2023 年 46 巻 7 号 p. 955-963
2023 年 46 巻 7 号 p. 955-963
Anticancer drugs exhibit many side effects, including skin pigmentation, which often lowers patient QOL. However, the mechanism of pigmentation caused by anticancer drugs remains unknown. The purpose of this study was to elucidate the mechanism of anticancer drug-induced skin pigmentation using 5-fluorouracil (5-FU), a widely used anticancer drug. Specific pathogen-free, 9-week-old Hos:HRM-2 male mice were intraperitoneally administered 5-FU daily for 8 weeks. Skin pigmentation was observed at the end of the study. Mice treated with 5-FU were also administered inhibitors of cAMP, α-melanocyte-stimulating hormone (α-MSH), and adrenocorticotropic hormone (ACTH) for analysis. Administration of oxidative stress, nuclear factor-kappa B (NF-κB), cAMP, and ACTH inhibitors reduced pigmentation in 5-FU-treated mice. These results indicate that the oxidative stress/NF-κB/ACTH/cAMP/tyrosinase pathway plays an important role in pigmentation in 5-FU-treated mice.
The skin, which acts as a barrier to the outside world, is constantly exposed to environmental stressors, such as UV radiation and dryness. Facial skin is particularly prone to the formation of spots, wrinkles, and sagging when exposed to sunlight. Dark spots are pigmentation abnormalities, where melanin pigment deposits on the epidermis. Melanin is produced by melanocytes present in 1 out of 10 cells in the basal layer of the epidermis. Various mechanisms underlying the synthesis of excess melanin due to environmental stress have been reported. Reactive oxygen species (ROS) are generated when the skin is exposed to stressful stimuli. ROS promote the production of proteins, such as endothelin-1, stem cell growth factor, α-melanocyte-stimulating hormone (α-MSH), cyclooxygenase, interleukin (IL)-1α, and macrophage migration inhibitory factor. Among these, the most widely studied is α-MSH, which binds to melanocortin 1 receptor (MC1R) on the membrane, leading to an increase in cAMP and the subsequent activation of protein kinase A/cAMP response element binding protein/microphthalmia-associated transcription factor signaling, thereby increasing tyrosinase enzyme activity.1–4) Melanin synthesis is initiated by the catalytic reaction of tyrosinase with tyrosine as the substrate. Tyrosine is converted to dopaquinone/dopachrome/5,6-dihydoroxyindole-2-carboxylic acid, eventually forming melanin, which is transported to keratinocytes.5) Thus, abnormalities involving cutaneous pigmentation induced by cutaneous irritants, such as UV, are well documented. However, UV is not the only factor that causes skin abnormalities, and skin is also affected by allergies and internal diseases.6,7)
Anticancer drugs are a factor that affects the skin. Skin damage induced by anticancer drugs has a minimal direct impact, but psychological distress due to changes in appearance reduces patient QOL and complicates compliance with anticancer treatment.8) Skin disorders caused by anticancer agents can occur in various forms, including acneiform eruptions, dry skin, and paronychia. Many preventive and therapeutic methods have been studied for skin disorders to improve treatment with anticancer drugs.8–11) However, although there are some case reports on pigmentation, which is among these skin disorders,12,13) the underlying causes have not been investigated, and treatment methods have not been established.
The future development of anticancer drugs should investigate the cause of pigmentation and take countermeasures to facilitate treatment with effective anticancer drugs. The purpose of this study was to investigate the mechanism of pigmentation as a side effect of anticancer drugs using 5-fluorouracil (5-FU), a widely used anticancer agent.
Specific pathogen-free (SPF), 9-week-old Hos:HRM-2 male mice (colored hairless mouse; Japan SLC, Inc., Hamamatsu, Shizuoka, Japan) were used for the experiments. These mice have melanocytes in the auricle, eyes, toes, tail, and skin around the buttocks, and they can synthesize melanin. All mice were individually housed in cages at a controlled temperature of 23 ± 1 °C under SPF conditions with a 12-h light/dark cycle. All animals were allowed free access to laboratory chow (rodent diet EQ 5L37; Japan SLC, Inc.) and water during the experiments. We used five animals per group and repeated the experiments three times. This study strictly followed the recommendations and guidelines for the Care and Use of Laboratory Animals of Suzuka University of Medical Science (Approval No. 34/October 7, 2017). All surgical procedures were performed under pentobarbital anesthesia, and every effect was adjusted to minimize animal suffering.
5-FU TreatmentApproximately 15 mg/kg of 5-FU (Kyowa Kirin Co., Ltd., Tokyo, Japan) in saline was intraperitoneally injected to mice once daily for 8 weeks. The solvent-injected animals were administered saline. Based on the method described by Huang et al.,14) concentrations were set to 5, 15, and 30 mg/kg, and the lowest effective concentration given to mice was adopted.
MC1R Antagonist [Agouti Signaling Protein (ASIP)] TreatmentApproximately 10 nM of ASIP (R&D Systems, Minneapolis, MN, U.S.A.) in 1% dimethyl sulfoxide (DMSO) was intraperitoneally injected to mice three times per week for 8 weeks. The solvent-injected animals were administered 1% DMSO.15)
cAMP Antagonist {α-[2-(3-Chlorophenyl) hydrazinylidene]-5-(1,1-dimethylethyl)-β-oxo-3-isoxazolepropanenitrile (ESI-09)} TreatmentApproximately 20 mg/kg of ESI-09 (Cayman, Ann Arbor, MI, U.S.A.) in a solution of DMSO: phosphate-buffered saline (PBS; ratio 1 : 7) was intraperitoneally injected to mice three times per week for 8 weeks. The solvent-injected animals were administered a solution of DMSO : PBS (ratio 1 : 7).16)
Adrenocorticotropic Hormone (ACTH) Receptor Antagonist [ACTH (11-24)] TreatmentApproximately 20 µg/kg of ACTH (11-24) (MedChemExpress, Monmouth Junction, NJ, U.S.A.) in saline was intraperitoneally injected to mice three times per week for 8 weeks. The solvent-injected animals were administered saline.17)
Nuclear Factor-kappa B (NF-κB) Inhibitor [4-Methyl-N1-(3-phenyl-propyl)-benzene-1,2-diamine (JSH-23)] TreatmentApproximately 1 mg/kg of JSH-23 (MedChemExpress) in 0.5% sodium carboxymethyl cellulose (CMC) was orally administered to mice once daily for 8 weeks. The solvent-injected animals were administered 0.5% CMC.18)
ROS Inhibitor (N-Acetyl-L-cysteine (NAC)) TreatmentNAC (200 mg/kg; Nacalai Tesque, Kyoto, Japan) in 0.08% DMSO was intraperitoneally injected to mice once daily for 8 weeks. The solvent-injected animals were administered 0.08% DMSO.19)
Preparation and Staining of the Skin around the ButtocksWe obtained samples from the skin around the buttocks after 8 weeks of starting the experiment. Specimens were fixed in phosphate-buffered paraformaldehyde (4%), embedded in frozen Tissue-Tek, an optimal cutting temperature compound, and cut into 5-µm sections. The sections were subjected to immunostaining; the staining method is described in details in our previous study.20) Briefly, the specimens were incubated with rabbit polyclonal anti-tyrosinase (1 : 100; Cell Signaling Technology Inc., Danvers, MA, U.S.A.) primary antibodies. The samples were then washed and incubated with fluorescein isothiocyanate-conjugated anti-rabbit (1 : 30; Dako Cytomation, Glstrup, Denmark) secondary antibodies. The expression levels were evaluated immunohistochemically using a fluorescence microscope. Other sections were stained using 3,4-dihydroxyphenylalanine (DOPA). DOPA-positive melanocytes in the skin around the buttocks were stained as described in a previous study.21) The skin around the buttocks was washed with PBS and incubated in PBS containing 0.1% L-DOPA at 37 °C (Sigma-Aldrich Chemical Co., St. Louis, MO, U.S.A.).
Measurement of ACTH, α-MSH, and cAMP Levels in the Skin around the Buttocks of 5-FU-Treated MiceSamples from the skin around the buttocks were collected the last day of the experiment, as described in a previous study.22) Briefly, 100 mg of skin was rinsed with PBS and homogenized, and the supernatant was collected for the assay. The ACTH, α-MSH, and cAMP levels in the skin samples were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (ACTH and α-MSH: Abcam, Cambridge, MA, U.S.A.; cAMP; MyBioSource, San Diego, CA, U.S.A.) according to the manufacturers’ instructions. Optical density was measured using a microplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.).
Statistical AnalysisAll data are presented as means ± standard deviation (S.D.). Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, U.S.A.) was used to analyze the statistical significance of the data, with one-way ANOVA followed by Tukey’s post-hoc test using SPSS software version 20 (SPSS Inc., Chicago, IL, U.S.A.). Results with p values *< 0.05 or **< 0.01 were considered statistically significant.
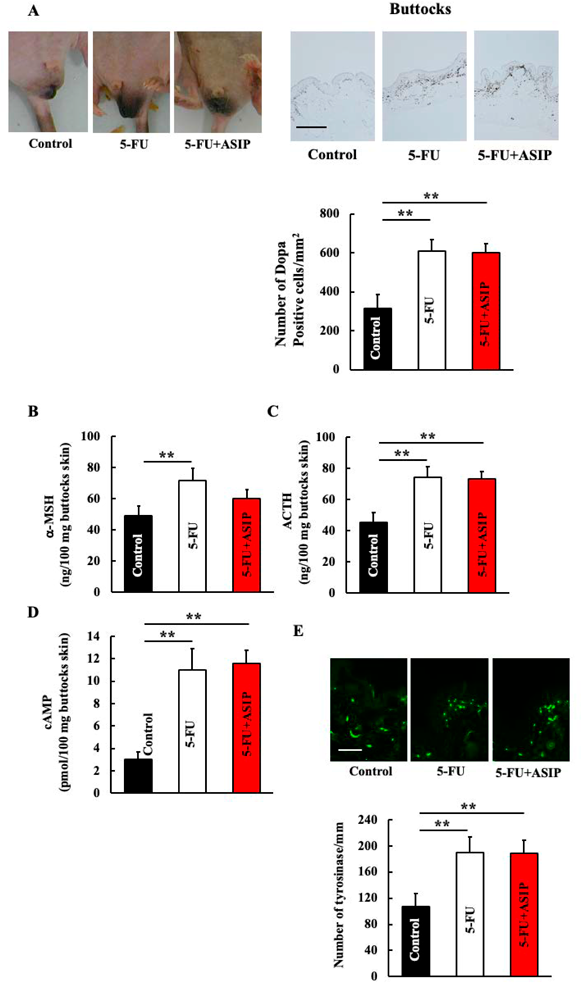
Eight weeks of 5-FU administration increased DOPA-positive melanocytes in the skin around the buttocks of mice (Fig. 1A). Furthermore, increased ACTH, α-MSH, and cAMP levels and tyrosinase expression were observed following 5-FU treatment (Figs. 1B–E).

Values are presented as the mean ± S.D. of five animals. * p < 0.05, ** p < 0.01. Data show one representative experiment performed on five animals. Scale bar = 100 µm. 5-FU, 5-fluorouracil; α-MSH, α-melanocyte-stimulating hormone; ACTH, adrenocorticotropic hormone; S.D., standard deviation.
We administered ASIP, an α-MSH inhibitor, to examine the effects of α-MSH. No difference in pigmentation was observed between 5-FU-treated and ASIP + 5-FU-treated mice (Fig. 2A). In addition, α-MSH, ACTH, and cAMP levels and tyrosinase expression in the skin around the buttocks did not differ between the two groups of mice (Figs. 2B–E).
Values are presented as the mean ± S.D. of five animals. * p < 0.05, ** p < 0.01. Data show one representative experiment performed on five animals. Scale bar = 100 µm. ASIP, α-MSH inhibitor; α-MSH, α-melanocyte-stimulating hormone; ACTH, adrenocorticotropic hormone; 5-FU, 5-fluorouracil; S.D., standard deviation.
ESI-09, a cAMP inhibitor, was administered to evaluate the effects of cAMP. Pigmentation was reduced in ESI-09 + 5-FU-treated mice compared to 5-FU-treated mice (Fig. 3A). The cAMP level and tyrosinase expression also decreased in ESI-09 + 5-FU-treated mice, compared to 5-FU-treated mice (Figs. 3D, E). However, the levels of ACTH and α-MSH in the skin around the buttocks did not differ (Figs. 3B, C).

Values are presented as the mean ± S.D. of five animals. * p < 0.05, ** p < 0.01. Data show one representative experiment performed on five animals. Scale bar = 100 µm. ESI-09, cAMP inhibitor; α-MSH, α-melanocyte-stimulating hormone; ACTH, adrenocorticotropic hormone; 5-FU, 5-fluorouracil; S.D., standard deviation.
We administered ACTH (11-24), an ACTH inhibitor, to study the effects of ACTH. Pigmentation was reduced in ACTH (11-24) + 5-FU-treated mice compared to 5-FU-treated mice (Fig. 4A). Similarly, cAMP levels and tyrosinase expression also decreased (Figs. 4D, E). However, the amount of α-MSH and ACTH in the skin around the buttocks did not differ between the two groups of mice (Figs. 4B, C).

Values are presented as the mean ± S.D. of five animals. * p < 0.05, ** p < 0.01. Data show one representative experiment performed on five animals. Scale bar = 100 µm. ACTH (11-24), ACTH inhibitor; α-MSH, α-melanocyte-stimulating hormone; ACTH, adrenocorticotropic hormone; 5-FU, 5-fluorouracil; S.D., standard deviation.
To elucidate the mechanism underlying the increase in ACTH levels, we used JSH-23 to inhibit the activity of NF-κB, a transcription factor that increases the ACTH levels. Pigmentation was reduced in JSH-23 + 5-FU-treated mice compared with that in 5-FU-treated mice (Fig. 5A). Concurrently, the ACTH and cAMP levels also decreased in the JSH-23 + 5-FU-treated mice compared with those in 5-FU-treated mice (Figs. 5B, C).

Values are presented as the mean ± S.D. of five animals. * p < 0.05, ** p < 0.01. The data show one representative experiment performed on five animals. Scale bar = 100 µm. JSH-23, NF-κB inhibitor; ACTH, adrenocorticotropic hormone; 5-FU, 5-fluorouracil; S.D., standard deviation.
Finally, we used NAC to inhibit oxidative stress, which is considered to be the most upstream factor of 5-FU-induced pigmentation. Pigmentation was reduced in NAC + 5-FU-treated mice, compared with that in 5-FU-treated mice (Fig. 6A). ACTH and cAMP levels also decreased in NAC + 5-FU-treated mice compared with those 5-FU-treated mice, and their levels did not differ from those in the control group (Figs. 6B, C).

Values are presented as the mean ± S.D. of five animals. * p < 0.05, ** p < 0.01. The data show one representative experiment performed on five animals. Scale bar = 100 µm. NAC, ROS inhibitor; ACTH, adrenocorticotropic hormone; 5-FU, 5-fluorouracil; S.D., standard deviation.
In this study, mice treated with 5-FU showed an increase in skin pigmentation around the buttocks. In addition, increases in ACTH and cAMP levels and tyrosinase expression were observed in pigmented skin samples.
Pigmentation generally occurs through the production of melanin in melanocytes. Melanin synthesis occurs in melanosomes, a group of intracellular organelles in melanocytes, where tyrosine is converted by tyrosinase to catalyze the reaction.5) α-MSH is known to regulate the activity of melanocytes.22) α-MSH is a POMC-derived hormone that is secreted by the pituitary gland, as well as by keratinocytes in the skin. Melanocortin receptors are expressed in melanocytes and are involved in melanin biosynthesis. The 5-FU formulations (5-FU, capecitabine, and tegafur) used in this experiment have caused many cases of pigmentation.23) Therefore, we first administered ASIP to examine the role of MSH in melanocyte activation. ASIP is an α-MSH inhibitor that acts as an inverse agonist on specific melanocortin receptors (MCR) related to α-MSH.24) The pigmentation effect of 5-FU was not reversed, and α-MSH was not involved in 5-FU-induced pigmentation. Although ASIP has been reported to decrease cAMP levels,24) this was not observed in this study.
Next, ESI-09, a cAMP inhibitor, was administered to examine whether cAMP affects 5-FU-induced pigmentation. ESP-09 targets the cAMP-binding domain of exchange protein directly activated by cAMP (EPAC)/cAMP-regulated guanine nucleotide exchange factor (GEF) and inhibits cAMP-dependent EPAC/GEF activity.25) Administration of ESI-09 decreased the pigmentation induced by 5-FU, indicating the involvement of cAMP activation in this process.
Thus, we continued to investigate substances that activate cAMP. Addison’s disease, which is caused by adrenal insufficiency, increases ACTH production and causes skin pigmentation.26) ACTH is a stress hormone that is excised from POMC and secreted in response to stress. Herein, 5-FU administration increased ACTH production, suggesting its involvement. In addition, the administration of ACTH (11-24), an inhibitor ACTH inhibitor,17) decreased pigmentation and cAMP levels, suggesting that 5-FU causes an increase in pigmentation via ACTH. Oxidative stress is involved in the occurrence of significant 5-FU side effects, such as gastrointestinal toxicity and myelotoxicity.27) Furthermore, intraperitoneal administration of 5-FU increases oxidative stress in local tissues, which activates NF-κB to promote inflammation.28) Inflammation is known to increase the release of ACTH.29,30) Hence, these findings suggest that the administration of 5-FU may increase ACTH secretion by inducing intracellular inflammation.
Based on the above, we investigated the mechanism of the increase in ACTH levels. In this study, 5-FU administration increased blood levels of IL-6 and inducible nitric oxide synthase, which are inflammatory markers (data not shown). This inflammation activates the transcription factor NF-κB and increases the ACTH levels.29,30) Therefore, administering an NF-κB inhibitor suppressed the increase in ACTH levels and alleviated the increase in pigmentation (Fig. 5). Furthermore, 5-FU administration is known to increase oxidative stress,28) which in turn, increases inflammatory markers.28) In this study, inhibiting oxidative stress abrogated the increases in ACTH and cAMP levels (Fig. 6). These results indicate that administration of 5-FU causes an increase in pigmentation, starting with the induction of oxidative stress.
In this study, 5-FU administration induced an increase in pigmentation, and the mechanism involved the activation of the 5-FU/oxidative stress/NF-κB/ACTH/cAMP/tyrosinase pathway. However, inhibition of oxidative stress abolished both ACTH and cAMP, but could not completely suppress the increase in pigmentation. Thus, other mechanisms are also conceivable, which should be further investigated in the future.
This study was supported by JSPS KAKENHI (Grant No. 18K06082).
The authors declare no conflict of interest.