2024 Volume 47 Issue 1 Pages 272-278
2024 Volume 47 Issue 1 Pages 272-278
Gold-based nanoparticles hold promise as functional nanomedicines, including in combination with a photothermal effect for cancer therapy in conjunction with chemotherapy. Here, we synthesized hollow gold nanoparticles (HGNPs) exhibiting efficient light absorption in the near-IR (NIR) region. Several synthesis conditions were explored and provided monodisperse HGNPs approximately 95–135 nm in diameter with a light absorbance range of approximately 600–720 nm. The HGNPs were hollow and the surface had protruding structures when prepared using high concentrations of HAuCl4. The simultaneous nucleation of a sacrificial AgCl template and Au nanoparticles may affect the resulting HGNPs. Diethyldithiocarbamate (DDTC) is metabolized from disulfiram and is a repurposed drug currently attracting attention. The chelation of DDTC with copper ion (DDTC-Cu) has been investigated for treating glioma, and here we confirmed the cytotoxic effect of DDTC-Cu towards rat C6 glioma cells in vitro. HGNPs alone were biocompatible and showed little cytotoxicity, whereas a mixture of DDTC-Cu and HGNPs was cytotoxic in a dose dependent manner. The temperature of HGNPs was increased by NIR-laser irradiation. The photothermal effect on HGNPs under NIR-laser irradiation resulted in cytotoxicity towards C6 cells and was dependent on the irradiation time. Photothermal therapy by HGNPs combined and DDTC-Cu was highly effective, suggesting that this combination approach hold promise as a future glioma therapy.
Gliomas are malignant brain tumors of the central nervous system and comprise approximately 80% of primary brain tumors.1,2) The best characterized and most aggressive form of glioma is glioblastoma multiforme, a grade IV glioma associated with an inferior prognosis, and high recurrence and mortality rates. Chemotherapy is an essential treatment modality for gliomas, together with surgery and radiation therapy. However, efficient drug delivery to the tumor site is hindered by the blood–brain barrier (BBB), which controls the transport into the brain of various substances, including drug molecules. While several approaches for efficient drug delivery can be proposed (i.e., specific ligand-spiked drugs/nanomedicine for BBB,3) micro/nanobubbles plus ultrasound and, nose-to-brain pathway approaches to escape BBB4)), local chemotherapy agents, such as carmustine wafers, can deliver an anticancer drug directly and do not need to transfer though the BBB, allowing a high drug concentration at the tumor site. However, poor drug penetration and chemo-resistance limit its application.5) This limitation is being addressed using nanomedicine, which is the application of nanotechnology and nanoparticle/nanomaterial-based drugs designed to target brain tumor cells, allowing the efficient delivery of antitumor drugs and/or nanocarriers to the required site.6–9)
Photothermal therapy (PTT) is a minimally invasive treatment approach through which cancer cells are heated using light. Various nanomedicines utilizing PTT have been investigated.10–12) For example, near-IR (NIR) light (700–1000 or 1100 nm) can penetrate body tissues more deeply than visible light which wavelength affects light absorption by blood and tissues.13,14) Combination therapies using an NIR-laser and an absorbent has been investigated for cancer treatment, as various nanomaterials and compounds have their effect on efficient light absorption (e.g., graphene nanosheets,15,16) indocyanine green,17) a boron dipyrromethene derivative18)), and gold nanoparticles have been studied in particular due to their attractive tuning properties achieved by the adjustment of surface plasmon resonance. For example, gold nanoparticles with large aspect ratios (e.g., gold nanorods,19,20) nanostars,21,22) nanoshells23,24) and nanocages25,26)) can absorb light with wavelengths longer than visible light. Hollow gold nanoparticles (HGNPs) have a cavity in each nanoparticle, similar to gold nanoshells and nanocages, and are a type of functional gold nanoparticle with a wide range of tunable (550–950 nm) absorption bands.27) Several types of HGNPs have nanoporous surface structures. One-step preparation of HGNPs is reported,28) which is advantageous not only from a cost-effective standpoint but also for practical application due to the facile preparation of HGNPs. HGNPs are being investigated for their utility in fuel cell catalysis as they exhibit enhanced catalytic properties.28) HGNPs are also being studied catalytically for environmental remediation for the reduction of 4-nitrophenol,29) and as electrochemical supercapacitors for next-generation electrical storage devices.30) Gold nanoparticle-decorated hollow and core-shell metal nanoparticles are also reportedly used for photocatalysis.31,32) The applications of HGNPs include as sensors for bioassays27) and in PTT for drug delivery.33,34) PTT using HGNPs holds promise in enhancing the effects of cancer therapies, including glioma therapy in combination with chemotherapy.
Diethyldithiocarbamate (DDTC) is a metabolite of disulfiram (DSF) and is being investigated for cancer therapy following complexation with copper (DDTC-Cu). DSF is an anti-alcohol-abuse drug used in the treatment of alcohol dependence. It has well-established safety profile and therapeutic effect at Food and Drug Administration-recommended dosage.35) DSF as a drug for drug repositioning, is promising for several diseases including cancer. DSF and DSF-Cu therapy are in clinical trials for the treatment of various kinds of cancers, including glioma.36) The chelation of copper with DSF and DDTC has a potent anticancer effect and can induce the production of reactive oxygen species and improve drug resistance, regulating oncogenetic signaling pathways (e.g., mitogen activated protein kinase, nuclear factor-kappa B).36,37) Skrott et al. proposed that DDTC-Cu can bind with nuclear protein localization-4 (NPL4) and induce aggregation, disabling the vital p97-NPL4-ubiquitin-fusion degradation protein 1 pathway and causing cell death.38) Conventional anticancer drugs hinder therapeutic effects due to drug resistance. Despite continuous efforts to search for new drugs, the development of a new anticancer drug requires a significant investment for success. Repurposing drugs is shedding light on the drug development process. Furthermore, DDTC-Cu was incorporated into liposomes or phase-change material nanoparticles composed of phospholipids in a nanomedicine investigation.39,40)
In this study, we focused on the preparation of HGNPs, which hold promise for PTT, and characterized their physicochemical, optical and photothermal properties. The cytotoxic effect of DDTC-Cu was then assessed on a glioma cell line in vitro. The combination of HGNPs and DDTC-Cu has not been investigated for glioma therapy yet as far as we know. Thus, the combined therapeutic effect against glioma of HGNPs with NIR-laser irradiation and DDTC-Cu was investigated as future glioma NIR-responsive nanomedicines and chemotherapies.
Chloroauric acid (HAuCl4), silver nitrate (AgNO3), copper(II) chloride, DDTC, sodium sulfite and ammonia water (25%) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Polyvinylpyrrolidone K30 (PVP, Wt. 40000), DDTC-Cu, and hydroquinone were purchased from Tokyo Chemical Industry (Tokyo, Japan).
Synthesis of HGNPsAs a typical experiment, we used the previously reported one-step template method for synthesizing HGNPs, with modifications.28) AgNO3 (10 mM, 600 µL), hydroquinone (90 mM, 400 µL) and PVP solution (1%, 45 mL) were mixed in a beaker with stirring at 0 °C (ice bath) for 3 min, then 600 µL HAuCl4 (10, 20, 30, 40 mM) was dropped in at a controlled speed (50, 100, 150, or 200 µL/min) and the samples were further stirred for 30 min. Then, 1 mL of concentrated ammonia (25%) water was added to remove the sacrificial AgCl template. The solution was centrifuged at 5000 rpm for 5 min and the samples were collected and washed with water twice. The HGNPs prepared using 20 mM HAuCl4 dropped into the reaction mix at a rate of 50 µL/min were evaluated in subsequent experiments.
Particle Size MeasurementThe mean particle size and polydispersity index (PDI) for the HGNPs samples dispersed into water were measured by dynamic light scattering method using a ZetaSizer Nano series (Malvern Instruments, Malvern, U.K.). The HGNPs samples dispersed into water (800 µL) were loaded into disposal cuvette (BRAND GMBH & CO KG, Wertheim, Germany). and then the samples were measured at a temperature set at 25 °C.
Optical PropertiesUV-visible (Vis) spectra of the samples from 400 to 1000 nm were obtained by scanning the absorbance of the samples using a plate microplate reader (Nivo 3S; PerkinElmer, Inc., Waltham, MA, U.S.A.).
Transmission Electron Microscopy (TEM)Samples dispersed in water were loaded on a carbon-coated grid and observed by TEM (JEM-1400Plus; JEOL, Tokyo, Japan).
Temperature Monitoring under NIR-Laser IrradiationHGNPs samples (500 µL, 6.3, 12.4, 18.4, 24.5, or 36.6 µg/mL) in 1.5 mL microtubes were irradiated with a 660 nm laser at 1.5 W/cm2 for 10 min (MDL-XD-660-3W NIR laser, Changchun New Industries Optoelectronics Tech. Co., Ltd., Changchun, China), and the temperatures of the samples were recorded at 30 s intervals using a thermograph (CPA-0170; CHINO Corporation, Tokyo, Japan).
For intermittent NIR-laser irradiation, a HGNPs sample was exposed to NIR-laser light for 5 min, then the sample was passively cooled at room temperature, then exposed again to laser light. This “on and off” cycle of NIR-laser irradiation was conducted three times while monitoring the temperature of the sample.
Cell CultureA C6 rat glioma cell line was obtained from American type culture collection (Rockville, MD, U.S.A.). The cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, supplemented with penicillin, in a cell culture dish and incubated at 37 °C in a CO2 incubator.
WST-8 AssayAs a typical experiment, cells were seeded at a density of 1 × 104 cells per well (100 µL) into 96-well plate and incubated for 24 h. The cells were treated with DDTC (0, 0.2, 0.3, 0.4, 0.5, 1.0, 1.5, or 2.0 µg/mL), DDTC-Cu (0, 0.2, 0.3, 0.4, 0.5, 1.0, 1.5, or 2.0 µg/mL), HGNPs (0, 6.3, 12.4, 18.4, or 24.5 µg/mL), or mixtures of HGNPs (12.4 µg/mL) and different concentrations of DDTC-Cu (0, 0.2, 0.3, 0.4, 0.5, 1.0, 1.5, or 2.0 µg/mL) and incubated for 1 h. To treat cells with laser light, the cells were irradiated using the NIR-laser (660 nm, 1.5 W/cm2) for 2–5 min, then the cells were washed with PBS and cultured for 24 h. Cell viability was determined using a Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan), following the manufacturer’s protocol. The absorbance of the samples was measured using a plate reader (Nivo 3S; wavelength, 450 nm).
Here, we synthesized hollow HGNPs as shown in the Graphical Abstract. HGNPs were synthesized using an AgCl template method.28) In this method, gold ions are reduced to Au on the surface of the AgCl template, and then AgCl is removed through a washing process. In our study, one step preparation method was adopted for its facile preparation. During this process, AgCl nuclei likely formed within the nanoparticles, and Au nucleation in the solution was initiated simultaneously using the reducing agent hydroquinone. The surface of the nanoparticle is then coated simultaneously with the surfactant PVP. The sacrificial AgCl template and PVP are removed using ammonium water and subsequent washing steps.
We first investigated the synthesis of HGNPs by changing the experimental conditions, as shown in Table 1. The concentration and the rate of dropping HAuCl4 into the reaction mix were altered to determine if these conditions affect the nucleation of AgCl and Au, and thus the resulting HGNPs. The particle size, PDI, and morphology of the HGNPs are shown in Table 1 and Fig. 1. We typically obtained monodisperse HGNPs with a particle size in the range of 100–130 nm and a PDI of around 0.1, except when using condition 1. Condition 1 used the slowest dropping rate and the lowest concentration of HAuCl4 and resulted in pronounced precipitation and thus we did not measure the particle size. TEM showed that this sample comprised non-uniform nanoparticles (Fig. 1a) and a hollow structure, perhaps due to insufficient Au, leading to the nucleation and growth of cubic AgCl.
| Condition | Conc of HAuCl4 (mM) | Dropping rate (µL/min) | Particle size (nm) | PDI | Peak wavelength (nm) |
|---|---|---|---|---|---|
| 1 | 10 | 50 | ND | ND | ND |
| 2 | 10 | 100 | 98.2 ± 2.6 | 0.113 ± 0.016 | 660 |
| 3 | 10 | 150 | 94.9 ± 2.1 | 0.116 ± 0.011 | 643 |
| 4 | 10 | 200 | 101.3 ± 4.5 | 0.104 ± 0.012 | 661 |
| 5 | 20 | 50 | 106.9 ± 4.6 | 0.089 ± 0.017 | 716 |
| 6 | 20 | 100 | 111.6 ± 4.4 | 0.094 ± 0.017 | 693 |
| 7 | 30 | 50 | 121.7 ± 5.6 | 0.085 ± 0.020 | 702 |
| 8 | 40 | 50 | 130.7 ± 7.6 | 0.129 ± 0.016 | 606 |
The mean particle size and PDI for the HGNPs (n = 4) and the peak wavelength of light absorbance. ND, No data.
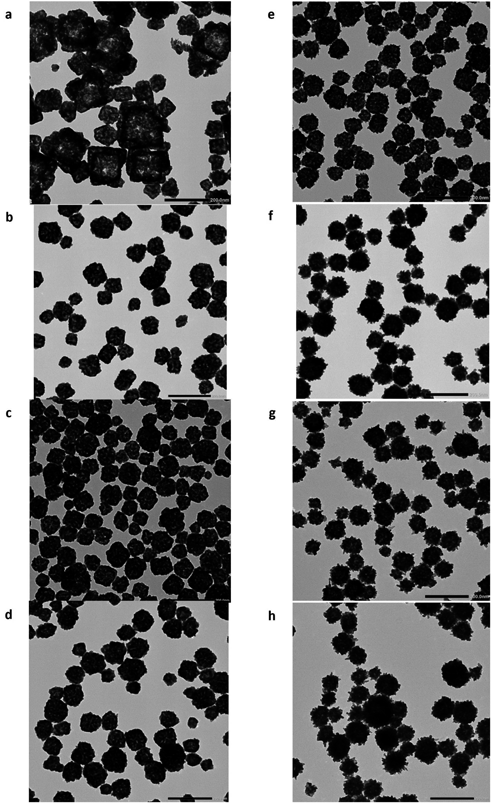
(a–h) HGNPs prepared using conditions 1–8, described in Table 1. The detailed preparation of HGNPs is described in Materials and Methods.
The use of higher concentrations of HAuCl4 (conditions 5–8, Figs. 1e–h) resulted in HGNPs with remarkable protruding structures, like gold nanostars (also called gold nano-urchins), suggesting that the Au protrusions on the nanoparticle surface grew after formation of the core nanoparticle. Since these protruding structures with high aspect ratios can affect light absorbance, HGNPs obtained using conditions 5–7 exhibited longer wavelength absorption peaks compared with those obtained using conditions 2–4 (Table 1). In contrast, HGNPs samples prepared using the highest concentration of HAuCl4 (condition 8) exhibited slightly larger particle sizes and PDI, and the lowest wavelength absorption peak of the samples prepared. Higher gold concentrations might inhibit the nucleation of AgCl, leading to the formation of low porosity HGNPs that absorb light of lower wavelength. We used an NIR-laser with a wavelength of around 660 nm because this wavelength has previously been used for photodynamic therapy using a photosensitizer (talaporfin sodium) to treat glioma and brain cancers in a clinical setting.41,42) As the laser power differs from that used in this study, further experiment regarding laser power is necessary. Overall, good HGNPs that absorb light with a higher wavelength (660 nm <) could be obtained under conditions 2, 4, 5, 6, and 7. In this study, we selected the HGNPs prepared under condition 5, which exhibited the highest peak wavelength in the UV-Vis spectrum (716 nm). This choice was made because the higher wavelength is advantageous for better penetration through body tissues, making it useful for future PTT with a NIR laser of this wavelength, although the current photodynamic therapy against glioma uses a 660 nm laser.
HGNPs can be prepared under restricted experimental conditions in another article.30) The concentration of hydroquinone for the preparation of HGNPs was previously investigated and the particle size of the resulting HGNPs was inversely related to the concentration (270–450 nm). This earlier report suggested that the concentration of reducing agent affected the balance between the growth rate of the sacrificial AgCl and Au particles, affecting the particle size.
Photothermal Effect of HGNPsHGNPs samples were irradiated using the NIR-laser, and the effect of irradiation time on the temperature increase was investigated (Fig. 2). The temperature increase correlated positively with the increase in HGNP concentration. For example, when the concentration of HGNPs was 12.4 µg/mL, the temperature was 43.4 ± 0.5 °C at 2 min, 49.8 ± 1.0 °C at 3 min, 54.5 ± 0.8 °C at 4 min, and 58.5 ± 0.5 °C at 5 min. This temperature profile is effective for inducing irreversible cell damage (> 50 °C) and for apoptosis (41–45 °C) caused by hyperthermic conditions.43) The temperature increase stopped around 80 °C when higher concentration HGNPs samples were irradiated, possibly due to the ambient temperature. These results suggest that the current experimental conditions could be suitable for photothermal cancer therapy. Regarding the light absorbance of DDTC-Cu, it had peaks around 447 nm and UV region (< 300 nm).44) Thus, we consider that the influence of NIR-laser on the DDTC-Cu is minimum.

The data represent the mean ± standard deviation (n = 3).
Furthermore, we investigated the effect of intermittent NIR-laser irradiation on temperature increase to better understand the stability of the HGNPs (Supplementary Fig. 1). The peaks of the temperature rise in the second and third cycles were 9% and 15.6% lower compared to the first cycle (30 °C, 5 min), indicating that the nanocarriers tolerated NIR irradiation well. The decreasing temperature increases may be due to slight changes in the nanostructure of the HGNPs caused by NIR irradiation, decreasing the light-to-heat conversion efficiency. Overall, these results suggest that repeated treatment of tumors might be possible.
Cytotoxic Effect of HGNP-Based Photothermal Therapy, DDTC-Cu-Based Chemotherapy, and Their Combination for Treatment in VitroDDTC, a metabolite of DSF, inhibits the proliferation of cancer cells, including glioma. The treatment with DDTC exhibited moderate cytotoxicity on C6 glioma cells in the current condition (Fig. 3a, 70.5%; 2 µg/mL). The presence of DDTC-Cu on the viability of glioma cells was assessed to understand the effect of copper ion (Fig. 3b). DDTC is a potent chelator of divalent metal ions such as copper.37) DDTC-Cu exhibited a cytotoxic effect at relatively higher concentrations (48.3%, 1 µg/mL; 15.4%, 2 µg/mL).

The data represent the mean ± standard deviation (n = 3).
The toxicity of HGNPs without NIR-laser irradiation was assessed to help understand the biocompatibility of gold-based nanoparticles (Fig. 3c). No remarkable cytotoxicity was observed, although cell viability slightly decreased at the highest concentration (90.8%, 24.5 µg/mL). In contrast, the mixture of DDTC-Cu and HGNPs without NIR-laser irradiation was cytotoxic in a dose dependent manner (Fig. 3d). The mixing of DDTC-Cu and HGNPs tended to cause aggregation at high concentration, suggesting that moderate aggregation might increase the total size of the complex and increase the amount of cellular uptake of DDTC, resulting in a pronounced cytotoxic effect on glioma cells. Ly et al. mentioned that the interaction of DDTC-Cu with gold nanoparticles through adsorption, detecting the copper ions in electroplating industrial wastewater.45) This description may partially support our explanation about the aggregation.
The outcome of PTT using the cytotoxic effect of HGNPs combined with NIR-laser irradiation is shown in Fig. 4a. The degree of cytotoxicity was dependent on the irradiation time (73.8%, 3 min; 50.7%, 4 min, 12.2%, 5 min). The temperature increase caused by the absorption of laser energy and its conversion into heat (Fig. 2) effectively killed the cells, while the treatment with HGNPs without NIR-laser irradiation did not exhibit remarkable cytotoxicity (Fig. 3c). PTT using HGNPs and NIR-laser irradiation combined with DDTC-Cu is shown in Fig. 4b and provided a clear cytotoxic effect (< 20%) in vitro, whereas DDTC-Cu and HGNPs without NIR-laser irradiation (Fig. 3d) and PTT alone (Fig. 4a) exhibited a moderate cytotoxic effect. The combination of DDTC-Cu and HGNPs might increase the total size of the complex and increase cellular uptake, resulting in a remarkable cytotoxic effect as well as the results in Fig. 3d. Alternatively, the cytotoxic effect is due to increased sensitivity to chemotherapy by PTT. The in vitro results of combined treatment hold promise in the treatment of glioma. We speculate that the prolonged treatment with PTT (e.g., 5 min NIR-laser irradiation) also has a high therapeutic effect.

The data represent the mean ± standard deviation (n = 3).
Future glioma therapy might involve conducting nanoparticle-based photothermal therapy after the uptake of HGNPs by glioma cells to avoid affecting normal cells. Localized therapy, which can involve surgical resection, is effective and thus PTT treatment would likely be conducted by NIR-laser irradiation and monitoring the temperature of the treated brain tissue. This therapeutic system may be effective for cancer prevention. Several barriers must be overcome prior to successful PTT. It is difficult to treat glioma cells completely due to cancerous cells infiltrating non-cancerous tissue. The incorporation of an imaging probe into HGNPs for theranostics, which is the combination therapy of therapeutics and diagnostics for personalized medicine, can visualize the distribution of nanoparticles and may improve the accurate therapy.43) The PTT using NIR-laser has limitations in terms of permeability into body tissue. The development of a fiber type NIR-laser that can reach deeper regions might help irradiation against glioma. Combining PTT with other treatment modalities, such as chemotherapy, could destruct tumor cells, improving the therapeutic outcome.43) Clinical trials of DDTC-Cu against glioma as a new chemotherapy in combination with PTT may prove this approach to be effective.
In conclusion, we developed HGNPs using a facile one-step method. The particle size and optical properties of HGNPs differ depending on surface roughness and porosity, which can be changed by controlling the dropping rate and concentration of HAuCl4. HGNPs with appropriate photothermal properties were suitable for NIR-laser-based PTT, and in the presence of DDTC-Cu, a remarkable cytotoxic effect against glioma cells was observed in vitro. Further investigations, such as in vivo experiments, are necessary to assess the therapeutic effect and safety of this approach. The results of the current study provide useful information regarding HGNPs and chemotherapy using DDTC-Cu as a combined treatment modality to treat glioma.
This work was supported in part by JST SPRING, Grant Number: JPMJSP2130.
The authors declare no conflict of interest.
This article contains supplementary materials.