong DH, Han M, Yang SK, Byeon JS, Ye BD, Park SH, Hwang SW, Shim TS, Jo KW. Incidence of active tuberculosis within one year after tumor necrosis factor inhibitor treatment according to latent tuberculosis infection status in patients with inflammatory bowel disease. J. Korean Med. Sci., 33, e292 (2018).
- 18) Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int. J. Tuberc. Lung Dis., 2006, 1127–1132 (2006).
- 19) Ramos GP, Stroh G, Al-Bawardy B, Faubion WA, Papadakis KA, Escalante P. Outcomes of treatment for latent tuberculosis infection in patients with inflammatory bowel disease receiving biologic therapy. Inflamm. Bowel Dis., 24, 2272–2277 (2018).
- 20) American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm. Rep., 49 (RR-6), 1–51 (2000).
- 21) Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum., 55, 19–26 (2006).
- 22) Brassard P, Lowe AM, Bernatsky S, Kezouh A, Suissa S. Rheumatoid arthritis, its treatments, and the risk of tuberculosis in Quebec, Canada. Arthritis Rheum., 61, 300–304 (2009).
- 23) De Keyser F. Choice of biologic therapy for patients with rheumatoid arthritis: the infection perspective. Curr. Rheumatol. Rev., 7, 77–87 (2011).
- 24) Tokuda H, Harigai M, Kameda H, Tomono K, Takayanagi N, Watanabe A, Tasaka S, Suda T, Tateda K, Kadota J. Consensus statements for medical practice: biological agents and lung disease [abridged English translation by the Japanese Respiratory Society]. Respir. Investig., 55, 229–251 (2017).
- 25) International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull. World Health Organ., 60, 555–564 (1982).
- 26) Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, Menzies D, Horsburgh CR Jr, Crane CM, Burgos M, LoBue P, Winston CA, Belknap R. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm. Rep., 69, 1–11 (2020).