2024 Volume 47 Issue 1 Pages 166-174
2024 Volume 47 Issue 1 Pages 166-174
Neuropilin-1 (NRP1), a transmembrane glycoprotein, plays an important role in the malignant progression of gliomas; however, its role in chemoresistance is not fully understood. In this study, we observed the effects of NRP1 on the stemness and chemoresistance of glioma cells and the mediating role of Yes-associated protein (YAP). We constructed NRP1 overexpressing LN-229 glioma cells. Cells were treated with recombinant NRP1 protein (rNRP1) and the YAP inhibitor Super-TDU when necessary. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to detect the sensitivity of cells to temozolomide (TMZ). Sphere and clone formation assays were performed to detect the sphere- and clone-forming abilities of cells. Western blotting was performed to detect cellular CD133, CD44, p-LATS1, and p-YAP protein expression. Immunofluorescence and flow cytometry were used to detect the subcellular localization of YAP and apoptosis, respectively. We found that both NRP1 overexpression and rNRP1 treatment enhanced self-renewal, TMZ resistance, and CD133 and CD44 protein expression in LN-229 cells. NRP1 overexpression and rNRP1 treatment also induced LATS1 and YAP dephosphorylation and YAP nuclear translocation. Super-TDU inhibits NRP1 overexpression-induced enhanced self-renewal and TMZ resistance in LN-229 cells. Our study suggests that NRP1 induces increased stemness in glioma cells, resulting in chemoresistance, and that this effect is associated with YAP activation.
Gliomas originate from glial and neuronal cells and are the most common primary intracranial malignant tumors.1) According to the WHO pathological classification, gliomas can be classified into grades I–IV; patients with grade I–II gliomas have a better prognosis, while those with grades III–IV have a worse prognosis.2) Clinical treatments for gliomas include surgery, radiotherapy, and chemotherapy, and some patients also receive targeted therapy.3) Chemotherapy is an essential means of prolonging the survival of glioma patients, especially the application of the alkylating agent temozolomide (TMZ), which not only prolongs patient survival but improves their QOL to some extent.4) However, chemotherapeutic agents are only applied for a period before tumor cells eventually acquire resistance, ultimately leading to treatment failure.5) Therefore, resolving chemotherapy resistance is the key to the clinical treatment of gliomas.
Glioma stem cells have tumor-initiating roles, which account for a relatively small proportion of the glioma cell population, but have self-renewal and multidirectional differentiation potential.6,7) Modern biological studies have shown that glioma stem cells have a role in inducing tumor angiogenesis,8) invasion,9) and metastasis.10) Studies have confirmed that an increased proportion of stem cells in the glioma cell population, i.e., enhanced stemness, is closely associated with cellular chemoresistance.11,12) Yes-associated protein (YAP) is a downstream signaling molecule of the Hippo signaling pathway and its activity is inhibited by LATS1/2 phosphorylation.13,14) When LATS1/2 is in a non-phosphorylated state, YAP undergoes dephosphorylation and enters the nucleus to participate in the regulating malignant cell behavior.13,14) Several studies have shown that YAP is an influential factor in tumor cell stemness and may be involved in regulating stem cell properties in various malignant tumors, including gliomas.15,16) Neuropilin-1 (NRP1) is a type I transmembrane glycoprotein that is overexpressed in gliomas and is associated with poor prognosis.17) NRP1 is also an important regulator of tumor cell stemness and can affect cell self-renewal capacity.18) Studies have confirmed that NRP1 is a critical molecule in regulating the invasive capacity of glioblastoma stem-like cells in vitro.19) It has also been found that NRP1 is associated with TMZ resistance in glioblastoma.20) However, whether NRP1 regulates the stemness and chemoresistance of glioma cells through YAP is unclear.
LN-229 cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. LN-229 cells were treated with NRP1 recombinant protein (rNRP1; MedChem Express, Monmouth Junction, NJ, U.S.A.), TMZ (MedChem Express), or the YAP inhibitor Super-TDU (MedChem Express) for 24 or 72 h, according to experiment. TMZ, rNRP1, and Super-TDU were prepared as 100 mM, 300 mg/mL, and 10 mM master mixes with dimethyl sulfoxide, respectively, and stored at −80 °C.
NRP1 Plasmid TransfectionThe plasmid containing NRP1 was constructed by Hoyuan Biotech (Shanghai) (China). LN-229 cells were transfected using Lipofectamine 3000 (Absin, China), according to the manufacturer’s instructions.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) AssayCells inoculated in 96-well plates were incubated for 4 h with 20 µL MTT (5 mg/mL) after treatment with the compound as indicated. The culture solution in the well plate was aspirated and 150 µL of dimethyl sulfoxide was added to dissolve the crystals. The plates were placed in an iMark enzyme marker (Bio-Rad, Hercules, CA, U.S.A.) and the absorbance was read at 570 nm.
Sphere Formation AssayStem cell spheres were prepared in DMEM/F12 containing 20 ng/mL epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and 2% B27. LN-229 cells were cultured in stem cell spheres for 9 d in ultra-low adsorption plates after treatment. The formation of cell spheres was observed and photographed using an IX53 inverted microscope (Olympus Corporation, Japan).
Clone Formation AssayLN-229 cells were inoculated into 6-well plates at 300 cells/well, treated as indicated, and cultured for 14 d. The culture medium was aspirated and cells were fixed and stained with 4% paraformaldehyde and 0.1% crystal violet staining solution (Solarbio Life Science, China) for 10 min. The staining solution was washed away with phosphate-buffered saline (PBS), and photographs of clone formation in each well were taken with an IX53 inverted microscope.
Western BlottingCells were lysed with lysis buffer (Solarbio Life Science), followed by centrifugation at 13000 × g for 20 min at 4 °C. The supernatant was subjected to protein quantification using a bicinchoninic acid assay kit (Solarbio Life Science) and prepared as a protein sample containing 25 µg/10 µL. Total protein was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, U.S.A.). The PVDF membrane was blocked with 5% skimmed milk for 4 h, followed by overnight incubation with primary antibodies against NRP (Catalog Number: 3725S, 1 : 1000), CD133 (Catalog Number: 64326S, 1 : 1000), CD44 (Catalog Number: 3570S, 1 : 1000), LATS1 (Catalog Number: 3477S, 1 : 1000), YAP (Catalog Number: 14074S, 1 : 1000), p-LATS1 (Catalog Number: 8654S, 1 : 1000), p-YAP (Catalog Number: 13008S, 1 : 1000), β-actin (Catalog Number: 4967S, 1 : 2000), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Catalog Number: 2118S, 1 : 2000) (Cell Signaling Technology, Danvers, MA, U.S.A.). The following day, primary antibodies were conjugated with anti-mouse (Catalog Number: 7076S, 1 : 3000) or anti-rabbit (Catalog Number: 7074S, 1 : 3000) immunoglobulin G (IgG)-horseradish peroxidase (HRP) secondary antibodies (Cell Signaling Technology). Protein bands were imaged using an enhanced chemiluminescence (ECL) luminescent solution (Millipore).
ImmunofluorescenceCells were fixed with 4% paraformaldehyde (Solarbio Life Science) for 15 min and subsequently permeabilized with 0.5% Triton X-100 (Solarbio Life Science) for 20 min. Cells were blocked in an immunocytostaining blocking solution (Solarbio Life Science) for 30 min and incubated with primary antibody at 4 °C overnight. Nuclei were stained with a fluorescent secondary antibody conjugated to the primary antibody for 1 h, followed by incubation with 4′,6-diamidino-2-phenylindole (Solarbio Life Science) for 5 min. The slices were sealed with an antifluorescence quenching solution and the acquired images were observed under an IX-71 fluorescence microscope (Olympus Corporation).
Flow CytometryCells were collected in flow tubes and centrifuged to remove the culture medium. Cells were resuspended with 200 µL Binding Buffer (Solarbio Life Science), and 5 µL Annexin V-fluorescein isothiocyanate (FITC) (Solarbio Life Science) was added and incubated for 10 min at room temperature, away from light. The cell suspension was centrifuged and the liquid was removed, and 200 µL Binding Buffer was added to resuspend the cells. Finally 5 µL of propidium iodide (Solarbio Life Science) was added to the suspension. Apoptosis was detected using the CytoFLEX LX flow cytometer (Beckman Coulter, Brea, CA, U.S.A.).
Statistical AnalysisEach experiment was performed at least three times independently and data were expressed as mean ± standard deviation. Statistical analysis between two groups was performed using the Student’s t-test. Statistical differences between multiple groups were analyzed using one-way ANOVA followed by the Bonferroni post-hoc test. p < 0.05 was considered a statistically significant difference.
We exogenously transfected the NRP1 plasmid into LN-229 cells and successfully overexpressed the NRP1 protein (Fig. 1A). MTT assay results showed that the sensitivity of cells to TMZ was reduced after transfection with the NRP1 plasmid (Fig. 1B). The sensitivity of LN-229 cells to TMZ was also reduced after pretreatment with rNRP1 (Fig. 1C).

(A) LN-229 cells were transfected with NRP1 plasmid and Vector. Western blotting detected NRP1 protein expression. ** p < 0.0001 vs. Vector. Sample size, n = 3. (B) The MTT method was used to detect the sensitivity of cells to TMZ (75–2000 µM) in each group. ** p = 0.0049 vs. Vector. Sample size, n = 3. (C) LN-229 cells were treated with rNRP1 (75, 150, 300 ng/mL) for 24 h. The sensitivity of cells to TMZ was detected using an MTT assay. * p = 0.0483, ## p = 0.0021, && p < 0.0001 vs. Control. Sample size, n = 3.
Using a sphere formation assay, we found that either transfection with the NRP1 plasmid or treatment with rNRP1 enhanced the sphere formation ability of LN-229 cells (Figs. 2A, B). We also used a clone formation assay and found that transfection of the NRP1 plasmid or rNRP1 treatment enhanced LN-229 cell clone formation ability (Figs. 2C, D). This suggests that NRP1 may be involved in regulating glioma cell stemness.

LN-229 cells were transfected with (A) NRP1 plasmid or (B) rNRP1 treatment for 24 h, and the number and diameter of cell spheres were measured after 7 d of incubation using stem cell sphere culture medium. LN-229 cells were transfected with (C) NRP1 plasmid or (D) rNRP1 treatment for 24 h, and a plate cloning assay was performed to detect cell clone formation ability. ** p = 0.0002, && p = 0.0018 vs. Vector. ## p < 0.0001, $$ p = 0.0047 vs. Control. Sample size, n = 3.
To further investigate the effect of NRP1 on glioma cell stemness, we first treated LN-229 cells with rNRP1 and observed the changes in stem cell marker expression. The results showed that rNRP1 treatment increased the expression of cell stemness markers CD133 and CD44 (Fig. 3A). Upregulation of CD133 and CD44 expression was also observed in LN-229 cells transfected with the NRP1 plasmid (Fig. 3B). Immunofluorescence assays to detect CD133 and CD44 expression in the cell spheres revealed similar results (Fig. 3C).

(A) LN-229 cells were treated with rNRP1 (75, 150, 300 ng/mL) for 24 h. Western blotting detected CD133 and CD44 protein expression. CD133: * p = 0.0104, ** p < 0.0001 vs. Control. CD44: * p = 0.0107, ** p = 0.0002, ## p < 0.0001 vs. control, Sample size, n = 3. (B) LN-229 cells were transfected with NRP1 plasmid and Western blotting detected CD133 and CD44 protein expression. ** p < 0.0001, ## p < 0.0001 vs. vector. Sample size, n = 3. (C) Cells in each group were cultured with stem cell sphere culture medium for 7 d, and CD133 and CD44 protein expression in cell spheres was detected by immunofluorescence.
We observed proteins related to the Hippo/YAP signaling pathway and found that either rNRP1 treatment or transfection of the NRP1 plasmid downregulated the phosphorylation levels of LATS1 and YAP in LN-229 cells (Figs. 4A, B). Immunofluorescence revealed that transfection with the NRP1 plasmid increased the nuclear distribution of YAP (Fig. 4C).

(A) LN-229 cells were treated with rNRP1 (75, 150, 300 ng/mL) for 24 h and Western blotting detected p-LATS1 and p-YAP protein expression. ** p < 0.0001 vs. vector. Sample size, n = 3. (B) LN-229 cells were transfected with NRP1 plasmid and Western blotting detected p-LATS1 and p-YAP protein expression. * p = 0.0006, ** p < 0.0001 vs. Control. Sample size, n = 3. (C) LN-229 cells were transfected with NRP1 plasmid and YAP subcellular distribution was detected by immunofluorescence.
To confirm the role of YAP in the NRP1-induced self-renewal of glioma cells, we treated LN-229 cells transfected with the NRP1 plasmid with the YAP inhibitor Super-TDU and observed changes in cellular sphere and clone formation ability. The results showed that the enhanced sphere and clone formation induced by NRP1 overexpression was inhibited by super-TDU treatment (Figs. 5A, B). Moreover, upregulation of CD133 and CD44 protein expression induced by NRP1 overexpression was inhibited by Super-TDU (Fig. 5C).

LN-229 cells transfected with NRP1 plasmid were treated with the YAP inhibitor Super-TDU (5 µM), and cell spheroidogenicity and plate cloning assays were performed to detect (A) cell spheroidogenicity and (B) clone formation, respectively. Sample size, n = 3. (C) LN-229 cells transfected with NRP1 plasmid were treated with YAP inhibitor Super-TDU (5 µM), and CD133 and CD44 protein expression was detected by Western blotting. ** p < 0.0001 vs. Vector; ## p < 0.0001 vs. NRP1 plasmid. Sample size, n = 3.
Flow cytometry results revealed that both apoptosis and proliferation inhibition induced by TMZ were reduced in LN-229 cells transfected with the NRP1 plasmid (Figs. 6A, B). Super-TDU treatment of LN-229 cells transfected with NRP1 plasmid enhanced apoptosis and proliferation (Figs. 6A, B).
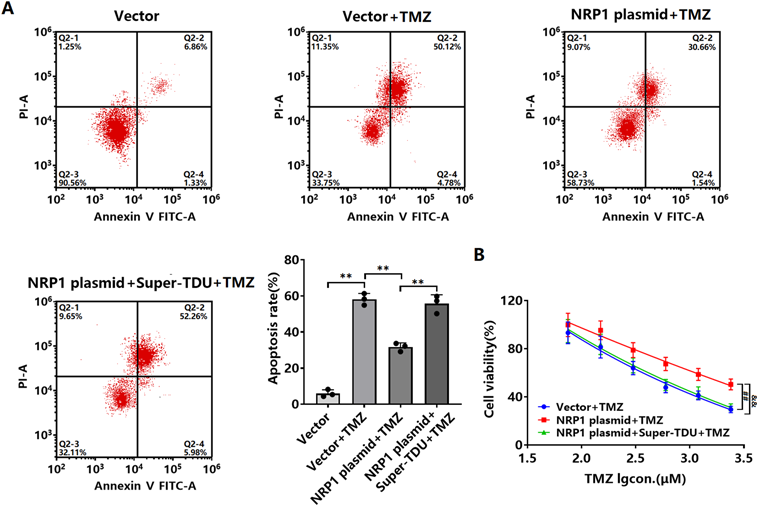
LN-229 cells transfected with NRP1 plasmid were treated with Super-TDU. (A) Apoptosis was detected by flow cytometry and (B) cell viability was detected by MTT assay. ** p < 0.0001 vs. Vector; ** p < 0.0001 vs. vector + TMZ; ** p < 0.0001 vs. NRP1 plasmid + TMZ. ## p = 0.0004 vs. vector + TMZ; && p = 0.0005 vs. NRP1 plasmid + TMZ. Sample size, n = 3.
NRP1, a receptor specific for semaphorin 3 and vascular endothelial growth factor (VEGF) 165, plays a vital role in regulating the nervous system.21) Under pathological conditions, NRP1 is an essential protein involved in the malignant progression of gliomas. Hepatocyte nuclear factor 4γ promotes glioma cell proliferation by inducing NRP1 transcription, while increased NRP1 transcription reduces apoptosis.22) Overexpression of NRP1 enhances autocrine hepatocyte growth factor/scatter factor (HGF/SF)/c-Met signaling activation, thus increasing the proliferative viability of glioma cells.23) NRP1 interacts with G-alpha interacting protein/regulator of G-protein signaling 19-interacting proteins, thereby enhancing glioma cell proliferation and invasion.24) However, the biological role of NRP1 in glioma is not fully understood. We found increased resistance to TMZ in glioma LN-229 cells following exogenous overexpression of NRP1. In addition, LN-229 cells treated with recombinant NRP1 protein similarly showed increased resistance to TMZ. This suggested that NRP1 induces TMZ resistance in glioma cells. Studies have reported that soluble NRP1 and secreted NRP1 are the two forms of NRP1 present and have different biological roles.25) Subsequent studies on the roles of the two forms of NRP1 in gliomas may provide further insight into the biological functions of NRP1.The RNA-binding protein Lin28B binds to the 3′UTR of NRP1 and increases the stability and expression of NRP1 mRNA, which in turn activates the Wnt/β-catenin signaling pathway and induces enhanced stem cell properties in gastric cancer cells.26) Jimenez-Hernandez et al.27) isolated NRP1-positive lung cancer cell lines using flow cytometry and found that cell migration, cloning, and self-renewal were enhanced, accompanied by upregulation of stem cell marker expression. Recent studies have shown that NRP1 enhances the stem cell properties of breast cancer cells, thereby increasing their resistance to radiation therapy.18) These results suggest that NRP1 enhances stem cell properties in some tumors. However, its effect on the stem cell properties of glioma cells remains unclear. Our results showed that either NRP1 overexpression or rNRP1 treatment enhanced the self-renewal ability of LN-229 cells. Additionally, NRP1 overexpression or rNRP1 treatment enhanced CD133 and CD44 protein expression. Previous studies have shown that CD133 and CD44 are stem cell markers for various malignant tumors, including gliomas, and play essential roles in maintaining cellular stem cell properties.28,29) Interestingly, NRP1 enhanced glioma cell stem cell properties in our study. The enhancement of stem cell properties in malignant tumors is often accompanied by cellular resistance to chemotherapeutic agents.30,31) These suggest that NRP1 induces TMZ resistance by enhancing the stem cell properties of glioma cells.
YAP, as an effector molecule of the Hippo signaling pathway, plays an important role in maintaining tumor cell stem cell properties. Studies have shown that SOX-2 maintains retinoblastoma stem cell properties by activating Hippo/YAP signaling.32) Park et al.33) found that YAP expression levels were associated with the stem cell properties of hepatocellular carcinoma cells under hypoxia. The transcription factor brachyury maintains malignant tumor cell stemness by maintaining YAP stability and protein synthesis.34) Additionally, YAP plays an important role in the chemoresistance of several malignant tumors. Studies have reported that YAP promotes chemoresistance in small-cell lung cancer cells by inducing Rest protein expression.35) It was also shown that erythropoietin-producing hepatocellular receptor A2 induces chemoresistance in gastric cancer cells by increasing the stability and nuclear expression of YAP.36) Recent studies have shown that SOX13 promotes thyroid cancer cell proliferation, migration, and chemoresistance via the TRIM11/YAP signaling axis.37) In the present study, rNRP1 treatment and NRP1 overexpression both induced LATS1 and YAP dephosphorylation in LN-229 cells. NRP1 overexpression increases nuclear translocation of YAP, and possibly YAP activation, in LN-229 cells. Further studies revealed that inhibition of YAP suppressed NRP1 overexpression-induced self-renewal of LN-229 cells as well as CD133 and CD44 protein expression. Inhibition of YAP activity suppresses TMZ resistance in NRP1 overexpressing LN-229 cells. These results suggest that NRP1 enhances stemness in glioma cells by activating YAP, thereby increasing the resistance to chemotherapy. It has been reported in the literature that NRP1 interacts with VEGF-A and GIPC1 to regulate phosphatidylinositol 3-kinase (PI3K)/PDK1, α6/β4-integrin, FAK, Src, and LATS1 signaling, which increases the accumulation of YAP/∆Np63α and promotes epidermal cancer stem cell survival, angiogenesis, and tumor formation.38) It is suggested that VEGF-A and GIPC1 may participate in the biological regulation of NRP1 as upstream targets of YAP. However, whether VEGF-A and GIPC1 are involved in NRP1-induced stemness and chemoresistance in glioma cells needs to be further investigated. Currently, we only performed NRP1 overexpression studies on one type of glioma cells. In order to validate the experimental results, we will further experiment on glioma cells with high NRP1 expression using RNA interference technology.
In conclusion, our study revealed the signaling mechanism by which NRP1 induces glioma cell stemness and chemoresistance via YAP. The inhibition of YAP may be a novel strategy for treating chemoresistance in NRP1-overexpressing gliomas.
This work was supported by Cangzhou Key R&D Program Guidance Project (No.222106045).
The authors declare no conflict of interest.
This article contains supplementary materials.