2023 Volume 46 Issue 9 Pages 1310-1315
2023 Volume 46 Issue 9 Pages 1310-1315
Recently, microneedling as a cosmetic product has attracted attention as one way to improve skin barrier function and moisturizing function to reduce wrinkle formation. However, some cases of erythema and edema have been reported as side effects. In order to develop safer microneedle cosmetics, we investigated whether microneedles can improve skin barrier function and moisturizing function even when applied in a non-invasive manner that does not penetrate the stratum corneum. We established the condition of non-penetrating microneedle application on reconstructed human full-thickness skin models and examined the effect on the skin models when microneedles were applied under this condition. Microneedle application increased the gene expression of serine palmitoyltransferase long chain base subunit (SPTLC) 3, filaggrin, and transglutaminase 1. The amount of ceramide produced by SPTLC was also increased by microneedle application. Gene expression of filaggrin-degrading enzymes and the amount of free amino acids, a product of filaggrin degradation, were also increased by microneedling. These results suggest that non-invasive microneedle application can improve skin barrier function and moisturizing function by increasing the amount of ceramide and natural moisturizing factors.
As the outermost layer of the body, the skin not only protects against external damage and stress, but also prevents water loss from the body. As skin components such as collagen and elastic fibers diminish with age, the skin loses its elasticity and wrinkles begin to form.1) There is a close relationship between skin moisture content and wrinkles, and it is believed that maintaining the skin’s moisture content by improving the skin barrier function and moisturizing function will lead to wrinkle reduction.2–4) Ceramide and natural moisturizing factors are considered as factors that control skin barrier function and moisturizing function. Ceramides are composed of sphingoid bases and saturated fatty acid moieties, and are classified into 12 major types in the human stratum corneum according to their combination.5) Ceramides are the main component of intercellular lipids and form a lamellar structure with the corneocyte in the stratum corneum, thereby protecting against external stimuli and retaining moisture in the skin.6,7) In addition, natural moisturizing factor is a general term for moisturizing factors that hold water in the stratum corneum, and more than 50% of them are composed of amino acids and amino acid metabolites.8)
Recently, cosmetic microneedles have attracted attention for their ability to reduce the appearance of wrinkles and rejuvenate skin by replenishing hyaluronic acid and collagen.9)
Microneedles are microscopic needle structures that penetrate the stratum corneum but do not reach the dermis and are characterized by their ability to be applied painlessly. Microneedles have been studied to overcome the skin barrier by penetrating the stratum corneum to deliver drugs or to induce tissue remodeling associated with injury healing.9) In fact, microneedling with or without active ingredients has been reported to improve stratum corneum barrier function and wrinkles.10,11) However, microneedling has also been reported to be associated with erythema and edema as side effects of the puncture.10,12)
We initiated this study to investigate whether non-invasive application of microneedles that do not penetrate the stratum corneum can also improve skin barrier function and wrinkles. To our knowledge, this is the first study to apply microneedles using the concept of non-penetration of the stratum corneum.
Reconstructed human full-thickness skin models, T-Skin were purchased from EPISKIN (Lyon, France). The culture medium was included. The solid microneedle patch made of polylactic acid is 8 mm in diameter and which has a 500 µm length of needles with a density of 152 needles/cm2. Esterified omega-hydroxy sphingosine ceramide (Cer [EOS]), non-hydroxy sphingosine ceramide (Cer [NS]), non-hydroxy phytosphingosine ceramide (Cer [NP]), alpha-hydroxy sphingosine ceramide (Cer [AS]), and alpha-hydroxy phytosphingosine ceramide (Cer [AP]) as standard samples were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, U.S.A.). The springs were purchased from TRUSCO NAKAYAMA Corp. (Tokyo, Japan).
Cell Culture and Microneedle ApplicationT-skin was cultured in the supplied medium at 37 °C with 5% CO2. The medium was changed daily. After 24 h of preincubation, microneedles were placed on the skin models and different loads (1, 2 or 3 N) were applied with a spring. One newton corresponds to 1 kg·m/s2. The control group was cultured under natural conditions without microneedle and spring application.
Measurement of Transepidermal Water Loss (TEWL)After 24 h of microneedle application at 1–3 N load, the skin models were removed from the incubator and placed on a clean bench at room temperature for 30 min. Once the microneedles were removed from the skin models, TEWL was measured three times using a VapoMeter (Delfin Technologies Ltd., Kuopio, Finland).
RNA Extraction and Quantitative Real-Time PCRAfter 8 h of microneedle application at 1 N load, the microneedles were removed from the skin models. The skin models were then hollowed out with a φ8 mm biopsy punch (BP-80F, Kai Industries, Gifu, Japan) to collect the skin directly under the microneedle, which was stored at 4 °C in RNAprotect Tissue Reagent (QIAGEN, Venlo, Netherlands). RNA was extracted from the skin samples according to the standard protocol of the RNA extraction kit (RNeasy Fibrous Tissue Mini Kit 50, QIAGEN). RNA concentration was measured by UV spectrophotometry (Simplinano, Biochrom Ltd., Cambridge, U.K.). RNA samples were diluted with ribonuclease (RNase)-free water to equalize the concentration. Target gene primers and β-actin primers (Thermo Fisher Scientific, Waltham, MA, U.S.A.) were mixed with PCR reagents (TaqMan™ RNA-to-CT™ 1-Step Kit, Thermo Fisher Scientific) and RNase-free water in specified amounts and measurements were performed using an Applied Biosystems® 7500 fast real-time PCR system (Thermo Fisher Scientific). Reactions were incubated in a 96-well plate at 48 °C for 15 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The results were normalized to β-actin and analyzed by the ΔΔCt method. Target genes were interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), serine palmitoyltransferase long chain base subunit (SPTLC) 1, SPTLC2, SPTLC3, filaggrin, transglutaminase 1 (TGM1), collagen 1A1, collagen 4A6, collagen 7A1, discoidin domain receptors 2 (DDR2), fibroblast growth factor (FGF) 1, FGF2, hyaluronan synthases (HAS) 1, HAS2, HAS3, integrin α6, involucrin, laminin subunit (LAM) A3, LAMC1, calpain 1, caspase 14, and bleomycin hydrolase. Assay ID for each primer is shown in Supplementary Table S1.
Ceramide Extraction and Quantification by High-Performance TLC (HPTLC)After 8 h of microneedle application at 1 N load, the microneedles were removed from the skin models and the skin models were incubated for another 64 h. The skin models were then hollowed out with a φ8mm biopsy punch and stored at −80 °C. The epidermis of the skin samples was soaked in 0.15 mL methanol and homogenized with TissueRuptor (QIAGEN). Then 0.3 mL chloroform was added and sonicated for 10 min. The solutions were filtered through a filter (SLLG033NB, Merck Milipore, Burlington, MA, U.S.A.), the solvent was removed under nitrogen gas, and the dried samples were redissolved in 0.1 mL chloroform:methanol solution (2 : 1, v/v). The extracted ceramide solution (5 or 20 µL) was spotted to HPTLC plate (Silica gel 60, Merck KGaA, Darmstadt, Germany) using a capillary pipette (Ringcaps 10 µL, Hirschmann, Eberstadt, Germany).13) The samples were developed twice with chloroform:methanol:acetic acid (190 : 9 : 1, v/v). The HPTLC plate was dried and immersed in a staining solution (10% copper sulfate/8% phosphoric acid reagent), then heated on a hot plate at 180 °C for 10 min. The resulting bands were photographed and quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, U.S.A.). The quantitative value was calculated from a calibration curve for each ceramide obtained from the same plate. The amounts of the various ceramides that were measurable were added together and considered the total ceramide content.
Quantification of Free Amino Acid ContentAfter 8 h of microneedle application at 1 N load, the microneedles were removed from the skin models and the skin models were incubated for another 64 h. The skin models were then hollowed out using a φ8 mm biopsy punch and stored at −80 °C. The epidermis of the skin samples was soaked in 2 mL of 80% methanol to which 0.01 mL of norvaline solution (50 mmol/L, Waters, Milford, MA, U.S.A.) was added as an internal standard and then homogenized. The supernatant from the centrifuged samples was filtered through a 0.2 µm pore filter. 0.08 mL of the extract was dried in a centrifugal concentrator, and 0.08 mL of the boric acid buffer provided in the derivatization kit (AccQ-Tag Ultra, Waters) was added to dissolve the amino acids by stirring and sonication. 0.02 mL of derivatization reagent was added, immediately stirred, and heated at 55 °C for 10 min. After cooling, the solution was transferred to a vial for HPLC. A Waters AccQ Tag Ultra column (2.0 mm i.d. × 150 mm, 1.7 µm particles, Waters) was used, and the flow rate was 0.44 mL/min. The absorbance of each peak was measured at 260 nm. Amino acid content was corrected for epidermal weight. The amounts of alanine (Ala), arginine (Arg), glutamic acid (Glu), glycine (Gly), histidine (His), and serine (Ser), which are the major components of filaggrin, were added together and considered as the amount of filaggrin main constituent amino acids.14)
Data AnalysisAll results are presented as means and standard errors. In each figure legend, “n” stands for number of samples. Statistical analysis was performed using Dunnett’s multiple comparison test or Welch’s t-test.
Reconstructed human full-thickness skin models were cultured with microneedle application at loads of 1, 2, or 3 N for 24 h and then TEWL was measured to see if the microneedle damaged the skin barrier. There was no difference in TEWL between the tissue with microneedle application at 1 N load and the control tissue (Fig. 1B). Whereas, TEWL was increased in the tissue with microneedle application at 2 or 3 N load compared to the control tissue (p < 0.05). Next, mRNA expression levels of inflammatory cytokines were measured after 8 h of microneedle application at 1 or 2 N load. IL-6 mRNA expression levels were decreased in tissue with microneedle application at 1 N load and increased in tissue with microneedle application at 2 N load compared to the control tissue (Fig. 1C, p < 0.01). TNF-α mRNA expression levels were not different in tissue with microneedle application at 1 N load and increased in tissue with microneedle application at 2 N load compared to the control tissue (Fig. 1D, p < 0.01).
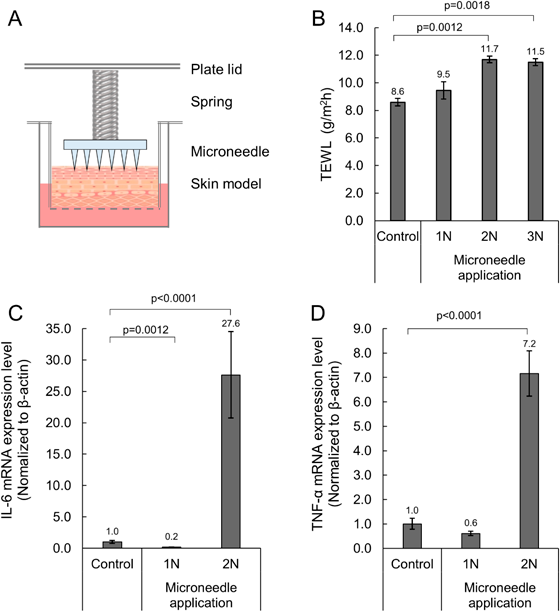
Statistical analysis was performed using Dunnett’s test (vs. control). TEWL, transepidermal water loss; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
After 8 h of microneedle application at 1 N load, mRNA expression levels of 19 genes related to skin barrier function and moisturizing function were measured. mRNA expression of SPTLC3, filaggrin, and TGM1 were significantly increased in microneedle-treated tissue compared to the control tissue (Fig. 2, p < 0.05). On the other hand, there were no changes in the mRNA expression of collagen 1A1, collagen 4A6, collagen 7A1, DDR2, FGF1, FGF2, HAS1, HAS2, HAS3, integrin α6, involucrin, LAMA3, LAMC1, SPTLC1, SPTLC2 (data not shown).
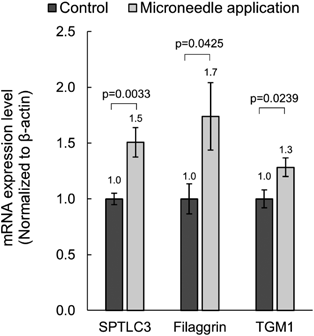
Statistical analysis was performed using Welch’s t-test. SPTLC3, serine palmitoyltransferase long chain base subunit 3; TGM1, transglutaminase 1.
After 8 h of microneedle application followed by 64 h of incubation, epidermal ceramide (ceramide [EOS], [NS], [NP], [AS], and [AP]) content was determined by HPTLC. Ceramide [EOS], [NS], [NP], and total ceramide content were significantly increased in microneedle-treated tissue compared to the control tissue (Figs. 3A, B, Supplementary Fig. S1, p < 0.05). Ceramide [AP] content was below the detection limit.
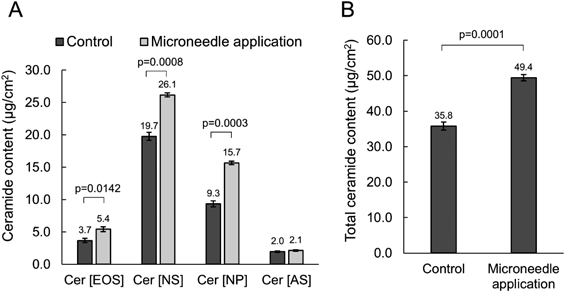
Statistical analysis was performed using Welch’s t-test. Cer [EOS], esterified omega-hydroxy sphingosine ceramide; Cer [NS], non-hydroxy sphingosine ceramide; Cer [NP], non-hydroxy phytosphingosine ceramide; Cer [AS], alpha-hydroxy sphingosine ceramide; Cer [AP], alpha-hydroxy phytosphingosine ceramide.
The mRNA expression levels of calpain 1, caspase 14, and bleomycin hydrolase were measured after 8 h of microneedle application at 1N load. Caspase 14 and bleomycin hydrolase mRNA expression levels were significantly increased in microneedle-treated tissues compared to the control tissue (Fig. 4A, p < 0.05).

Individual data values are shown in Supplementary Table S2. Statistical analysis was performed using Welch’s t-test.
The free amino acid content in the epidermis was then determined by HPLC after 8 h of microneedle application at 1N load followed by 64 h of incubation. The results showed that His, phenylalanine (Phe), and tryptophan (Trp) were significantly increased by microneedle application (Fig. 4B, Supplelmentary Table S2, p < 0.05). The amount of cysteine was below the detection limit. The amounts of Ala, Arg, Glu, Gly, His, and Ser, which are the major components of filaggrin, were added together and considered as the amount of filaggrin main constituent amino acids. The amount of filaggrin constituent amino acids was significantly increased by microneedle application (Fig. 4C, p < 0.05).
Microneedling with puncture has been reported to improve skin barrier function and wrinkles through wound-related remodeling.10,11) However, microneedling has also been reported to cause erythema and edema as side effects.10,12) We wondered if microneedles could also improve skin barrier function by applying them in a non-invasive manner that does not penetrate the stratum corneum.
First, we investigated the conditions for application of microneedles that do not penetrate the stratum corneum in a reconstructed human full-thickness skin model. Application of the microneedle at a spring load of 1 N did not increase TEWL or mRNA expression of inflammatory cytokines. It is known that application of general microneedles increases TEWL.15) Thus, this result suggests that microneedle application at 1 N load does not penetrate the stratum corneum and does not damage the skin in the reconstructed human full-thickness skin models. Although TEWL and inflammatory cytokine mRNA expression increased when microneedles were applied at 2 and 3 N loads, we believe that this is a phenomenon unique to the skin model and would not damage the skin in clinical use. In fact, experiments on extracted human skin have confirmed that TEWL does not increase at loads up to 20 N (data not shown). In this paper, a 1N load was adopted as a non-invasive condition that does not cause damage to the skin model and subsequent tests were performed. To our knowledge, this is the first report of microneedling under conditions where the needle does not penetrate the stratum corneum.
We performed RT-PCR to test whether mRNA expression levels of genes related to skin barrier function and moisturizing function were altered after microneedle application at 1N load for 8 h. The results showed that the mRNA expression levels of SPTLC3, filaggrin, and TGM1 were significantly increased in microneedle-treated tissue compared to the control. We tested whether the increase in these mRNA expression levels varied with microneedle application time. mRNA expression levels were measured after 1, 3, or 8 h of microneedle application at 1N load, and the strongest increase in mRNA expression was observed at 8 h (Supplementary Fig. S2).
SPTLC3 is an enzyme that synthesizes the basic skeleton of ceramide from L-serine and acyl-CoA.16) Ceramide is the major component of intercellular lipids, which form a lamellar structure in the stratum corneum to retain moisture and protect the skin from external stimuli.6,7) We measured ceramide content in the epidermis and found that microneedle application significantly increased ceramide [EOS], [NS], [NP], and total ceramide content. There was no increase in ceramide [AS], but this may be due to its low content in the skin model. These results suggest that microneedle application increased ceramide content via upregulated gene expression of SPTLC3.
Filaggrin is considered a histidine-rich protein consisting mainly of Ala, Arg, Glu, Gly, His, and Ser and is known as a source of natural moisturizing factors (NMF).14,17) Profilaggrin is synthesized in the granular layer as a component of keratohyalin granules. Profilaggrin is degraded to monomeric filaggrin by proteases. Filaggrin is further degraded by calpain 1, caspase 14, and bleomycin hydrolase to free amino acids, which are considered NMF.18) Furthermore, filaggrin-derived histidine is converted by histidine deaminase to trans-urocanic acid, which has UV-absorbing effects and is also known to act as NMF.17,19) The gene expression levels of three filaggrin-degrading enzymes were measured, and microneedle application significantly increased the gene expression level of caspase-14 and bleomycin hydrolase. In addition, the amount of free amino acids in the epidermis was measured, and microneedle application increased the amount of His as well as the amount of filaggrin main constituent amino acids. These results suggest that microneedle application increases the gene expression of filaggrin, caspase-14, and bleomycin hydrolase, and increases the amount of free amino acids as NMF. Microneedle application also increased the amount of Phe and Trp, but these are not filaggrin main constituent amino acids, so the mechanism of their increase needs to be further investigated.
TGM1, together with filaggrin, contributes to the maturation of the cornified envelope, a membrane-like structure that surrounds corneocytes.20) TGM1 cross-links filaggrin, involucrin, and loricrin to form the cornified envelope. The cornified envelope contributes to skin barrier function by physically solidifying the stratum corneum and providing a foundation for intercellular lipids.
Thus, it is speculated that non-invasive microneedle application improves skin barrier function and moisturizing function by increasing the amount of ceramide and natural moisturizing factors in the skin. Maturation of the cornified envelope with increased TGM1 gene expression by microneedles may also contribute to improve skin barrier function. The present results are not inconsistent with the literature showing improved skin barrier function and anti-wrinkle effects with microneedle application under puncture conditions and suggest that these effects can be mimicked under safer, non-puncture conditions. Previous studies on microneedle have suggested that tissue remodeling during the healing process of microscopic wounds improves the skin barrier function.10,11) However, this study suggests that the skin barrier function is improved even under conditions that do not cause wounding. Furthermore, there have been no reports to date of increases in SPTLC3, filaggrin and TGM1 gene expression, epidermal ceramide content or free amino acid content with invasive microneedle application. This suggests that microneedle application under non-puncture conditions may contribute to the improvement of skin barrier function by a different mechanism than under puncture conditions. It has been reported that non-invasive external stimuli such as temperature, humidity, and tensile force can affect the inside of the skin,21,22) and this study suggests that a novel stimulation method using microneedles can also affect the inside of the skin. It has been reported that mechanical stretching of cultured skin tissue activates the mitogen-activated protein kinase (MAPK) pathway,22) and activation of the MAPK pathway has been reported to increase filaggrin and SPTLC gene expression.23,24) Applying microneedle to the epidermis without puncturing is thought to provide the similar stimulation as stretching, thus it is suggested that the microneedle may act via the MAPK pathway.
Only one type of microneedle was used in this study. Further research is needed to determine if changing the conditions of the microneedles will alter their effect on the skin barrier function and moisturizing function. This study used reconstructed human full-thickness skin models as an alternative to animal and clinical studies. However, the optimal conditions for microneedle application may vary from model to model. Therefore, the effects of microneedles on skin barrier function, moisturizing function, and wrinkle grade should be further investigated in clinical studies.
We applied microneedles to reconstructed human full-thickness skin models under non-puncture conditions. The results showed that microneedle application increased the gene expression of SPTLC3, filaggrin and TGM1, as well as the content of epidermal ceramide and free amino acids. These results suggest that non-invasive microneedle application contributes to the improvement of skin barrier function and moisturizing function.
Kota Sakuraba, Yukio Kojima, Takaaki Terahara and Hidekazu Kuma are employees of Hisamitsu Pharmaceutical Co., Inc.
Yoshihiro Tokudome received consulting fees from Hisamitsu Pharmaceutical Co., Inc.
This article contains supplementary materials.