2023 Volume 46 Issue 9 Pages 1184-1193
2023 Volume 46 Issue 9 Pages 1184-1193
Febrile seizures are seizures accompanied by a fever and frequently occur in children six months to five years of age. Febrile seizures are classified as simple or complex, and complex febrile seizures increase the risk of temporal lobe epilepsy after growth. Therefore, it is important to interfere with epileptogenesis after febrile seizures to prevent post-growth epilepsy. The present study challenged nutritional intervention using docosahexaenoic acid (DHA). Febrile seizures were induced in mice at the age of 10 d using a heat chamber, and seizure sensitivity was examined using pentylenetetrazol (PTZ) administration after growth. PTZ increased the seizure score and shortened the latency in the complex febrile seizure group compared to the control, hyperthermia and simple febrile seizure groups. Mice in the complex febrile seizure group showed abnormal electroencephalograms pre- and post-PTZ administration. Therefore, seizure susceptibility increases the episodes of complex febrile seizures. DHA supplementation after febrile seizures clearly suppressed the increased seizure susceptibility due to complex febrile seizures experienced in infancy. DHA also attenuated microglial activation after complex febrile seizures. Taken together, DHA suppressed microglial activation following complex febrile seizures, which may contribute to protecting the brain from post-growth seizures. The intake of DHA in infancy may protect children from high fever-induced developmental abnormalities.
Fever often occurs during childhood, and it is primarily caused by infection, which sometimes triggers febrile seizures. Febrile seizures are the most common type of convulsion in children and fall into two categories: simple febrile seizures and complex febrile seizures.1) Simple febrile seizures are the main form of febrile seizures, and the prognosis is often good when they develop. Complex febrile seizures have one or more of the following characteristics: duration greater than 15 min, more than one seizure in 24 h, or focal features. Having complex febrile seizures in childhood increases the risk of temporal lobe epilepsy after growth.2,3) Recent research revealed that the incidence of epilepsy after growth increases 7 times when simple febrile seizures occur 3 times or more.4) Adults with temporal lobe epilepsy commonly have a history of prolonged febrile seizures during childhood.5)
Epileptogenesis is the process by which the previously normal brain network is functionally altered toward increased seizure susceptibility, which enhances the probability of generating spontaneous recurrent seizures.6) Febrile seizures, especially febrile status epilepticus, may cause epileptogenesis. Hyperpolarization-activated, cyclic nucleotide-gated channels are modulated by prolonged experimental febrile seizures, which elicits profound consequences on the excitability of the hippocampal network.7) Experimental febrile seizures induced an upregulation of gamma-aminobutyric acid-A receptors in neonatally generated granule cells, and their hyperactivation, which is regulated by Na+K+2Cl− co-transporter (NKCC1), reversed the direction of granule cell migration.8) Genetic epilepsy with febrile seizures plus (GEFS+) is a complex autosomal dominant disorder caused by mutations in SCN1A (a voltage-gated sodium channel), which suggests that sodium current is involved in epileptogenesis.9) Brain immune cells and microglia are involved in the onset of febrile seizures and epileptogenesis. Fever upregulated transient receptor potential vanilloid type 1 (TRPV1) in microglia, and the stimulation of TRPV1 promoted microglia activation and indirectly enhanced seizure susceptibility by inhibiting the neuroprotective effects of microglial transforming growth factor-beta1 via interaction with toll-like receptor 4.10) Microglia are activated 24 h after febrile seizures to produce inflammatory mediators, such as tumor necrosis factor alpha, which leads to neuroinflammation.11) We previously reported that treatment with levetiracetam after status epilepticus prevented the development of spontaneous recurrent seizures via the inhibition of neuroinflammation in pilocarpine-induced status epilepticus mice.12) Therefore, neuroinflammation elicited by microglial activation may play a fundamental role in epileptogenesis formation and the post-growth increase in seizure sensitivity after febrile seizures.
Proper medication for complex febrile seizures, especially when the seizures progress to febrile status epilepticus, is necessary. Febrile seizures are generally treated with benzodiazepines and occasionally with barbiturates. Although preventing epileptogenesis events using pharmacological agents may be important for the treatment of secondary epilepsy after febrile seizures, the use of drugs in infants should be avoided as much as possible considering the effects on development, and nutritional intervention important. We previously reported that maternal intake of docosahexaenoic acid (DHA) during pregnancy and infancy significantly increased the amount of DHA and significantly prolonged febrile seizure latency and increased the body temperature at which the first seizure occurred in a mouse model of febrile seizure.13) DHA may induce estradiol in the brain and suppress febrile seizures. Estrogen suppresses excessive microglial activity.14) We also showed that ingestion of DHA in 8-week-old male mice increased the levels of estradiol in cerebral cortices and suppressed the onset of pentylenetetrazol (PTZ)-induced convulsions.15)
The action of DHA in epileptic patients is controversial. Several studies observed protective effects of DHA on convulsive seizures, but DHA administration did not suppress the seizures.16) However, the action of DHA in epilepsy may depend on the dose administered, and a high dose of DHA effectively reduced the number of seizures.17) The present study constructed a mouse model of increased seizure sensitivity after febrile seizures and investigated the effects of DHA on post-growth seizure sensitivity.
All animal procedures were performed in accordance with the Fundamental Guidelines for the Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The Animal Care and Use Committee of Hiroshima University approved the experimental protocols (No. C18-29-5). Pregnant ICR mice were purchased from SLC (Shizuoka, Japan). The mice were maintained on a 12 : 12 h light/dark cycle (light on from 8:00 a.m. to 8:00 p.m.) and had free access to water and food. We used a simple randomization method.18) Pregnant mice were housed in separate cages, and four to five adult mice were housed in the same cage. No sample calculation was performed, and the sample size was based on our previous study.13)
Febrile Seizure InductionFebrile seizures were induced according to our previous report.13) A glass cylinder with an inner diameter of 10 cm, a height of 17.5 cm, and a thickness of 0.5 cm was placed on a square glass plate with filter paper spread over 12.5 cm. The temperature in the glass chamber was maintained by turning an HL-1 heat lamp (Physitemp instruments LLC, NJ, U.S.A.) on and off with a TCAT-2AC controller (Physitemp instruments LLC). Male 10-d-old mice were acclimated in a glass chamber for 10 min at room temperature (25 °C), and the rectal temperature of the mice was measured using a BAT-12 microprobe thermometer (Physitemp instruments LLC). The mice were placed in a glass chamber preheated to 33 °C. The rectal temperature of the mouse was measured every 3 min, and seizures were classified and scored after the rectal temperature exceeded 38.5 °C. We used Racine’s score: Stage 0, normal; 1, face washing; 2, tail shaking; 3, myoclonic jerking; 4, myoclonic jerking and rearing; 5, clonic convulsion; 6, tonic and clonic convulsion; and 7, unconsciousness.19) Mice with stage 3 or 4 seizures once were defined as the simple febrile seizure group, and mice with stage 5 or higher seizures twice were defined as the complex febrile seizure group. The hyperthermia group received treatment with pentobarbital intraperitoneally (10 mg/kg), Sigma-Aldrich Co. LLC, St. Louis, MO, U.S.A. to suppress the seizures during heating. The mice were kept in the home cage until the experiments were performed.
Electroencephalogram (EEG) MeasurementFor EEG recording surgery, mice were anesthetized with isoflurane. Mouse EEG screws with wire leads (8403NS, Pinnacle Technologies, Inc., Lawrence, KS, U.S.A.) were used as cortical electrodes. The EEG screws were placed over the left frontal cortex (L-F) as the reference and the left/right parietal cortex (L-/R-P). The fourth wire was placed over the right frontal cortex (R-F) through burr holes in the skull as the ground. EEG screws with wire lead were connected to the head mount (8235-SM, Pinnacle Technologies) and fixed to the skull using dental cement (UNIFAST III, GC Corp., Tokyo, Japan). Buprenorphine subcutaneously (0.05 mg/kg) was used as the opioid analgesic in post-surgical treatment. Mice recovered from isoflurane anesthesia and were placed on a heat plate overnight to maintain body temperature. After 5–7 d of recovery from the surgery, EEG signals were recorded using the Pinnacle 8200 system (Pinnacle Technologies). The head mount was connected to a preamplifier tethered to an analog-digital converter box. The voltage differential between the pair of electrodes from L-F and L-/R-P was amplified with 0.5 Hz high-pass filtering and 100 Hz low-pass filtering and recorded. EEG signals were sampled at 400 Hz. Simultaneous video was recorded at 30 frames/s using IP color cameras (Vcam01, JSEED Inc.) and PnP Super Client free software.
Electroencephalographic Spectral AnalysisFast Fourier power spectral analysis of the EEG signal was performed using Sirenia Seizure Pro software with a Hanning window (Pinnacle Technologies) applied to reduce spectral leakage. The band widths for the full, delta, theta, alpha, beta, and gamma frequency bands were set as 0–1000, 0.5–4, 4.5–7.5, 8–13, 13–30, and 35–45 Hz, respectively. Only root-mean-square values from the left hemisphere were used for analysis.
Preparation of Control and DHA DietsSoybean oil (Nisshin OilliO, Tokyo, Japan) was added to AIN-93G (a standard purified diet without fat, Oriental Yeast Co., Ltd., Tokyo, Japan) to a final concentration of 7% (w/w), which was designed as a control diet. DHA (97% purity) was kindly provided by Bizen Chemical Co., Ltd. (Okayama, Japan). DHA was added to the control diet to a final concentration of 4% (w/w) of the total fat (DHA supplemented diet: final concentration of DHA in the diet was 0.28%). The lipid contents of soybean oil have been reported.15)
PTZ Challenge for Measurement of Seizure SusceptibilityMice were placed in a plastic chamber (15 × 15 × 30 cm). Their behavior was observed before and after the administration of PTZ (Sigma-Aldrich). Once the mice displayed a resting posture, they were intraperitoneally injected with 40 mg/kg PTZ. The control mice received a saline injection. Mice were observed for 15 min for convulsive behavior following treatment. Convulsions were classified and scored as previously reported15): 1, immobilization; 2, facial, vibrissal and forelimb clonus (short myoclonic jerks); 3, myoclonic jerking consisting of a whole body jerk with or without irregular, bilateral forelimb movements; 4, generalized clonic seizures with a kangaroo posture; and 5, generalized tonic–clonic seizures with loss of posture tone. One week after 40 mg/kg PTZ administration, the mice were treated with 60 mg/kg PTZ and observed for 15 min for scoring. The latency from the intraperitoneal administration of 60 mg/kg PTZ until the onset of the first Stage 5 convulsion was calculated.
Determination of DHA Contents in the Cerebral CortexDHA contents in the cerebral cortex were determined according to our previous report.13) Briefly, the cerebral cortex was homogenized in saline, and methanolic 5% hydrochloric acid was added to the homogenate. The mixture was incubated for 2 h at 100 °C, and the resulting methyl derivatives were extracted with n-hexane as GC-MS samples. The samples were separated and analyzed by the Sgilrny 7890GC/5975MSD GC-MS system (Agilent, Santa Clara, CA, U.S.A.) equipped with an HP-INNOWAX capillary column (Agilent, 19091 N-133I, Length: 30 m, Diam: 0.250 mm, Film: 0.25 µm). The initial oven temperature was 100 °C, which was increased to 240 °C at a rate of 15 °C/minute, and this temperature was maintained for 10 min.
Measurement of Estradiol Levels in the Cerebral Cortex17β-Estradiol was measured according to our previous report.15) Briefly, the steroid extracts were applied to a C18 Amprep solid-phase column (Amersham Biosciences, Arlington Heights, IL, U.S.A.), and 17β-estradiol was separated using a normal-phase HPLC system. The purified 17β-estradiol was quantified using an EIA kit (Cayman Chemical, Ann Arbor, MI, U.S.A.).
Evaluation of CD68 Expression in MicrogliaMicroglia were separated according to our previous report.20,21) Briefly, the brain was perfused by cold phosphate buffered saline (PBS). The cerebral cortex was isolated, dissected, minced and digested for 45 min at 37 °C in the presence of 400 U/mL collagenase (Sigma-Aldrich) and 30 U/mL deoxyribonuclease (DNase) I (Thermo Fisher Scientific) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% FBS. The cells were pelleted via centrifugation at 500 × g for 10 min at 4 °C. The pellet was resuspended in 37% isotonic Percoll (GE Healthcare, Chicago, IL, U.S.A.) and carefully layered on top of 70% isotonic Percoll, followed by centrifugation. The collected cells from the boundary between 37 and 70% Percoll were blocked with an anti-mouse antibody for CD16/CD32 against Fc receptors (BD Biosciences, San Jose, CA, U.S.A.) for 10 min on ice. Dead cells were stained with propidium iodide. Cells were incubated for 30 min with anti-mouse antibodies against CD11b fluorescein isothiocyanate (FITC) (1/50 dilution, Biolegend, San Diego, CA, USA, RRID:AB_312789), CD45 PE (1/50 dilution, Biolegend, RRID:AB_312970) and CD68 PE/Cyanine7 (1/50 dilution, Biolegend, RRID:AB_2562948) on ice. Stained cells were analyzed with a CytoFLEX S flow cytometer (Beckman Coulter, Indianapolis, IN, U.S.A.). Microglia were defined as CD11b+/CD45low, and myeloid infiltrates were defined as CD11b+/CD45high. CD68 fluorescence in the microglial population was measured and the values of control groups were set to 1 to compare between the experimental groups.
StatisticsAll data are presented as the means ± standard deviation (S.D.). All data were analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, U.S.A.). Student’s t test, one-way ANOVA with Dunnett’s corrected multiple comparison tests, and two-way ANOVA with Tukey’s corrected multiple comparison tests were used to determine significant differences between the means of two or more independent groups. The F value calculated from ANOVA is described in each figure legend, the p value is indicated in each figure or legend, and differences were considered significant when p values were less than 0.05.
We previously reported that febrile seizures were induced in 10-d-old mice (P10) using a heat chamber, and seizure severity was evaluated using Racine’s scoring.13) To categorize the severity of febrile seizures in this study, we defined mice with stage 3 or 4 seizures that occurred once as the simple febrile seizure group and mice with stage 5 or higher seizures twice as the complex febrile seizure group. The hyperthermia group was treated with pentobarbital to suppress seizures during heating. At 8 and 9 weeks of age, 40 and 60 mg/kg PTZ, respectively, were intraperitonially administered to investigate seizure susceptibility (Fig. 1A). When 40 mg/kg PTZ was administered, most mice in the control, hyperthermia and simple febrile seizure groups showed stage 1 seizures, and there was no difference in seizure scores between these 3 groups (Fig. 2A). In contrast, greater than half of the mice in the complex febrile seizure group showed stage 5 severe seizures after 40 mg/kg PTZ injection (Fig. 2A). When 60 mg/kg PTZ was administered, the seizure score was not different between the control, hyperthermia, simple febrile seizure and complex febrile seizure groups (Fig. 2B). The latency to stage 5 seizures was significantly shortened in the complex febrile seizure group compared to the other 3 groups (Fig. 2C). Therefore, seizure susceptibility increased by episodes of complex febrile seizures.
(A) The protocol to evaluate post-growth sensitivity to convulsion after febrile seizures. (B) The protocol to evaluate the effects of DHA supplementation on seizure sensitivity. FS: febrile seizures, 40 PTZ: 40 mg/kg PTZ i.p., 60 PTZ: 60 mg/kg PTZ i.p.

Ten-day-old mice were placed in a chamber and irradiated with an infrared lamp to induce febrile seizures then grown up to 8 weeks of age. (A) After the administration of 40 mg/kg PTZ i.p., seizures were scored according to the Racine classification. The reported values are the means ± S.D. (n = 7 animals in each group). Data were analyzed using one-way ANOVA [F(3, 24) = 15.14, p < 0.0001] followed by Dunnett’s multiple comparisons test. (B) After administration of 60 mg/kg PTZ i.p., seizures were scored according to the Racine classification. The reported values are the means ± S.D. (n = 7 animals in each group). (C) The latency from the administration of 60 mg/kg PTZ i.p. until the onset of the first Stage 5 convulsion was calculated. The reported values are the means ± S.D. (n = 7 animals in each group). Data were analyzed using one-way ANOVA [F(3, 20) = 7.713, p = 0.0013] followed by Dunnett’s multiple comparisons test.
We measured EEG to evaluate brain excitability after febrile seizures. There was no difference in baseline amplitude between the control, hyperthermia, simple febrile seizure and complex febrile seizure groups, but the complex febrile seizure group tended to have larger baseline amplitudes than the other groups (Figs. 3A, B). We measured the number and amplitude of spikes that occurred for 5 min. A recorded signal with more than 4 times higher amplitude of the baseline was defined as a spike. Although there was no significant difference in the number of spikes between the 4 groups, the amplitude of spikes observed in the complex febrile seizure group was significantly larger than the control group (Figs. 3A–D). To investigate the obtained EEG in more detail, the EEGs for an arbitrary minute were converted into a power spectrum. There was no difference in any frequency between the control, hyperthermia, and simple febrile seizure groups (Figs. 3B, E). However, EEGs from 4 to 14 Hz in the complex febrile seizure group had higher power than the control group (Figs. 3B, E), which indicates that the amplitude of this frequency may be high after febrile seizures. Taken together, mice with a history of severe febrile seizures showed basal abnormal EEG, which was a shift of the excitatory-inhibitory balance toward excitability, after growth.

Ten-day-old mice were placed in a chamber and irradiated with an infrared lamp to induce febrile seizures then were grown up to 8 weeks of age. The electrodes were placed on the cerebral cortex to record the potential. (A) Representative EEGs of the control, hyperthermia, simple febrile seizure and complex febrile seizure groups. The average of baseline EEGs (B), number of spikes (C) and spike amplitude were calculated. The reported values are the means ± S.D. (n = 5 animals in each group). Data were analyzed using one-way ANOVA [F(3, 16) = 4.302, p = 0.0209] followed by Dunnett’s multiple comparisons test. (E) The EEGs for every minute were converted into a power spectrum. The reported values are the means ± S.D. (n = 5 animals in each group).
We examined EEGs after the administration of 40 mg/kg PTZ. There was no significant difference in baseline amplitude or the number of spikes after PTZ challenge between the control, hyperthermia, simple febrile seizure and complex febrile seizure groups (Figs. 4A–C). Notably, the spike amplitude observed after PTZ administration in the complex febrile seizure group was significantly larger than the control group (Figs. 4A, D). The power spectrum showed that the complex febrile seizure group had higher power from 4 to 20 Hz frequency than the other 3 groups (Fig. 4E). Therefore, severe febrile seizure episodes affect the amplitude of convulsive EEG after growth.

Ten-day-old mice were placed in a chamber and irradiated with an infrared lamp to induce febrile seizures then were grown up to 8 weeks of age. The electrodes were placed on the cerebral cortex, and EEGs were monitored after intraperitoneal administration of 40 mg/kg PZT. (A) Representative EEGs of the control, hyperthermia, simple febrile seizure and complex febrile seizure groups. The average of baseline EEGs (B), number of spikes (C) and spike amplitude were calculated. The reported values are the means ± S.D. (n = 5 animals in each group). Data were analyzed using one-way ANOVA [F(3, 16) = 5.599, p = 0.0081] followed by Dunnett’s multiple comparisons test. (E) The EEGs for every minute were converted into a power spectrum. The reported values are the means ± S.D. (n = 5 animals in each group).
We investigated the effects of DHA on post-growth increased seizure sensitivity after febrile seizures. Two DHA supplementation protocols were used: maternal DHA intake from P10 to P20, and DHA supplementation from 5 weeks to 6 weeks of age (Fig. 1B). Febrile seizures were induced at P10, and 40 and 60 mg/kg PTZ was intraperitonially administered at 8 and 9 weeks or age, respectively, to examine the effects of DHA on seizure susceptibility.
When 40 mg/kg PTZ was administered, the control group rarely showed seizure behavior, but the complex febrile seizure group developed approximately stage 4 seizures (Fig. 5A). DHA intake just after febrile seizures (from P10 to P20) significantly reduced seizure severity, but late intake of DHA (from 5 to 6 weeks old) did not affect the seizure score (Fig. 5A). PTZ administration at a dose of 60 mg/kg clearly shortened the latency in the complex febrile seizure group compared to the control group (Fig. 5B). DHA intake just after febrile seizures significantly prolonged the seizure latency, which was shortened by the experience of complex febrile seizures (Fig. 5B). Late intake of DHA had no effect on seizure latency (Fig. 5B). Collectively, DHA supplementation as early as possible after febrile seizures may attenuate increased seizure sensitivity after growth.

Ten-day-old mice were placed in a chamber and irradiated with an infrared lamp to induce complex febrile seizures. DHA was supplemented according to the protocol shown in Fig. 1B: DHA intake from P10 to P20 (DHA P10) or from 5 weeks old to 6 weeks old (DHA 5w). (A) After administration of 40 mg/kg PTZ i.p., seizures were scored according to the Racine classification. The reported values are the means ± S.D. (n = 7 animals in each group). The data were analyzed using two-way ANOVA [F(1, 36) = 17.83, p = 0.0002] with Tukey's corrected multiple comparison tests. (B) The latency from the administration of 60 mg/kg PTZ i.p. until the onset of the first Stage 5 convulsion was calculated. The reported values are the means ± S.D. (n = 7 animals in each group). The data were analyzed using two-way ANOVA [F(1, 36) = 11.48, p = 0.0017] with Tukey’s corrected multiple comparison tests.
We previously reported that DHA administration increased estradiol concentrations in the brain due to the upregulation of CYP19 expression.15) The DHA concentration in the cerebral cortex at P12 in the DHA-supplemented group was significantly higher than the control group (Fig. 6A). Estradiol levels in the cerebral cortex at P12 in the DHA-supplemented group were also higher than the control group (Fig. 6B), which indicated a positive correlation between DHA and estradiol concentrations in the brain. Therefore, DHA supplementation upregulated estradiol synthesis. When we measured microglial activity in the cerebral cortex using CD68 expression as an index, microglia were activated 1 d after complex febrile seizure induction, and this activation lasted for 1 week (Fig. 7A). DHA intake significantly suppressed microglial activation elicited by complex febrile seizures at 1 week (Fig. 7B). These results suggest that DHA and/or estradiol synthetically stimulated by DHA suppresses microglial activation following complex febrile seizures, which may contribute to protecting the brain from post-growth seizures.

Ten-day-old mice were placed in a chamber and irradiated with an infrared lamp to induce complex febrile seizures. DHA was supplemented after febrile seizure induction. The cerebral cortex was isolated at P12, and the concentrations of (A) DHA and (B) estradiol were measured. The reported values are the means ± S.D. (n = 5 animals in each group). The data were analyzed using Student’s t tests.
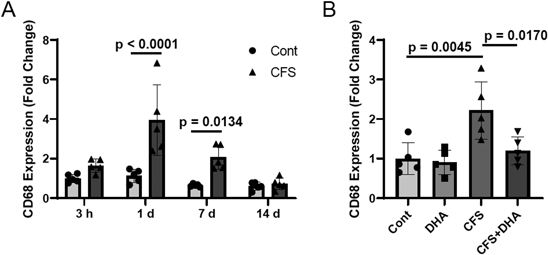
Ten-day-old mice were placed in a chamber and irradiated with an infrared lamp to induce complex febrile seizures. DHA was supplemented after febrile seizure induction. The cerebral cortex was isolated, and CD68 expression in CD11b+CD45low microglia was measured using flow cytometry. (A) Time course of CD68 expression in the control and complex febrile seizure groups. The reported values are the means ± S.D. (n = 5 animals in each group). The data were analyzed using two-way ANOVA [F(1, 32) = 31.32, p < 0.0001] with Tukey’s corrected multiple comparison tests. (B) Effects of DHA supplementation on CD68 expression 1 week after febrile seizure induction. The reported values are the means ± S.D. (n = 5 animals in each group). The data were analyzed using two-way ANOVA [F(1, 16) = 12.61, p = 0.0027] with Tukey's corrected multiple comparison tests.
The present study created 3 experimental groups, hyperthermia, simple febrile seizures and complex febrile seizures, to examine seizure susceptibility after growth. A low dose of PTZ increased the seizure score, and a high dose of PTZ prolonged seizure latency only in the complex febrile seizure group. Abnormal EEG was observed in young adult mice in the complex febrile seizure group. EEG amplitude was also potentiated in the complex febrile seizure group when PTZ was administered. Based on these findings, complex febrile seizures experienced in childhood may develop into increased seizure sensitivity after growth. Dubé et al. showed that adult rats subjected to experimental prolonged febrile seizures during infancy developed abnormal EEGs in the hippocampus and cortex.22) Our results support their observation that severe or prolonged febrile seizures induce abnormal neuronal activity even after growth. Typical behaviors associated with limbic seizures are sudden cessation of activity, head bobbing or jerk, facial automatisms, prolonged immobility with staring, and alternating or bilateral clonus. We could not observe seizure behaviors until 10 weeks of age after complex febrile seizures. Garcia-Curran et al. reported that 40% of rats with febrile status epilepticus developed spontaneous seizures, but spontaneous seizures were sparse (fewer than one seizure per week), and their onset typically occurred in young adult or adult rats.23) They used rats in their study, and their core temperature was increased using a regulated stream of heated air to induce febrile seizures. These differences in species and methodology may be involved in the occurrence ratio of spontaneous seizures after growth.
The intake of DHA during infancy increased the amount of DHA in the cerebral cortex of the offspring and decreased seizure severity, but administration of DHA at a young age for 1 week did not show any effect on seizure susceptibility. DHA is well taken up in the developing brain. The formation of the blood–brain barrier, which interferes with the transport of DHA into the brain, occurs during embryonic days 11 to 17, and the blood–brain barrier during infancy is immature.24) Therefore, blood–brain barrier permeability may be involved in DHA transport to the brain. The DHA uptake rate into the brains of humans was estimated to be 20 mg/d/kg brain in late pregnancy, 7.6 mg/d/kg brain from 0 to 6 months after birth and 2.7 mg/d/kg brain in healthy adults.25–27) This difference could determine the effects of DHA on seizure sensitivity. The intake of DHA in adult mice rarely increased the DHA concentration in the brain compared with mice during development.13,28)
Estradiol is a female sex steroid that has several beneficial effects, such as prevention of bone loss29) and decreasing the risk of coronary disease,30) as well as essential functions in female reproduction. The brain possesses an inherent endocrine system and synthesizes steroid hormones, including estradiol, and the brain is also affected by estradiol. Treatment with a high concentration of estradiol (10–100 µg/g weight) shortened seizure latency in rats,31) and ovariectomized rats supplied with 2 µg/d estradiol had prolonged latency to convulsions caused by kainic acid administration.32,33) Therefore, estradiol concentration may be an important factor in defining the action of estradiol on convulsions. Estradiol has several antioxidative actions.34) Antioxidative enzymes, such as catalase, superoxide dismutase and glutathione peroxidase 1, are transcriptionally activated by estrogen receptor signaling. Estradiol affects mitochondrial antioxidative enzymes or respiratory complexes to decrease reactive oxygen species by enhancing mitochondrial efficiency. Estradiol suppresses microglial activation, which attenuates neuroinflammation. Treatment of microglia with estradiol reduced the expression of inflammatory mediators, such as MCP-1 and tumor necrosis factor (TNF)-alpha, induced by lipopolysaccharide.14) Notably, CYP19 (CYP19A, an enzyme for estrogen synthesis from testosterone) inhibition exacerbated reactive gliosis,35) which showed the importance of synthesized estradiol in microglial inactivation. These actions of estradiol may contribute to the suppression of seizures.
DHA acts as a retinoid X receptor (RXR) agonist, and we previously reported that RXR increased the expression of CYP19.36) Therefore, DHA-dependent RXR activation is induced in the cerebral cortex in pups and may be involved in the upregulation of brain estradiol levels. The CYPIL-aromatase gene has several RXR half sites in the promoter region, which may be responsible for the induction of CYP19 expression by an RXR agonist.36)
Neuroinflammation induced by microglial activation plays an important role in epileptogenesis.37,38) We reported that Ly6G+Ly6C+ neutrophils and microglia were involved in the induction of acute brain inflammation after status epilepticus in pilocarpine-treated mice.39,40) The present study evaluated CD11b+CD45low microglial activity by measuring CD68 expression. CD68 expression significantly increased 1 d to 1 week after complex febrile seizures, and this increase recovered to basal levels. Notably, DHA administration clearly suppressed upregulated CD68 expression and attenuated the increase in seizure sensitivity in adulthood after complex febrile seizures, which suggest that DHA suppresses post-growth excessive neuronal activity by inhibiting microglial activation caused by complex febrile seizures in infancy. Several pro-inflammatory mediators released from microglia are involved in epileptogenesis and subsequent spontaneous seizures. Interleukin (IL)-1β may be the focus. Notably, IL-1β levels were elevated only in rats that developed spontaneous limbic seizures after febrile status epilepticus.41) Further study is needed to reveal the relationships between fever and/or seizure-induced epileptogenesis and neuroinflammation.
We previously reported that DHA supplementation during pregnancy and infancy decreased the susceptibility to febrile seizures in offspring.13) The present study revealed that DHA intake during infancy after febrile seizures normalized excessive neuronal activity and reduced seizure susceptibility after growth. Therefore, DHA intake during development suppressed the onset of febrile seizures and the temporal lobe epilepsy that developed due to severe and prolonged febrile seizures in childhood. DHA intake shows no critical risk, even in pregnancy in human.42) The use of an enteral DHA emulsion until 36 weeks of postmenstrual age was associated with modestly higher full-scale intelligence quotient scores at 5 years of age than control feeding.43) Taken together, DHA supplementation during pregnancy and infancy provide large advantages in supporting healthy development.
In conclusion, we revealed that DHA supplementation in infancy suppressed the increased seizure sensitivity that developed due to complex febrile seizures in childhood. DHA acts on the developing brain to attenuate neuroinflammation, which plays a fundamental role in epileptogenesis formation. Intake of DHA in pregnancy to infancy has great benefit in protecting children from high fever-induced developmental abnormalities.
The authors thank Mr. Yoshihisa Misawa, Bizen Chemical Co., Ltd. for his kind support of experiments on DHA supplementation and Ms. Nami Ishihara and Kaede Namba for their technical assistance during the course of this study.
This work was supported by KAKENHI Grants from the Japan Society for the Promotion of Science (Grant Numbers: 21K06702 to KI and 20H04341 to YI) and the Food Science Institute Foundation (Ryoushoku-kenkyukai) (Grant Number: 2018A02 to Y.I.).
Conceptualization, YI; methodology, SK, KI and YI; investigation, SK, KI and YI; visualization, SK, KI and YI; funding acquisition, KI and YI; project administration, KI and YI; writing—original draft, YI; and writing—review and editing: SK, KI and YI. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.
All data generated or analyzed in this study are included in this published article.