Abstract
Patients with diabetes mellitus (DM) often experience complications such as peripheral arterial disease (PAD), which is thought to be caused by vascular damage resulting from increased oxidative stress. Dipeptidyl peptidase-4 inhibitors have been reported to reduce oxidative stress, although the exact mechanism remains unclear. This study aimed to investigate the impact of long-term (6 weeks) anagliptin treatment at a dose of 200 mg/kg/d against oxidative stress in the femoral artery of Otsuka Long-Evans Tokushima Fatty (OLETF) rats using a well-established animal model for type 2 DM. Serum toxic advanced glycation end-products concentrations and blood glucose levels after glucose loading were significantly elevated in OLETF rats compared to Long-Evans Tokushima Otsuka (LETO) rats but were significantly suppressed by anagliptin administration. Plasma glucagon-like peptide-1 concentrations after glucose loading were significantly increased in anagliptin-treated rats. Superoxide production and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in femoral arteries were significantly increased in OLETF rats compared to LETO rats but were significantly decreased by anagliptin administration. The expressions of NADPH oxidase components (p22phox in the intima region and p22phox and gp91phox in the media region) in the femoral artery were significantly increased in OLETF rats compared to LETO rats but were significantly suppressed by anagliptin administration. Furthermore, the femoral artery showed increased wall thickness in OLETF rats compared to LETO rats, but anagliptin administration reduced the thickening. This study suggests that long-term anagliptin administration can reduce oxidative stress in femoral arteries and improve vascular injury.
INTRODUCTION
Patients with diabetes mellitus (DM) are susceptible to developing peripheral arterial disease (PAD) in their lower extremities. PAD is an atherosclerotic disease and, in severe cases, may lead to amputations. The 5-year survival rate for patients with PAD and intermittent claudication is as low as 70%,1) and the primary causes of death among these patients are ischemic heart disease and cerebrovascular disease.2–4) Endothelial dysfunction plays an important role in the etiology of PAD in patients with DM, with enhanced oxidative stress thought to be the underlying cause.5,6) In general, factors that contribute to oxidative stress encompass increased production of advanced glycation end-products (AGEs), activation of the polyol pathway, activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and increased protein kinase C, as well as the uncoupling of the xanthine oxidase and endothelial nitric oxide synthase (eNOS).7–10) However, the availability of effective strategies to inhibit superoxide production remains limited.
AGEs, which are elevated in hyperglycemia, are formed through nonenzymatic reactions of reducing sugars like glucose with amino groups of proteins. This reaction results in the formation of Schiff bases and Amadori compounds, followed by slow and irreversible processes of dehydration, condensation, oxidation, and reduction. AGEs have been recognized to be associated with the onset and progression of complications related to diabetes mellitus, including nephropathy, retinopathy, and atherosclerosis. Among the various types of AGEs, glyceraldehyde-derived AGEs, referred to as toxic AGEs (TAGE) and generated from glucose/fructose metabolic intermediates, have been shown to bind to the receptor of AGEs (RAGE), playing a significant role in the onset and development of diabetic vascular complications. The TAGE-RAGE pathway has been reported to induce oxidative stress via NADPH oxidase.11,12) However, the relationship between this pathway and PAD remains unclear.
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat has been widely used as a model for human Type 2 DM (T2DM) in various studies.13) It has been suggested that NADPH oxidase activation and eNOS uncoupling may serve as possible mechanisms.14,15) Similarly, in a rat model of Type 1 DM induced by streptozotocin administration, increased superoxide production was observed in the femoral artery, attributed to NADPH oxidase activation and eNOS uncoupling.16) In the femoral artery of a rat model of T2DM, elevated serum TAGE levels and activation of mitochondrial respiratory chain complex II have been reported to contribute to increased superoxide production.17) However, the involvement of NADPH oxidase in this process remains unknown.
Anagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, is used for treating T2DM. It works by stimulating insulin secretion in a glucose-dependent manner via inhibiting the breakdown of incretin hormones like glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide. Clinical studies in humans have yielded inconsistent findings regarding the effects of DPP-4 inhibitors on endothelial function in large blood vessels. Some reports suggest improvement in vascular endothelial function18–22) while others present scattered evidence of no improvement.23,24) In addition, the specific effects of anagliptin have not yet been reported. In animal studies, anagliptin has been shown to significantly reduce monocyte/macrophage accumulation, smooth muscle cell proliferation, and inflammatory cytokines, improving atherosclerotic lesions in the aorta of apolipoprotein E-deficient mice.25) However, reports on the impact of DPP-4 inhibitors on oxidative stress are scarce, and their effects on femoral arteries have not been fully elucidated.
In this study, we aimed to investigate the impact of long-term anagliptin administration on vascular injury caused by oxidative stress in the femoral artery of OLETF rats, a well-established model for human T2DM.
MATERIALS AND METHODS
MaterialsAnagliptin was provided by Sanwa Kagaku Kenkyusho Co., Ltd. (Nagoya, Japan). Dihydroethidium was purchased from Molecular Probes, Inc. (Eugene, OR, U.S.A.). The superoxide-sensitive chemiluminescent dye 8-amino-5-chloro-7-phenylpyrrole[3,4-d]pyridazine-1,4-(2H,3H)dione sodium salt (L-012) was purchased from Wako Pure Chemical Corporation, Ltd. (Osaka, Japan). Goat anti-p22phox polyclonal antibody and goat anti-gp91phox polyclonal antibody were purchased from Santa Cruz Biotechnology, Inc. (Catalog Number: sc-11712, sc-5827, Dallas, TX, U.S.A.). Donkey anti-goat immunoglobulin G H&L (Alexa Fluor® 488) was purchased from Invitrogen Corp. (Catalog Number: A-11055, Carlsbad, CA, U.S.A.). All other reagents used in this study were commercially available.
The composition of the Krebs solution used in this study was as follows: 137.4 mM NaCl, 5.9 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 15.5 mM NaHCO3, 1.2 mM KH2PO4, and 11.5 mM glucose. The solution was then bubbled with a mixture of 95% O2 and 5% CO2 to achieve a pH range of 7.3–7.4. The Krebs-Henseleit buffer contained 118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 11 mM glucose (pH 7.4).
Experimental AnimalsThis study was performed in accordance with the Guidelines for the Conduct of Animal Experiments issued by Nagoya City University and was approved by the Committee on the Ethics of Animal Experiments within the institution (ethical approval number and date: H23-P-20, December 12, 2011). Male OLETF rats, which serve as a model for T2DM, were used as the experimental group, while nondiabetic Long-Evans Tokushima Otsuka (LETO) rats of the same origin were used as the control group. OLETF (n = 14) and LETO (n = 14) rats were obtained from Hoshino Laboratory Animals, Inc. (Bandou, Japan). All rats were fed standard laboratory chow (CE-2; CLEA Japan, Inc., Tokyo, Japan) and given free access to drinking water ad libitum. They were housed in pairs in a specific pathogen-free facility with a controlled temperature (23 ± 2 °C) and a 12-h light/dark cycle. Starting at 24 weeks of age, oral anagliptin dissolved in free drinking water was administered to LETO and OLETF rats for 6 weeks. After a preliminary study, the optimal dose was determined to be 200 mg/kg/d. Prior to tissue collection, the rats were fasted overnight, anesthetized with sevoflurane (Maruishi Pharmaceutical Co., Osaka, Japan), and euthanized by exsanguination. Blood samples were collected from the heart and centrifuged at 1470 × g and 4 °C for 15 min to obtain sera, which were stored at −80 °C until further analysis. The femoral artery was immediately excised and placed in Krebs solution, and any connective tissue was carefully removed. The femoral artery was then stored in Krebs solution at 4 °C until use.
Biochemical AnalysisWhole blood was used to measure glucose levels and glycated hemoglobin A1c (HbA1c) percentages. Fasting blood glucose levels were measured using the glucose oxidase method using a glucometer (Sanwa Kagaku Kenkyusho Co., Ltd., Nagoya, Japan), while HbA1c percentages were measured using a DCA Vantage Analyzer (Siemens Healthcare Diagnostics Inc., Camberley, U.K.). Serum TAGE levels were measured using a competitive enzyme-linked immunosorbent assay with an immunopurified anti-TAGE antibody, as previously described.26,27)
Oral Glucose Tolerance TestIn this experiment, rats were fasted overnight and orally administered 1 g/kg of glucose. Blood glucose levels were measured at 0, 30, 60, and 120 min after glucose administration. Plasma samples were obtained by collecting blood from the tail vein at 0 and 30 min after glucose administration. The collected blood was then mixed with heparin (2.5–10 IU/mL) and aprotinin (105–420 KIU/mL). The mixture was allowed to stand at 4 °C for 90 min, and then centrifuged at 1470 × g for 15 min at 4 °C. The resulting supernatant was used for further analysis. The plasma insulin and plasma GLP-1 levels were measured using commercially available enzyme-linked immunosorbent assay kits (Catalog Nos. AKRIN-010T and AKMGP-011, respectively, Shibayagi, Gunma, Japan).
Superoxide ProductionSuperoxide production in the femoral artery was investigated using oxidative fluorescence staining with dihydroethidium, as previously described.15,17) The femoral artery segments were stored in an optimal cutting temperature compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) and used for these experiments. A frozen segment of the embedded femoral artery was cut into 10-µm-thick sections using a cryostat and then mounted on MAS-coated glass slides (Matsunami Glass Ind., Ltd., Osaka, Japan). The femoral arterial sections were incubated with Krebs–Henseleit buffer (pH 7.4) containing dihydroethidium (2 µM) in a CO2 incubator for 20 min at 37 °C. Images of the stained sections were captured using a confocal laser scanning microscope system (LSM-510, Carl Zeiss AG, Jena, Germany). The fluorescence intensity in each section was measured from ten randomly selected regions (8 × 8 pixels) and averaged using digital image analyzer software (ImageJ, National Institutes of Health, Bethesda, MD, U.S.A.).
NADPH Oxidase ActivityNADPH oxidase activity was assessed by adding a protein solution obtained from homogenized femoral arteries to the substrate NADPH (Sigma-Aldrich, St. Louis, MO, U.S.A.) and measuring the resulting superoxide using the chemiluminescence method with L-012 and a luminometer (Glomax®-Multi Detection System, Promega, Madison, WI, U.S.A.). Femoral arteries (14-mm-long ring segments) were homogenized using a glass homogenizer in 50 mM phosphate buffer (pH 7.0) containing 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) and Protease Inhibitor Cocktail (Sigma-Aldrich). The resulting homogenate was centrifuged at 45 × g for 10 min at 4 °C, and the supernatant obtained was used as the total protein solution. For the chemiluminescence measurements, a reaction solution consisting of 50 mM phosphate buffer (pH 7.0) containing 0.1 mM NADPH, 1 mM EGTA, 0.15 M sucrose, and 0.1 mM L-012 was used as a blank by measuring its chemiluminescence with a luminometer. Subsequently, samples were diluted to a concentration of 0.15 µg/mL (4.5 µg total protein) and subjected to chemiluminescence measurements at 37 °C for 30 min, with readings taken at 30 s intervals. The chemiluminescence of the sample was calculated by subtracting the blank measurement from the chemiluminescence obtained in the presence of the sample. Finally, NADPH oxidase activity was expressed as RLU/min/µg protein.
Immunohistochemical AnalysisPreparation of Tissue SectionsFemoral arteries were cut into 3-mm sections and fixed with 4% paraformaldehyde. These sections were incubated in 10 and 15% sucrose for 4 h each, followed by overnight incubation in 20% sucrose. The specimens were then embedded using an optimal cutting temperature compound and snap-frozen in liquid nitrogen. The frozen segments of the embedded femoral artery were then cut into 6-µm-thick sections using a cryostat and mounted on MAS-coated glass slides.
Fluorescent ImmunostainingThe effect of anagliptin on the NADPH oxidase component was investigated using fluorescence immunostaining. The sections were incubated overnight at 4 °C with goat anti-p22phox polyclonal antibody (1 : 100 dilution) or goat anti-gp91phox polyclonal antibody (1 : 100 dilution) as the primary antibody. The next day, the sections were rinsed with phosphate-buffered saline (pH 7.4) and incubated for 1 h at room temperature with a secondary antibody (1 : 500 dilution). Finally, the sections were mounted with Fluoromount/Plus (Diagnostic BioSystems, Pleasanton, CA, U.S.A.). Images were captured using the LSM 510. The fluorescence intensity in each section was measured from ten randomly selected regions (8 × 8 pixels) and averaged using digital image analyzer software (ImageJ, National Institutes of Health, Bethesda, MD, U.S.A.).
Hematoxylin–Eosin (H&E) StainingThe effect of anagliptin on vessel wall thickening caused by vascular injury was investigated using morphometric analysis. The sections were stained with H&E. After that, the tissue sample was dehydrated using ethanol and xylene and then sealed with Multi Mount 480 (Matsunami Glass Ind., Ltd.). Light-microscope images were captured using a digital camera (Nikon, DS-L2, Tokyo, Japan). The wall thickness, the vessel wall/outer diameter ratio, and the number of smooth muscle cell nuclei across the wall were then measured using Scion Image software. Statistical analysis
All results were presented as means with their respective standard deviations. The “n” values represented the number of rats used, with each rat contributing only one segment for a given experiment. For multiple comparisons, a one-way ANOVA was conducted, followed by Tukey’s post hoc test. Two-group comparisons were performed using paired t-tests, and statistical significance was defined as a p-value less than 0.05.
RESULTS
Biochemical ProfileAt 30 weeks of age, OLETF rats showed significant increases in body weight, fasting blood glucose levels, HbA1c percentages, and serum TAGE levels compared to LETO rats. Anagliptin-treated OLETF rats also had significantly higher body weight, fasting blood glucose levels, and HbA1c percentages compared to LETO rats, with no difference observed compared to OLETF rats. However, there was a significant decrease in serum TAGE levels in anagliptin-treated OLETF rats compared with untreated OLETF rats (Table 1).
Table 1. Biochemical Profile of Anagliptin-Treated Rats
| LETO | LETO + ANG | OLETF | OLETF + ANG |
|---|
| Body weight (g) | 509.7 ± 35.2 | 495.8 ± 22.9 | 612.0 ± 53.7aa, bb | 640.8 ± 38.9aa, bb |
| Fasting blood glucose (mg/dL) | 87.0 ± 8.2 | 97.9 ± 16.5 | 164.1 ± 34.5aa, bb | 143.6 ± 23.5aa, bb |
| HbA1c (%) | 3.5 ± 0.1 | 3.4 ± 0.1 | 5.0 ± 1.5aa, bb | 4.0 ± 0.2 |
| Serum TAGE (U/mL) | 13.3 ± 2.2 | 13.1 ± 2.3 | 21.4 ± 1.4aa, bb | 17.0 ± 1.7aa, bb, cc |
Data are expressed as means ± standard deviations (n = 7). aa, p < 0.01 vs. LETO rats; bb, p < 0.01 vs. anagliptin-treated LETO rats (LETO + ANG); cc, p < 0.01 vs. OLETF rats. Multiple comparisons were analyzed using a one-way analysis of variance followed by Tukey’s post hoc test. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty; ANG, anagliptin; HbA1c, glycated hemoglobin A1c; TAGE, toxic for advanced glycation end-products.
Blood glucose levels were significantly increased in OLETF rats compared to LETO rats at 0 (predose), 30, 60, and 120 min after oral administration of glucose. Although blood glucose levels were significantly higher in anagliptin-treated OLETF rats compared to LETO rats, there was a significant suppression in the increase of blood glucose levels at 60 min compared to OLETF rats and a trend (p = 0.096) toward suppression at 120 min (Fig. 1A). Plasma insulin levels at 0 and 30 min after oral glucose administration were significantly elevated at 30 min compared to 0 min in anagliptin-treated LETO rats. However, no significant differences were observed between 0 and 30 min in the other rats (Fig. 1B). Plasma GLP-1 levels at 0 and 30 min after oral glucose administration were significantly elevated at 30 min compared to 0 min in anagliptin-treated LETO and OLETF rats (Fig. 1C).
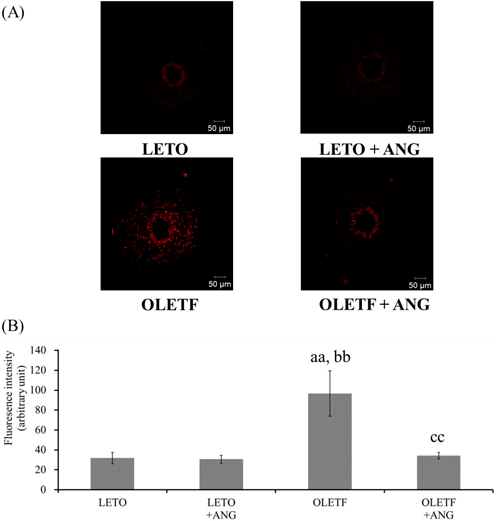
Superoxide ProductionSuperoxide production in the femoral artery was significantly increased in OLETF rats compared to LETO rats, while it was significantly decreased in anagliptin-treated OLETF rats compared to OLETF rats (Fig. 2).
NADPH Oxidase ActivityNADPH oxidase activity was significantly increased in OLETF rats compared to LETO rats, while it was significantly decreased in anagliptin-treated OLETF rats compared to OLETF rats (Fig. 3).
NADPH Oxidase ExpressionThe fluorescence intensities of p22phox in the intima region and p22phox and gp91phox in the media region were increased in the femoral arteries of OLETF rats compared to LETO rats. However, these fluorescence intensities were significantly reduced in anagliptin-treated OLETF rats compared to OLETF rats (Fig. 4).
Vessel Wall Thickening Due to Vascular InjuryThe vessel wall thickening of the femoral artery was assessed by measuring vessel wall thickness, vessel wall/outer diameter ratio, and smooth muscle cell count. The values of vessel wall thickness, vessel wall/outer diameter ratio, and smooth muscle cell count were significantly increased in OLETF rats compared to LETO rats. However, these values were significantly decreased in anagliptin-treated OLETF rats compared to OLETF rats (Fig. 5).
DISCUSSION
OLETF rats, a model animal for T2DM, are known to develop insulin resistance at 12 weeks of age, experience delayed hyperglycemic symptoms at 18 weeks of age, and be diagnosed with diabetes by 24 weeks of age. Furthermore, these rats are known to exhibit low plasma insulin levels at 60 weeks of age.13) In the present study, OLETF rats showed increased body weight, elevated HbA1c percentages, obesity, and hyperglycemia at 30 weeks of age. Notably, serum TAGE levels were significantly higher in OLETF rats than in LETO rats, indicating increased superoxide production within the femoral arteries of OLETF rats. Although the age was different, these findings align with previous reports regarding femoral arteries.17)
Measurement of NADPH oxidase activity using chemiluminescence assays revealed elevated activity in the femoral artery of OLETF rats. Furthermore, the expressions of p22phox and gp91phox, components of NADPH oxidase, were examined using fluorescence immunostaining. The findings revealed upregulation of p22phox in the intima region as well as p22phox and gp91phox in the media region of the femoral arteries in OLETF rats. Although the expression levels of p22phox, gp91phox, and p47phox, as well as the NADPH oxidase activity, have been reported to be increased in the coronary arteries of OLETF rats,14) this is the first report of such findings in the femoral arteries.
It has been suggested that TAGE are produced during postprandial hyperglycemic spikes and can cause damage to endothelial cells.28) In addition, TAGE, by binding to their receptor RAGE, have been shown to activate NADPH oxidase and increase intracellular superoxide production.11,12) In the present study, TAGE production was found to be increased during postprandial hyperglycemia, resulting in increased p22phox and gp91phox expressions and NADPH oxidase activity, leading to increased superoxide production. H&E staining of the vascular wall revealed its thickening in OLETF rats. Increased oxidative stress impairs vascular endothelial function and upregulates the expression of inflammatory cytokines, growth factors, and adhesion factors, which are thought to be involved in the development and progression of atherosclerosis.29) Therefore, the increased superoxide production is likely responsible for the vessel wall thickening.
In the present study, anagliptin was administered orally ad libitum to OLETF rats for six weeks, and its effects were assessed. The blood glucose levels at 60 min after glucose loading in the oral glucose tolerance test were significantly lower in anagliptin-treated OLETF rats than in OLETF rats. In addition, plasma GLP-1 levels were elevated 30 min after glucose loading, while no significant change in plasma insulin levels was observed. Since the rise in blood glucose levels was suppressed after 60 min, it is possible that insulin was secreted after 30 min. This suggests that anagliptin inhibits GLP-1 degradation and promotes insulin secretion, thereby suppressing post-glucose hyperglycemia and subsequent TAGE production. In addition, in the femoral arteries of anagliptin-treated OLETF rats, p22phox and gp91phox expressions and NADPH oxidase activity were suppressed compared to the untreated OLETF rats. These findings indicate that anagliptin suppresses superoxide production and reduces vascular wall thickening. This study is the first to investigate the effects of long-term anagliptin administration on vascular injury in femoral arteries, suggesting that the activation of NADPH oxidase is the primary mechanism underlying increased vascular superoxide production in vitro and in vivo.30–32) Notably, NADPH oxidase activity and expression, superoxide production, and vessel wall thickening were improved to a level similar to that in LETO rats. These findings suggest that NADPH oxidase activity and expression are the major contributors to superoxide production in femoral arteries and that anagliptin proves to be effective in mitigating femoral artery vascular injury.
The observed effects of anagliptin can also be attributed to the suppression of postprandial hyperglycemia. However, the usefulness of controlling blood glucose levels in PAD is currently uncertain. Although studies in humans have demonstrated that glycemic control reduces the risk of myocardial infarction and lower limb amputation,33,34) there is no evidence supporting a preventive effect of glycemic control specifically for PAD. Anagliptin has also been shown to alleviate inflammation and endothelial cell injury. For example, it inhibits H2O2-induced apoptosis of human umbilical vein endothelial cells,35) ameliorates high glucose-induced endothelial dysfunction by inhibiting the activation of nucleotide-binding domain, leucine-rich–containing family, pyrin domain-containing 3 inflammasomes,36) and suppresses neointimal hyperplasia by regulating superoxide dismutase-1/Ras homolog family member A/Jun N-terminal kinase signaling in the endothelial cells of the arterial wall.37) Therefore, the effects of anagliptin on femoral arteries may be pleiotropic and extend beyond the correction of blood glucose levels.
Further, DPP-IV inhibitors other than anagliptin are considered beneficial for vascular injury in the femoral artery.38,39) Conversely, there are no reports examining the relationship between TAGE, NADPH oxidase, and oxidative stress in the femoral artery. Anagliptin significantly differs from other DPP-IV inhibitors as it lowers LDL-cholesterol levels.40) However, whether this difference is related to the predominance of anagliptin in the femoral artery is unclear. Hence, in the future, the role of other DPP-IV inhibitors should be examined to validate the specific effects of anagliptin, as shown in this study.
In lower extremity occlusive arterial disease, there is currently a lack of evidence regarding the benefit of oral hypoglycemic agents. Unlike animal studies, clinical trials have failed to demonstrate a favorable effect of DPP-4 inhibitors in reducing cardiovascular outcomes in patients with T2DM.41–44) However, the antiatherosclerotic effects of DPP-4 inhibitors in the early stages of atherosclerosis have not been evaluated, as the majority of subjects enrolled in these studies already had cardiovascular disease, and the observation period was only a few years. Interestingly, Gaspari et al.45) reported that liraglutide, a GLP-1 receptor agonist, inhibited the progression of early-onset hypoxic atherosclerosis in Apoe−/− mice, while no significant effect of liraglutide on the progression of late-onset hypervascular atherosclerosis was observed. This study may provide a reasonable explanation for the failure of incretin-based therapy to prevent secondary events in patients with DM.42) Since the OLETF rats used in this study were in the early stages of T2DM, the earlier use of anagliptin may be beneficial.
The current study had two limitations. First, the RAGE expression in the femoral artery could not be evaluated. In the thoracic aorta of OLETF rats, an increased expression of RAGE genes and proteins and NADPH oxidase has been observed.46) Reportedly, the treatment with TAGE increased the production of superoxide in the femoral artery of OLETF rats.17) Additionally, these results suggest that RAGE is expressed in the femoral arteries of OLETF rats, although at different ages, and its expression is increased under hyperglycemic conditions. Second, we have not been able to investigate mechanisms other than NADPH oxidase as a source of oxidative stress in the femoral artery. eNOS uncoupling, in addition to NADPH oxidase, reportedly contributes to oxidative stress in the femoral artery of a rat model of T1DM.16) Furthermore, in the femoral artery of a rat model of T2DM, activation of mitochondrial respiratory chain complex II has been reported to be involved in increased superoxide production,17) and anagliptin may have an effect on these factors. Future studies are needed to clarify these mechanisms.
In conclusion, anagliptin has been shown to suppress TAGE production by inhibiting GLP-1 degradation and improving post-glucose tolerance, suppressing the expression and activation of NADPH oxidase components (p22phox and gp91phox), thereby reducing superoxide production and mitigating vascular damage. However, further prospective clinical studies are needed to evaluate the risk of vascular injury and validate the findings of this study.
Acknowledgments
This study was supported in part by Sanwa Kagaku Kenkyusho Co., Ltd. (Nagoya, Japan).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Kumakura H, Kanai H, Hojo Y, Iwasaki T, Ichikawa S. Long-term survival and fate of the leg in de novo intermittent claudication. Eur. Heart J. Qual. Care Clin. Outcomes, 3, 208–215 (2017).
- 2) Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg., 45 (Suppl. S), S5–S67 (2007).
- 3) Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ. Res., 116, 1509–1526 (2015).
- 4) Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob. Health, 7, e1020–e1030 (2019).
- 5) Taniwaki H, Shoji T, Emoto M, Kawagishi T, Ishimura E, Inaba M, Okuno Y, Nishizawa Y. Femoral artery wall thickness and stiffness in evaluation of peripheral vascular disease in type 2 diabetes mellitus. Atherosclerosis, 158, 207–214 (2001).
- 6) Suzuki E, Kashiwagi A, Nishio Y, Egawa K, Shimizu S, Maegawa H, Haneda M, Yasuda H, Morikawa S, Inubushi T, Kikkawa R. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care, 24, 2107–2114 (2001).
- 7) Fatehi-Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur. J. Pharmacol., 636, 8–17 (2010).
- 8) Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler. Thromb. Vasc. Biol., 25, 274–278 (2005).
- 9) Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes, 49, 1939–1945 (2000).
- 10) Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation, 105, 1656–1662 (2002).
- 11) Takeuchi M, Takino J, Yamagishi S. Involvement of TAGE-RAGE System in the Pathogenesis of Diabetic Retinopathy. J. Ophthalmol., 2010, 170393 (2010).
- 12) Ojima A, Matsui T, Maeda S, Takeuchi M, Yamagishi S. Glucose-dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm. Metab. Res., 44, 501–505 (2012).
- 13) Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes, 41, 1422–1428 (1992).
- 14) Kajikuri J, Watanabe Y, Ito Y, Ito R, Yamamoto T, Itoh T. Characteristic changes in coronary artery at the early hyperglycaemic stage in a rat type 2 diabetes model and the effects of pravastatin. Br. J. Pharmacol., 158, 621–632 (2009).
- 15) Kikuchi C, Kajikuri J, Hori E, Nagami C, Matsunaga T, Kimura K, Itoh T. Aortic superoxide production at the early hyperglycemic stage in a rat type 2 diabetes model and the effects of pravastatin. Biol. Pharm. Bull., 37, 996–1002 (2014).
- 16) Serizawa K, Yogo K, Aizawa K, Tashiro Y, Ishizuka N. Nicorandil prevents endothelial dysfunction due to antioxidative effects via normalisation of NADPH oxidase and nitric oxide synthase in streptozotocin diabetic rats. Cardiovasc. Diabetol., 10, 105 (2011).
- 17) Hori E, Kikuchi C, Nagami C, Kajikuri J, Itoh T, Takeuchi M, Matsunaga T. Role of glyceraldehyde-derived ages and mitochondria in superoxide production in femoral artery of OLETF rat and effects of pravastatin. Biol. Pharm. Bull., 40, 1903–1908 (2017).
- 18) van Poppel PC, Netea MG, Smits P, Tack CJ. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care, 34, 2072–2077 (2011).
- 19) Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K, Hokimoto S, Jinnouchi H, Ogawa H. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ. J., 77, 1337–1344 (2013).
- 20) Kubota Y, Miyamoto M, Takagi G, Ikeda T, Kirinoki-Ichikawa S, Tanaka K, Mizuno K. The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J. Korean Med. Sci., 27, 1364–1370 (2012).
- 21) Ayaori M, Iwakami N, Uto-Kondo H, Sato H, Sasaki M, Komatsu T, Iizuka M, Takiguchi S, Yakushiji E, Nakaya K, Yogo M, Ogura M, Takase B, Murakami T, Ikewaki K. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J. Am. Heart Assoc., 2, e003277 (2013).
- 22) Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, Shiraiwa T, Kosugi K, Umayahara Y, Yamamoto T, Yokoyama H, Kuribayashi N, Jinnouchi H, Gosho M, Shimomura I, Watada H. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care, 39, 139–148 (2016).
- 23) Hage C, Brismar K, Lundman P, Norhammar A, Rydén L, Mellbin L. The DPP-4 inhibitor sitagliptin and endothelial function in patients with acute coronary syndromes and newly detected glucose perturbations: A report from the BEGAMI study. Diab. Vasc. Dis. Res., 11, 290–293 (2014).
- 24) Maruhashi T, Higashi Y, Kihara Y, Yamada H, Sata M, Ueda S, Odawara M, Terauchi Y, Dai K, Ohno J, Iida M, Sano H, Tomiyama H, Inoue T, Tanaka A, Murohara T, Node K. Long-term effect of sitagliptin on endothelial function in type 2 diabetes: a sub-analysis of the PROLOGUE study. Cardiovasc. Diabetol., 15, 134 (2016).
- 25) Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori R, Fujitani Y, Watada H. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology, 154, 1260–1270 (2013).
- 26) Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol. Med., 6, 114–125 (2000).
- 27) Tahara N, Yamagishi S, Takeuchi M, Honda A, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, Ishibashi M, Hayabuchi N, Matsui T, Imaizumi T. Positive association between serum level of glyceraldehyde-derived advanced glycation end products and vascular inflammation evaluated by [(18)F]fluorodeoxyglucose positron emission tomography. Diabetes Care, 35, 2618–2625 (2012).
- 28) Tsunosue M, Mashiko N, Ohta Y, Matsuo Y, Ueda K, Ninomiya M, Tanaka S, Hoshiko M, Yoshiyama Y, Takeuchi M, Ueda S, Yamagishi S. An alpha-glucosidase inhibitor, acarbose treatment decreases serum levels of glyceraldehyde-derived advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin. Exp. Med., 10, 139–141 (2010).
- 29) Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA, 287, 2570–2581 (2002).
- 30) Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res., 74, 1141–1148 (1994).
- 31) Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest., 97, 1916–1923 (1996).
- 32) Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes, 51, 522–527 (2002).
- 33) Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ, 343 (jul26 1), d4169 (2011).
- 34) Hasan R, Firwana B, Elraiyah T, Domecq JP, Prutsky G, Nabhan M, Prokop LJ, Henke P, Tsapas A, Montori VM, Murad MH. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J. Vasc. Surg., 63 (Suppl.), 22S–28S.e1–2 (2016).
- 35) Li Q, Li J, Liu Y, Zhang M, Chen C. Anagliptin prevents apoptosis of human umbilical vein endothelial cells by modulating NOX-4 signaling pathways. Biomed. Pharmacother., 103, 1623–1631 (2018).
- 36) Jiang T, Jiang D, Zhang L, Ding M, Zhou H. Anagliptin ameliorates high glucose- induced endothelial dysfunction via suppression of NLRP3 inflammasome activation mediated by SIRT1. Mol. Immunol., 107, 54–60 (2019).
- 37) Li Q, Zhang M, Xuan L, Liu Y, Chen C. Anagliptin inhibits neointimal hyperplasia after balloon injury via endothelial cell-specific modulation of SOD-1/RhoA/JNK signaling in the arterial wall. Free Radic. Biol. Med., 121, 105–116 (2018).
- 38) Terawaki Y, Nomiyama T, Kawanami T, Hamaguchi Y, Takahashi H, Tanaka T, Murase K, Nagaishi R, Tanabe M, Yanase T. Dipeptidyl peptidase-4 inhibitor linagliptin attenuates neointima formation after vascular injury. Cardiovasc. Diabetol., 13, 154 (2014).
- 39) Akita K, Isoda K, Shimada K, Daida H. Dipeptidyl-peptidase-4 inhibitor, alogliptin, attenuates arterial inflammation and neointimal formation after injury in low-density lipoprotein (LDL) receptor-deficient mice. J. Am. Heart Assoc., 4, e001469 (2015).
- 40) Morimoto T, Sakuma I, Sakuma M, Tokushige A, Natsuaki M, Asahi T, Shimabukuro M, Nomiyama T, Arasaki O, Node K, Ueda S. Randomized evaluation of anagliptin vs. sitagliptin on low-density lipoproteiN cholesterol in diabetes (REASON) trial: a 52-week, open-label, randomized clinical trial. Sci Rep., 9, 8537 (2019).
- 41) Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med., 369, 1317–1326 (2013).
- 42) White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med., 369, 1327–1335 (2013).
- 43) Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med., 373, 232–242 (2015).
- 44) Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK. Effect of linagliptin vs. placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the Carmelina randomized clinical trial. JAMA, 321, 69–79 (2019).
- 45) Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(−/−) mouse model. Diab. Vasc. Dis. Res., 10, 353–360 (2013).
- 46) Matsui T, Nishino Y, Takeuchi M, Yamagishi S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol. Res., 63, 383–388 (2011).