2024 Volume 47 Issue 1 Pages 303-310
2024 Volume 47 Issue 1 Pages 303-310
Methotrexate (MTX) is an indispensable drug used for the treatment of many autoimmune and cancerous diseases. However, its clinical use is associated with serious side effects, such as lung fibrosis. The main objective of this study is to test the hypothesis that hydroxytyrosol (HT) can mitigate MTX-induced lung fibrosis in rats while synergizing MTX anticancer effects. Pulmonary fibrosis was induced in the rats using MTX (14 mg/kg/week, per os (p.o.)). The rats were treated with or without HT (10, 20, and 40 mg/kg/d p.o.) or dexamethasone (DEX; 0.5 mg/kg/d, intraperitoneally (i.p.)) for two weeks concomitantly with MTX. Transforming growth factor beta 1 (TGF-β1), interleukin-4 (IL-4), thromboxane A2 (TXA2), vascular endothelial growth factor (VEGF), 8-hydroxy-2-deoxy-guanosine (8-OHdG), tissue factor (TF) and fibrin were assessed using enzyme-linked immunosorbent assay (ELISA), immunofluorescence, and RT-PCR. Pulmonary fibrosis was manifested by an excessive extracellular matrix (ECM) deposition and a marked increase in TGF-β1 and IL-4 in lung tissues. Furthermore, cotreatment with HT or dexamethasone (DEX) significantly attenuated MTX-induced ECM deposition, TGF-β1, and IL-4 expression. Similarly, HT or DEX notably reduced hydroxyproline contents, TXA2, fibrin, and TF expression in lung tissues. Moreover, using HT or DEX downregulated the gene expression of TF. A significant decrease in lung contents of VEGF, IL-8, and 8-OHdG was also observed in HT + MTX- or DEX + MTX -treated animals in a dose-dependent manner. Collectively, the results of our study suggest that HT might represent a potential protective agent against MTX-induced pulmonary fibrosis.
Pulmonary disorder is considered a drastic global health concern. The WHO ranked it second in death, epidemiology, and cost of management.1) In fact, methotrexate (MTX) is a tetrahydrofolate reductase inhibitor that plays a pivotal role in the treatment of numerous tumors including breast, lung, head, and neck tumors. Moreover, it is the reference standard drug in the treatment of several autoimmune diseases such as rheumatoid arthritis and psoriasis. However, unfortunately, MTX exhibited serious adverse effects specifically on the pulmonary system including lung fibrosis.2)
Notably, the repeated usage of MTX causes the generation of reactive oxygen species (ROS) which leads to lung epithelial injury that represents a continuous pulmonary insult, and triggers the release of inflammatory cell mediators.3) In this milieu, various leukocytes are recruited in lung tissues, unleashing profibrotic cytokines such as interleukin-1 beta (IL-1β), and transforming growth factor beta 1 (TGF-β1). Conceivably, this status enhances fibroblast migration, proliferation, and activation, which promotes extracellular matrix (ECM) deposition in pulmonary tissues causing fibrosis.4) Additionally, the activated fibroblasts worsen the situation by releasing vascular endothelial growth factor (VEGF) that induces angiogenesis; this facilitates access for more inflammatory cells and enhances the deleterious changes in lung structure. Besides, IL-8, an angiogenic cytokine, has been found to be elevated in fibrotic lung tissue.5)
It is worth mentioning that injured pulmonary epithelium secretes the platelet aggregator cytokine, thromboxane A2 (TXA2), as well as, TF. The aforementioned cytokines play an axial role in activating the coagulation cascade that ends with the transformation of soluble fibrinogen into insoluble fibrin deteriorating pulmonary fibrosis.6,7)
Hydroxytyrosol (HT) is a potent antioxidant phenolic constituent in olive oil with multi-targeted beneficial effects including cardioprotective capabilities, and platelet function improvement. Besides, it has antimicrobial, antimycotic, antidiabetic, neuroprotective, anti-inflammatory, and anti-tumor potentials.8) In addition, HT has a notable antiproliferative activity against wide cancer cell lines, particularly, acute human leukemia T cells (Jurkat and HL60).9)
Therefore, our study has been designed to investigate the potential protective effects of HT against pulmonary fibrosis induced by MTX in rats.
Wistar adult male rats (160–180 g) were used in this study. We got them from the breeding colony at the animal house of the Egyptian Drug Authority (EDA), Cairo, Egypt. Food and water were given ad libitum, with 12 h light–dark cycles, and kept at 40–60% relative humidity and 21–24°C. Animals were adapted for 2 weeks in the animal house before the experiments. Unnecessary animal disturbances have been avoided. Animals were handled gently; squeezing, pressure, and the hard maneuver was avoided. This study was conducted in agreement with the ethical guidelines for investigations in laboratory animals and got approval from the Research Ethics Committee of NODCAR, (NODCAR/I/51/19) and comply with the Guide for the Care and Use of Laboratory Animals.10)
Ethical ApprovalAll procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted, and comply with the Guide for the Care and Use of Laboratory Animals.
Reagents and ChemicalsHydroxytyrosol was obtained from Sigma (St. Louis, MO, U.S.A.). Methotrexate (vial of 50 mg/2 mL) was acquired from Mylan Institutional LLC, U.S.A. Dexamethasone ampoules (8 mg/2 mL) were bought from Amriya Pharm (Alexandria, Egypt). The analytical grade for all other chemicals was used.
Induction of Pulmonary FibrosisFor experimental induction of pulmonary fibrosis, rats were given MTX (14 mg/kg/week, per os (p.o.)) for 2 weeks.11)
Experimental DesignIn this study, 96 adult male Wistar rats (160–180 g) were randomly allocated into 8 groups, twelve rats per each. Group 1; served as control group and received saline only. Group 2; rats received MTX (14 mg/kg, p.o.) once weekly for two successive weeks and served as the positive control.11) Group 3; rats were injected with DEX (0.5 mg/kg, intraperitoneally (i.p.)) once daily for two successive weeks.11) Group 4; rats were given HT (40 mg/kg, p.o.) once daily for two consecutive weeks.12) Group 5; rats received MTX (14 mg/kg/week, p.o.) and DEX (0.5 mg/kg/d, i.p.) concomitantly for 2 weeks.11) Groups 6–8; rats received MTX (14 mg/kg/week, p.o.) and concomitantly treated for 2 weeks with HT (10, 20, 40 mg/kg/d, p.o.), respectively.12,13)
Sample PreparationRats were sacrificed by decapitation under ether anesthesia, at the end of the experiment, and their lungs were promptly excised, washed with ice-cold saline, blotted dry, and weighed. A part of each lung was homogenized in phosphate buffer saline (PBS) (10% (w/v)), centrifuged (4000 rpm, 4 °C) for 15 min, and supernatants were frozen at −80 °C for further assessment of the oxidative stress biomarker (8-hydroxy-2-deoxy-guanosine; 8-OHdG), cytokines (IL-4, IL-8, and TGF-β1), TXA2 and hydroxyproline. Another part of the lung was processed for tissue factor gene expression. In addition, the third part from the lung was fixed in 10% formalin–saline and processed for histopathological examination using hematoxylin–eosin (H&E) stain and Masson's trichrome stain for detection of % fibrosis beside immunofluorescence assay for detection of the fibrin and tissue factor deposition in lung tissue.
Biochemical InvestigationsAssessment of Fibrotic Biomarkers in LungRat IL-4 and TGF-β1 were assessed according to the enzyme-linked immunosorbent assay (ELISA) kit manufacturer guide (MyBioSource®, San Diego, CA, U.S.A.; Catalog Nos. MBS494192 and MBS175833, respectively).
Estimation of Oxidative Stress in Lung8-OHdG is potentially the best non-invasive biomarker of oxidative damage to DNA. It was estimated in tissue homogenate according to the manufacturer’s instructions stated by MyBioSource®, (Catalog No: MBS269902) named (8-OHdG) ELISA Kit.
Assessment of Angiogenesis Biomarkers in LungAngiogenesis Biomarkers, VEGF and IL-8, were assessed according to the ELISA kit manufacturer guide (MyBioSource®; Catalog Nos. MBS2514825 and MBS262974, respectively).
Estimation of Pulmonary Collagen ContentHydroxyproline content of lung tissue was estimated in tissue homogenate according to the HYP ELISA kit manufacturer guide (MyBioSource®; Catalog No. MBS017427).
Assessment of Coagulation Biomarker in LungRat TXA2 was assessed according to the ELISA kit manufacturer guide (MyBioSource®; Catalog No. MBS2883902)
Quantitative RT-PCR for Tissue FactorTotal RNA was isolated from lung tissue using the RNeasy® Mini Kit. The isolated RNA was reverse transcribed into cDNA using a QuantiTect SYBR® Green PCR Kits as directed by the manufacturer. To analyse the expression of the target genes, quantitative real-time PCR was done using SYBR green PCR Master mix (Qiagen, Germany) according to the manufacturer's instructions. In a final reaction volume of 50 µL, 25 µL of QuantiTect SYBR Green PCR Master Mix, 22.5 µL dH2O, 2 µL primer pair mix (5 pmol/µL per primer), and 0.5 µL cDNA were added. Table 1 shows the primer sequences for the housekeeping gene.14,15) PCR reactions were run for 10 min at 95 °C to activate AmpliTaq DNA polymerase, then 40 cycles at 95 °C for 15 s (denaturing) and 60 °C for 1 min (annealing/extension). The results were presented in cycle threshold (Ct), which is the point at which the increasing fluorescence curve crosses a threshold value. The comparative Ct (ΔΔCt) method was used to determine the relative expression of the target gene. The ΔCt was obtained by subtracting the Ct of the tissue factor from that of the target gene, while ΔΔCt was acquired by subtracting the reference sample ΔCt (internal control) from that of the test sample. The 2−ΔΔCt was used to compute the relative expression ratios.16)
Lung tissues were harvested from different treatment groups, fixed in 10% formalin saline for 24 h and washed with tap water. Serial dilutions of alcohol (methyl, ethyl, and absolute ethyl) were used for dehydration. In xylene, specimens were cleared and embedded in paraffin for 24 h at 56 °C in a hot air oven. Paraffin beeswax tissue blocks were prepared for sledge microtome sectioning at a thickness of 4 microns. On glass slides, the obtained tissue sections were collected, deparaffinized, and stained by hematoxylin & eosin stain or Masson’s trichrome stain for examination through the light electric microscope.17)
The histopathological changes (peribronchiolar, perivascular and interalveolar fibrosis, collapse and emphysema of air alveoli) were scored from 1–5. Masson’s trichrome staining of slides from 6 rats per group was performed to quantify pulmonary fibrosis. From each slide, six representative images were obtained at 40×, and the area of the green-stained collagen fibers in the lung was measured by the Image J software.18)
Immunofluorescence AnalysisThe immunofluorescence staining was carried out as previously described.19) In brief, the tissue sections were deparaffinized in xylene and rehydrated in a series of ethanol strengths. Dako solution (0.01 M sodium citrate buffer, pH 6; Dako, CA, U.S.A.) was used to extract antigen in a microwave oven at 500 Watts for 20 min. After cooling, slices were washed for 9 min in PBST (0.05% tween 20 in PBS pH 7.4). Tissues were blocked with blocking buffer (PBS containing 1% bovine serum albumin (BSA) and 10% horse serum) at room temperature for 1 h after being fixed with p-formaldehyde (3.7% for 10 min). The primary antibodies; Anti-TF mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., OR, U.S.A.; Catalogue No. TF (H-9): sc-374441) and polyclonal rabbit Anti-Human fibrinogen antibody (Dako; Catalogue No. F 0111) were incubated in a concentration of 1 : 300 overnight at 4 °C. Following washing, tissue antigens were detected for 30 min using goat anti-mouse Alexa fluor 488 or goat anti-rabbit Cy3 secondary antibodies. The counterstain was carried out using 40,60-diamidino-2-phenylindole (DAPI) and rinsed with PBST for 30 min.
Finally, tissue slices were coated with Fluoromount G and examined using a Leica DM5000 B fluorescent microscope (Leica, Wetzlar, Germany). ImageJ/NIH software was used to measure the fluorometric intensity of at least 5 microscopic fields in each slice (minimum of two animals in each group).
Statistical AnalysisThe data were presented as mean ± standard deviation (S.D.). To evaluate statistical significance among groups, a one-way ANOVA was utilized, followed by a Tukey multiple comparisons post-test. The p ≤ 0.05 was used to determine whether or not a difference was significant. Furthermore, the Kruskal–Wallis test (non-parametric one-way ANOVA) was used to analyze the assigned scores in the histopathological investigation, followed by the Dunn's post hoc test. The GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA, U.S.A.) was used for statistical analysis and graph construction.
Our data demonstrated that administration of MTX significantly increased profibrotic cytokines like TGF-β1 (nearly 8 folds) and IL-4 (5 folds) in lung homogenate as compared to the normal control group. However, DEX markedly reduced TGF-β1 and IL-4 by nearly 59 and 50%, respectively as compared to MTX group. Moreover, HT (10, 20, and 40 mg/kg/d) significantly mitigated the fibrotic effect of MTX on profibrotic biomarkers and significantly decreased the elevated TGF-β1 (64, 65, and 77%, respectively) and IL4 (68, 69, and 70%, respectively) as compared to MTX group (Table 2).
| Group | Control | MTX | DEX | HT40 | MTX + DEX | MTX + HT10 | MTX + HT20 | MTX + HT40 |
|---|---|---|---|---|---|---|---|---|
| Parameters | ||||||||
| TGF-β1(pg/g tissue) | 26.7 ± 1 | 233.7 ± 1.4 a) | 34 ± 1.9 b) | 20.5 ± 1.3 b) | 95.1 ± 2.2 a,b) | 83.6 ± 2.4 a,b,c) | 82 ± 2 a,b,c) | 53.6 ± 2.2 a,b,c,d) |
| IL-4(pg/g tissue) | 3.7 ± 0.4 | 22.3 ± 0.8 a) | 4.4 ± 0.79 b) | 3.8 ± 0.5 b) | 11.2 ± 0.75 a,b) | 7.24 ± 0.8 a,b,c) | 7 ± 0.6 a,b,c) | 6.9 ± 0.5 a,b,c) |
| Hydroxyproline (µg/g tissue) | 4.4 ± 0.8 | 28.9 ± 0.9 a) | 4.4 ± 1 b) | 4.6 ± 1.1 b) | 15.8 ± 1.6 a,b) | 12.8 ± 1.6 a,b,c) | 8.6 ± 1.1 a,b,c,d) | 7.4 ± 1.1 a,b,c,d) |
- Data were analyzed by one-way ANOVA, followed by Tukey’s multiple comparison test. -Each bar represents the mean of 7 animals ± S.D. a) Significantly different from control group; b) Significantly different from MTX group; c) Significantly different from MTX + DEX group; d) Significantly different from MTX + HT10 group at p < 0.05. DEX, dexamethasone; HT, hydroxytyrosol; MTX, methotrexate; IL-4, interleukin-4; TGF-β1, transforming growth factor beta 1.
Our results revealed that MTX induced a substantial rise in collagen production, as reflected by a nearly 5-folds increase in hydroxyproline content in the lung homogenate relative to the normal control group. On the opposite side, DEX (0.5 mg/kg/d) or HT (10, 20, and 40 mg/kg/d), markedly hampered hydroxyproline content by approximately 45, 55, 70, or 75%, respectively, as compared to MTX group (Table 2).
Effects of Hydroxytyrosol on Angiogenesis Biomarkers in MTX-Treated RatsAngiogenesis (a crucial sign of fibrosis) was initiated in rats after MTX administration as represented by a significant increase in VEGF (around 1.5 times) and IL-8 (almost 7 times) in the lung homogenate as compared to the normal control group. On the other hand, treatment with DEX or HT (10, 20, and 40 mg/kg/d) significantly reduced the levels of VEGF (30, 30, 46, and 54%, respectively) and IL-8 (59, 58, 70, and 76%, respectively) as compared to MTX (positive control) group (Table 3).
| Group | Control | MTX | DEX | HT40 | MTX + DEX | MTX + HT10 | MTX + HT20 | MTX + HT40 |
|---|---|---|---|---|---|---|---|---|
| Parameters | ||||||||
| IL-8 (pg/g tissue) | 24.3 ± 2.3 | 190.9 ± 1.7 a) | 27.7 ± 1.8 b) | 25.2 ± 1 b) | 78.3±1.9 a,b) | 78.9 ± 2.2 a,b,c) | 57.9 ± 2.3 a,b,c) | 46.4 ± 2.5 a,b,c) |
| VEGF (pg/g tissue) | 37.7 ± 2.8 | 94.3 ± 5.6 a) | 37.2 ± 5.2 b) | 38.5 ± 6.7 b) | 65.4 ± 7.3 a,b) | 65.7 ± 5.9 a,b) | 51 ± 4.5 a,b,c,d) | 43 ± 5 b,c,d) |
- Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. -Each bar represents the mean of 7 animals ± S.D. a) Significantly different from control group; b) Significantly different from MTX group; c) Significantly different from MTX + DEX group; d) Significantly different from MTX + HT10 group at p < 0.05. DEX, dexamethasone; HT, hydroxytyrosol; MTX, methotrexate; IL-8, interleukin-8; VEGF, vascular endothelial growth factor.
Rats treated with MTX showed elevated oxidative stress manifested by a significant increase in 8-OHdG (252%) in lung homogenate in comparison with the group of normal control. In contrast, treatment with DEX or HT (10, 20, and 40 mg/kg/d) significantly attenuated oxidative stress caused by MTX (62, 65, 71, and 72, respectively) as compared to MTX treated group (Fig. 1).
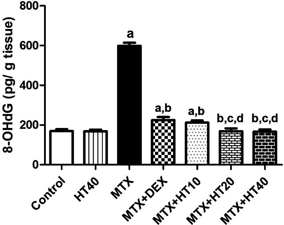
-Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. -Each bar represents the mean of 7 animals ± S.D. a Significantly different from control group; b Significantly different from MTX group; c Significantly different from MTX + DEX group; d Significantly different from MTX + HT10 group at p < 0.05. -DEX, dexamethasone; HT, hydroxytyrosol; MTX, methotrexate; 8-OHdG, 8-hydroxy-2'-deoxyguanosine.
Our data demonstrated that administration of MTX significantly increased TXA2 (around 9 folds) in lung homogenate as compared to the normal control group. However, DEX and HT (10, 20, and 40 mg/kg/d) significantly decreased the elevated TXA2 (56, 68, 79, and 85%, respectively) as compared to MTX group (Fig. 2).

-Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. -Each bar represents the mean of 7 animals ± S.D. a Significantly different from control group; b Significantly different from MTX group; c Significantly different from MTX + DEX group; d Significantly different from MTX + HT10 group; e Significantly different from MTX + HT20 group at p < 0.05. -DEX, dexamethasone; HT, hydroxytyrosol; MTX, methotrexate; TXA2; Thromboxane A2
In the normal control group, immunofluorescence examination of lung TF and fibrin revealed no or basal expressions for both proteins. On the other hand, treatment with MTX significantly increased peribronchiolar, perivascular and interalveolar tissue expression of TF and fibrin. In contrast, DEX or HT (10, 20, and 40 mg/kg/d), treatment in MTX-treated rats showed weak TF and fibrin protein expressions as compared to MTX alone (Fig. 3)

(A) Immunofluorescence staining of fibrin deposition and (C) tissue factor in pulmonary cells of normal rats and MTX (14 mg/kg/week, for 2 weeks), with or without HT40. (B, D) Quantitative image analysis of immunofluorescence staining expressed as fluorescence intensity was obtained from five fields in each rat section (minimally three rats in each group) using Image J software. a Significantly different from control group; b Significantly different from MTX-treated group using one-way ANOVA, followed by Tukey test for multiple comparison test at p < 0.05. -DEX, dexamethasone; HT, hydroxytyrosol; MTX, methotrexate; TF, tissue factor.
MTX induction of lung fibrosis resulted in a dramatic increase in tissue factor gene expression by almost 17 times as compared to the normal control group. In contrast, treatment with DEX and HT (10, 20, and 40 mg/kg/d) diminished MTX effect on gene expression shown as a marked reduction in tissue factor (40, 62, 85, and 92%, respectively) as compared to MTX group (Fig. 4).

-Data were analyzed by one-way ANOVA, followed by Tukey’s multiple comparison test. -Each bar represents the mean of 7 animals ± S.D. a Significantly different from control group; b Significantly different from MTX group; c Significantly different from MTX + DEX group; d Significantly different from MTX + HT10 group; e Significantly different from MTX + HT20 group at p < 0.05. -DEX, dexamethasone; HT, hydroxytyrosol; MTX, methotrexate; TF, tissue factor.
Histopathological alterations of lung tissues are demonstrated in Figs. 5 and 6. The histopathological investigation of lung sections (H&E; 40×) of the control group displayed a normal histological construction of the bronchioles and surrounding air alveoli (Fig. 5A). However, MTX-treated rats revealed deteriorating changes as manifested by fibrosis with collagen proliferation in the peribronchiolar, perivascular and interalveolar tissue with collapse and emphysema of the air alveoli (Fig. 5B). Treatment of rats with DEX (Fig. 5C) or HT (40 mg/kg/d) (Fig. 5D) clearly alleviated the histopathological alterations induced by MTX.

The HT40 treatment lessens MTX-induced histopathological changes and collagen content in rats’ fibrotic lungs. Histological sections of lung tissue were stained with H&E (A–D) and Masson trichrome (E–H). The images were captured under a light microscope. Original magnification, ×40. (A, E) Lung tissue sections of control animals showing normal histological structure of the bronchiole (b), blood vessels (b.v.) and air alveoli (a); thin lined interalveolar septa with well-organized alveolar space; (B, F) lung tissue sections of MTX-treated animals showed distorted lung morphologies: peribronchiolar, perivascular & interalveolar fibrosis (black arrows) with collapse in air alveoli (blue arrow heads) and emphysema in others, wider and thickened interalveolar septa, showing dense collagen accumulations (red arrows); (C, G) lung tissue sections of MTX + DEX-treated animals: showing normal histological structure; (D, H) lung tissue sections of MTX + HT40-treated animals: showing normal histological structure. (I) Quantitative analysis of ECM deposition was obtained from five fields in each rat section (minimally three rats in each group) using the Image J software, values are expressed as mean ± S.D., a Significantly different from control group; b Significantly different from MTX group, using one-way ANOVA, followed by the Tukey test for multiple comparison at p < 0.05. ANOVA, analysis of variance; DEX, dexamethasone; HT, hydroxytyrosol; ECM, extracellular matrix; MTX, methotrexate.

Histopathological alteration scores (represented as median with the interquartile range), a Significantly different from control group, b Significantly different from MTX group, using Kruskal–Wallis test (non-parametric one-way ANOVA), followed by Dunn post-hoc test for the multiple comparisons between the groups at p < 0.05.
Staining of lung autopsy samples collected from MTX fibrotic group with Masson’s trichrome showed a large increase in extracellular matrix deposition in the peribronchiolar tissue. However, sections obtained from MTX + DEX and MTX + HT40-treated rats displayed a lower deposition of extracellular matrix (Figs. 5E–H).
When alveolar epithelial damage occurs, coagulation and inflammatory cascades begin. Then, profibrotic cytokines magnify, and, as a result, ECM depositions enhance, disseminating to fibrotic lesions. Additionally, damaged epithelial cells release a wide range of growth factors and cytokines.20,21) Accordingly, mesenchymal cell activation, proliferation, and migration occur; and it is proposed that this is an end-stage process responsible for probable organ failure and malfunction.22) Furthermore, vascular remodeling is a distinctive process in pulmonary fibrosis, where angiogenesis takes place and causes cellular structure changes.23) The coagulation cascade orchestrates inflammatory and tissue healing responses through the formation of fibrosis, where tissue factor may act as a key element.24)
In addition, pulmonary fibrosis has multifactorial mechanisms, including inflammatory response, oxidative stress, and coagulation cascade activation.25) In the same context, it has been reported that HT has various pharmacological activities, such as anti-inflammatory, antioxidant, and antineoplastic effects.26) This study assesses the possible protective effect of HT on lung fibrosis induced by MTX. Moreover, hydroxytyrosol possesses potent anti-inflammatory and antioxidant effects and is abundantly available in the Mediterranean diet which includes many types of fruits and vegetables.27)
Reactive oxygen species can be major mediators of the breakdown of cellular structures, proteins, nucleic acids, and lipids as a result of oxidative alteration of macromolecules and enzyme deactivation. This, in turn, leads to aberrant cell activities.28) Thus, a rising number of scientists have recommended using antioxidants to reduce the inflammatory response caused by ROS in animals. Our data reveal that the administration of HT reduces oxidative damage as reflected when decreasing pulmonary content of 8-OHdG in MTX-damaged lung in a dose-dependent manner. Interestingly, Fuccelli et al.12) have reported that HT effectively exerts protective activity against lipopolysaccharide (LPS)-induced systemic inflammation and oxidative damage. In addition, Bernini et al.28) have mentioned that HT employs its antioxidant effects via direct (with redox activity) and indirect (regulation of antioxidant enzymes) mechanisms. Besides, hydroxytyrosol could regulate antioxidant enzymes through its ability to activate the nuclear factor-E2-related factor-2/antioxidant responsive element (Nrf2/ARE) pathway in vitro, leading to the increased transcription of both antioxidant enzymes (e.g., Cu/ZnSOD, MnSOD, peroxiredoxin 3 and 5, thioredoxin-2) and phase II detoxifying enzymes (e.g., glutathione (GSH) S-transferase, nicotinamide adenine dinucleotide (NAD)(P) H: quinone oxidoreductase 1 or GSH reductase).
Previous studies have also indicated that the most potent factor in fibroblast-myofibroblast transformation is TGF-β1, which, at low levels, triggers the proliferation of fibroblast. Despite the fact that normal wound healing is regulated by extensive interactions between pro-fibrotic and antifibrotic cytokines, chemokines, and other cell mediator proteins, tissue fibrosis is considered an unregulated and confused injury repair procedure.29,30) Data from our investigation show that HT treatment has reduced pulmonary collagen accumulation, as evidenced by a significant drop in lung hydroxyproline content, as well as decreased pulmonary contents of IL-4 and TGF-β1 in MTX-induced lung fibrosis. In line with these positive results, MTX treatment has improved histopathological scores in a dose-dependent pattern. These data are aligned with Razali et al.,31) who stated that HT has provided protection against SMAD and AKT pathway activation in the TGFβ1-induced epithelial–mesenchymal transition (EMT) respiratory epithelial cells (RECs).
In the normal track of the wound healing process, the angiogenesis process occurs under control. In contrast, in pulmonary fibrosis, it seems to be uncontrolled and causes severe lung architectural epithelium changes, where VEGF and IL-8 -angiogenic cytokines- trigger the angiogenesis process.32) Zhao et al.33) have mentioned that HT exerts anti-angiogenic effects, which are manifested by reduction of VEGF, because of HT ability to decrease the expression of NF-κB p65 gene. This, in turn, has reduced the production of NF-κB-regulated gene products such as VEGF.
Coagulation cascade activation is a normal response during the tissue repair process. However, it has a critical role in pulmonary fibrosis.34,35) Tissue factor is one of the most vital key players in the coagulation cascade. It has been reported that inhibition of tissue factor could mitigate nephrotic fibrosis.36) In the same context, our findings show that HT has significantly reduced TF gene expression and pulmonary content. Besides, HT notably decreased TXA2. This is compatible with the findings of Hu et al.37) who have mentioned that HT decreases platelets aggregation via quenching eicosanoid production, and, accordingly, hinders TXA2 production. Furthermore, compatible with these effects, treatment with HT has caused a significant reduction in fibrin deposition in lung tissue. These findings are consistent with those of D’Angelo et al.38) who have found that HT shows fibrinolytic activity and reduced platelet aggregation.
In addition, methotrexate-induced tissue factor expression is not routinely expressed by cells. Tissue factor is the primary initiator of the coagulation cascade, converting FX to FXa and activating prothrombin (FII) to form thrombin (FIIa).39,40) Thrombin stimulates clot development via encouragement of the activation of platelets, and the production of fibrin, which polymerizes into actin fibers; this causes the platelets to become sticky and seal off the leaking. The actin fibers mechanically stabilize the platelet clog.34) Excessive or uncontrolled clot development results in thrombosis, which prevents blood supply that nourishes and oxygenates cells, resulting in cell death.35)
In the present investigation, HT has dramatically reduced TF expression and fibrin deposition in pulmonary cells, preserving the natural structure and characteristics of the lung. These findings are supported by histological, biochemical, and other pulmonary indicators that show an improvement in lung tissue structure and function. Consequently, HT is crucial in preventing the activation of coagulation, which, in turn, prevents fibrin accumulation, inflammation, and tissue damage. Nonetheless, the empirical results reported herein should be considered in the light of a specific limitation which is studying the different parameters of pulmonary fibrosis affected by HT. Further studies should be done for more investigation of the molecular mechanism of HT on tissue factor-VEGF axis. In conclusion, HT has promising anti-inflammatory, antioxidant, and antifibrotic activities. These activities may explain its ability to mitigate MTX-induced pulmonary fibrosis in rats.
We are deeply thankful to Prof. Dr. Adel B. Khelosy, Department of Pathology, Faculty of Veterinary Medicine, Cairo University, and Mohamed G. Ewees, assistant lecturer, Department of Pharmacology & Toxicology, Al Azhar Faculty of Pharmacy, Cairo; for their kind help in histopathological and immunofluorescence investigation, respectively.
Mohamed F. Manie: Conceptualization, Methodology, Formal analysis, Visualization, Data curation, Writing–original draft.
El-Sayed M. El-Sayed: Conceptualization, Methodology, Formal analysis, Visualization, Data curation, Writing–original draft, Writing–review & editing.
Hala M. Fawzy: Conceptualization, Methodology, Writing–review & editing.
The authors declare no conflict of interest.
All data and materials can be freely obtained from the authors through correspondence.