2024 Volume 47 Issue 12 Pages 2003-2010
2024 Volume 47 Issue 12 Pages 2003-2010
Using 2-pyrazole oxazolidine-4-one derivative 1 as the lead compound, a series of novel 2-heteroaryl-oxazolidine-4-one derivatives were designed and synthesized by replacing pyrazole moiety with other heterocycles, including methyl pyrazole, oxazole, thiazole, triazole, and reverse pyrazoles, based on the principle of bioisosterism. The structures of target compounds were established by 1H-NMR, 13C-NMR, and electrospray ionization (ESI)-MS. Majority of these compounds showed moderate to good inhibitory activities against hepatitis B virus (HBV) DNA proliferation with low cytotoxicities. Especially, compound 7g showed the most potent anti-HBV activity with EC50 value of 0.059 µM, accompanied with better selectivity index (SI) value than that of lead compound. Our research revealed the structure–activity relationship (SAR) of these newly designed compounds and compound 7g was identified as the potential HBV capsid assembly modulator.
According to WHO, there were over 257 million hepatitis B virus (HBV) infections worldwide, with approximately 15% of the cases continued to develop into diseases such as liver cancer, cirrhosis, and liver fibrosis, and about 650000 people directly or indirectly died of Chronic hepatitis B (CHB) each year.1–3) Anti-HBV drugs on clinical were mainly divided into Nucleoside analogs (NAs), such as tenofovir, entecavir, etc., as well as interferon (IFN).4–6) Nucleoside analogs can effectively reduce the viral titer in patients, and are most widely used in clinic. However, long-term use will lead to drug resistance and a high recurrence rate after drug withdrawal. There are only about 20% of patients responded well to IFN treatment, which limited its clinical use. Therefore, it is of great significance to develop discover novel anti-HBV medicine to achieve the functional cure of hepatitis B.2,7,8)
HBV is a hepatotropic DNA virus consisting of an envelope and a nuclear shell. The nucleocapsid is composed of capsid proteins, HBV relaxed circular DNA (rcDNA), and HBV reverse transcriptase that binds to the 5′ end of the minus strand of rcDNA. Nucleocapsid assembly is the critical step for virus replication in human body9) and blocking capsid assembly with the potential to achieve functional cure of hepatitis B. Therefore, HBV capsid protein modulators were regarded as promising HBV therapeutic strategy, and several of them, including GLS-4, RO-68896787, ABI-H 0731, AB-423, JNJ-6379, GST-H141, NZ-4, NVR-3778, and ZM-H1505R, had entered clinical trials10–13) (Fig. 1).

Among those HBV capsid protein modulators, compound 1, a pyrazole-oxazolinone derivative similar to ZM-H1505R, attracted our attention due to its potent anti-HBV acitivity (EC50 = 11.8 nM).14) With the aim to get more potent and safe oxazolinone derivatives, the docking study of compound 1 with HBV core protein was performed to know its possible binding mode.
In our previous work, it was confirmed that the HBV core protein (5E0I) was the optimal model to carry out docking study due to its excellent performance to distinguish positive or negative HBV capsid modulators.15) As shown in Fig. 2, compound 1 has a nice fit in the pocket, and combined with the protein in a “V” shape mode. In the middle part, the oxygen of oxazolidine-4-one ring formed a critical hydrogen bond with the amide of Leu140. While the pyrazole core did not form any obvious interaction with the amino acid residues of protein, indicating the modification on this part was reasonable.

Therefore, using the bioisosterism strategy, seven novel 2-heteroaryl-oxazolidine-4-one derivatives 7a–7g were designed based on docking study of compound 1 with the protein, in which the central pyrazole ring was replaced with different five-member heterocycles, including methyl pyrazole, oxazole, thiazole, triazole or inverted pyrazole (Fig. 3), and the structure–activity relationship (SAR) of these compounds was discussed.

The intermediates 3a–3g was synthesized from different substrates and the detail was described in Supplementary Materials.16–20)
Intermediate 2 was obtained by condensation of p-nitrophenylethylamine with glycolic acid. The intermediate diaryl heterocyclic formate 3a–3g were reduced by diisobutyl aluminum hydride (DIBAL-H) to obtain diaryl heterocyclic methanol intermediates 4a–4g, which were then oxidized by Dess–Martin reagent to obtain diaryl heterocyclic formaldehyde intermediates 5a–5g, followed by condensation with compound 2 in the presence of p-toluenesulfonic acid in toluene provide compounds 6a–6g, which were finally reduced with stannous chloride to furnish target compounds 7a–7g21–25) (Fig. 4).

Reagents and conditions: (A) N-Methylacetylene toluene sulfonamide, 2-hydroxyacetic acid, CH2Cl2; (B) Diisobutyl aluminum hydride, CH2Cl2, −78 °C; (C) DMP, CH2Cl2; (D) p-Toluenesulfonic acid, intermediate 2, toluene, reflux over night; (E) SnCl2·H2O, EtOH, reflux.
In Vitro Anti-HBV Activity and Cytotoxicity of Target Compounds 7a–7gThe in vitro anti-HBV activities and cytotoxicity of target compounds were evaluated in HepG2.2.15 cells, the data was shown in Table 1.
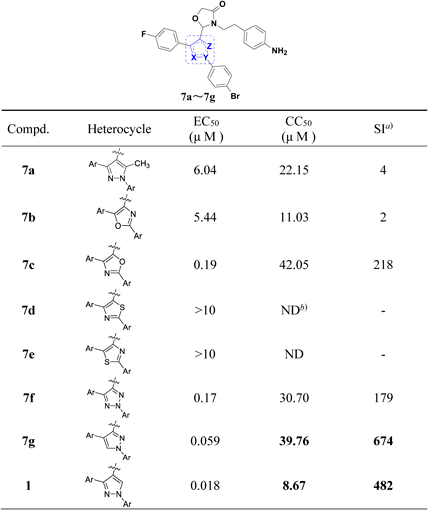 |
a) Selectivity index, SI = CC50 /EC50; b) ND = not detected.
As shown in Table 1, the modification on the pyrazole moiety of compound 1 caused dramatical effect on their anti-HBV activities. Methyl-pyrazole derivative 7a showed an EC50 value of 6.04 µM, which was more than 300 folds decrease in comparison with lead compound 1, indicating the Z part of heterocycle was not tolerable, even for methyl moiety. The thiazole derivatives 7d and 7e lost their activities (EC50 >10 µM), suggesting the introduction of sulfur atom in heterocycles was not suitable. Oxazole derivative 7b showed moderate anti-HBV activity with EC50 value of 5.44 µM, which was 300 folds decrease in comparison with lead compound 1. Triazole derivative 7f (EC50 = 0.17 µM) showed weaker activity than pyrazole derivatives but stronger than thiazole derivatives, which indicated that excessive electronegativity of heteroatoms in the central aromatic heterocycle would lead to a decrease in anti-HBV activity. Inverted pyrazole derivative 7g, 1,2,3-triazole derivatives 7f and oxazole derivative 7c showed good to excellent HBV inhibitory activities, with EC50 values of 0.059, 0.17, and 0.19 µM, respectively. The most potent compound 7g showed weak cytotoxicity with 50% cytotoxicity concentration (CC50) value of 39.76 µM, indicating its improved safety profiles compared with compound 1 (CC50 = 8.67).
Molecular DockingSimilarly, the molecular docking study of compound 7g with HBV core protein (5E0I) was performed to reveal its possible binding mode with HBV core protein.15) As shown in Fig. 5, the compound 7g has a nice fit with the binding pocket of protein. The figure also depicted that 7g combined with the protein in a “V” shape mode similar to compound 1. In the middle part, the oxygen of oxazolidine-4-one ring formed a critical hydrogen bond with the amide of Leu140. In the left part of “V” shape, the 4-F-phenyl ring formed an edge-to-face π–π interaction with Trp102 deep into the hydrophobic pocket.26) In the right part, the terminal 4-NH2-phenyl ring formed another edge-to-face π–π stacking with Trp125 in the solvent zone.

To get more potent HBV capsid modulators, the pyrazole-oxazolinone derivative 1 was employed as lead compound, and the central pyrazole ring was replaced with methyl pyrazole, oxazole, thiazole, triazole or inverted pyrazole to furnish a series of 2-heteroaryl-oxazolidine-4-one derivatives. The SAR study revealed that the central pyrazole was essential moiety for the anti-HBV activity and compound 7g with inverted pyrazole demonstrated good potential as HBV capsid modulator with better safety profiles than lead compound 1. Our research provides good basis for the develop novel 2-heteroaryl-oxazolidine-4-one derivatives as HBV capsid modulators.
All reagents were purchased from commercial suppliers in China and were used as received. Flash column chromatography was performed to purify the synthesized compounds over 200–300 mesh silica gel. Human liver cancer cell line HepG2.2.15 expressing HBV virus was purchased from Shanghai Bihe Biochemical Technology Co., Ltd. (China). GLS4 was homemade. Hepatitis B virus nucleic acid testing kit was bought from Hunan Shengxiang Biotechnology Co., Ltd. (China).
InstrumentsThe BRUKER AVANCE III 500 spectrometer (Bruker, Biotechnology) were used for 1H- and 13C-NMR. LC-mass spectrometry (LCQ-DECAXP) with multi-stage ion trap mass spectrometry (Finnegan Mass Spectrometry, U.S.A.) and Shimadzu LC/MS-2020 LC-mass spectrometry (Shimadzu Corporation, Japan) were used for analysis.
ChemistrySynthesis of 2-Hydroxyl-N-(4-nitrophenylethyl) Acetamide (2)A mixture of N-methylsulfonamide (MYTsA) (11.6 g, 55.5 mmol), 2-hydroxyacetic acid (3.3 mL, 55.5 mmol) and dichloromethane (50.0 mL) was stirred at room temperature for 2 h. After that, p-nitrophenylethylamine (9.2 g, 55.5 mmol) was added into the mixture and stirred until the raw material disappeared. The reaction mixture was concentrated in vacuum and the residue was purified by silica gel column chromatography to afford the intermediate 2 (85.1%, 10.6 g). White solid, 85.1% yield; 1H-NMR (500 MHz, dimethyl sulfoxide (DMSO)-d6) δ: 8.15 (d, J = 8.5 Hz, 2H), 7.85 (t, J = 5.5 Hz, 1H), 7.49 (d, J = 8.5 Hz, 2H), 5.48 (t, J = 5.5 Hz, 1H), 3.76 (d, J = 4.5 Hz, 2H), 3.39 (m, 2H), 2.90 (t, J = 7.0 Hz, 2H); electrospray ionization (ESI)-MS: m/z = 225.1 [M + H]+.
Synthesis of Intermediates 3a–3gFull details are given in Supplementary materials.
Synthesis of Intermediates 4a–4gA mixture of intermediate 3a (3.0 g, 7.7 mmol) and anhydrous dichloromethane solution (30 mL) was stirred at −78 °C under nitrogen atmosphere. Diisobutyl aluminum hydride (1.5 mol/L, 15.4 mL, 23.1 mmol) was added to the system slowly. The mixture was continually stirred at −78 °C until the raw material disappeared. The reaction was quenched by saturated NH4Cl solution, and the reaction mixture was extracted with dichloromethane (3 times). The organic layer was successively washed with water and saturated salt water, dried over Na2SO4, and filtered. The filtrate was concentrated in vacuum and the residue was purified by silica column chromatography to afford the intermediate 4a (95%, 2.64g). Compounds 4b–4g were prepared using the same method.
(1-(4-Bromophenyl)-3-(4-fluorophenyl)-5-methyl-1H-pyrazole-4-) methanol (4a): White solid, 95% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 7.91–7.88 (m, 2H), 7.75 (d, J = 9.0 Hz, 2H), 7.57 (d, J = 9.0 Hz, 2H), 7.31 (t, J = 9.0 Hz, 2H), 5.10 (s, 1H), 4.41 (d, J = 3.0 Hz, 2H), 2.37 (s, 3H); ESI-MS: m/z = 361.0 [M + H]+.
(2-(4-Bromphenyl)-5-(4-fluorophenyl)oxazole-4-) methanol (4b): White solid, 85% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 8.00 (d, J = 8.5 Hz, 2H), 7.91 (dd, J = 8.5, 5.5 Hz, 2H), 7.77 (d, J = 8.5 Hz, 2H), 7.41 (t, J = 9.0 Hz, 2H), 5.47 (t, J = 5.5 Hz, 1H), 4.57 (d, J = 5.0 Hz, 2H); ESI-MS: m/z = 348.0 [M + H]+.
(2-(4-Bromophenyl)-4-(4-fluorphenyl)oxazole-5-) methanol (4c): White solid, 83% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 8.10–8.06 (m, 2H), 7.78–7.74 (m, 2H), 7.70 (d, J = 8.5 Hz, 2H), 7.41 (t, J = 9.0 Hz, 2H), 5.69 (t, J = 5.5 Hz, 1H), 4.69 (d, J = 6.0 Hz, 2H); ESI-MS: m/z = 348.0 [M + H]+.
(2-(4-Bromophenyl)-4-(4-fluorophenyl)thiazole-5-) methanol (4d): White solid, 85% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 7.93 (d, J = 9.0 Hz, 2H), 7.80 (dd, J = 9.0, 5.5 Hz, 2H), 7.72 (d, J = 8.5 Hz, 2H), 7.35 (t, J = 9.0 Hz, 2H), 6.00 (t, J = 5.5 Hz, 1H), 4.79 (d, J = 5.5 Hz, 2H); ESI-MS: m/z = 364.0 [M + H]+.
(2-(4-Bromophenyl)-5-(4-fluorophenyl)thiazole-4-) methanol (4e): White solid, 82% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 7.88 (d, J = 9.0 Hz, 2H), 7.74 (dd, J = 9.0, 5.5 Hz, 2H), 7.65 (d, J = 8.5 Hz, 2H), 7.25 (t, J = 9.0 Hz, 2H), 5.98 (t, J = 5.5 Hz, 1H), 4.74 (d, J = 5.5 Hz, 2H); ESI-MS: m/z = 364.0 [M + H]+.
(2-(4-Bromophenyl)-5-(4-fluorophenyl)-2H-1,2,3-triazole-4-) methanol (4f): White solid, 89% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 8.04–8.00 (m, 4H), 7.79 (d, J = 8.0 Hz, 2H), 7.41 (t, J = 8.5 Hz, 2H), 5.69 (t, J = 5.5 Hz, 1H), 4.74 (d, J = 5.5 Hz, 2H); ESI-MS: m/z = 348.0 [M + H]+.
(1-(4-Bromophenyl)-4-(4-fluorobophenyl)-1H-pyrazole-3-) methanol (4g): White solid, 88% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 8.86 (s, 1H), 7.86 (d, J = 8.5 Hz, 2H), 7.80–7.77 (m, 2H), 7.72 (d, J = 9.0 Hz, 2H), 7.30 (d, J = 9.0, 2H), 5.42 (s, 1H), 4.57 (s, 2H); ESI-MS: m/z = 347.0 [M + H]+.
Synthesis of Intermediates 5a–5gA mixture of DMP (5.8 g, 20.7 mmol) and intermediate dichloromethane (15 mL) was added into the mixture of 4a (2.5 g, 6.9 mmol) and dichloromethane (15 mL). The mixture was then stirred at room temperature for 2 h. After that, the reaction mixture was extracted with ethyl acetate (3 times). The organic layer was concentrated in vacuum. The crude product was purified by silica column chromatography to afford the intermediate 5a (98%, 2.4 g). Compounds 5b–5g were prepared by the same method.
1-(4-Bromophenyl)-3-(4-fluorophenyl)-5-methyl-1H-pyrazole-4-formaldehyde (5a): White solid, 98% yield; 1H-NMR (500 MHz, CDCl3) δ: 10.04 (s, 1H), 7.71–7.66 (m, 4H), 7.40 (d, J = 8.5 Hz, 2H), 7.19 (t, J = 8.5 Hz, 2H), 2.65 (s, 3H) ESI-MS: m/z = 359 [M + H]+.
2-(4-Bromophenyl)-5-(4-fluorophenyl)oxazole-4-formaldehyde (5b): White solid, 95% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 10.05 (s, 1H), δ: 8.00 (d, J = 8.5 Hz, 2H), 7.91 (dd, J = 8.5, 5.5 Hz, 2H), 7.77 (d, J = 8.5 Hz, 2H), 7.41 (t, J = 9.0 Hz, 2H); ESI-MS: m/z = 346.0 [M + H]+.
2-(4-Bromophenyl)-4-(4-fluorophenyl)oxazole-5-formaldehyde (5c): White solid, 95% yield; 1H-NMR (500 MHz, DMSO-d6) δ: 10.32 (s, 1H), δ 8.10–8.06 (m, 2H), 7.78–7.74 (m, 2H), 7.70 (d, J = 8.5 Hz, 2H), 7.41 (t, J = 9.0 Hz, 2H); ESI-MS: m/z = 346.0 [M + H]+.
2-(4-Bromophenyl)-4-(4-fluorophenyl)thiazole-5-formaldehyde (5d): White solid, 85% yield; 1H-NMR (500 MHz, CDCl3) δ: 10.00 (s, 1H), 7.95 (d, J = 8.5 Hz, 2H), 7.80–7.77 (m, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.26–7.23 (m, 2H), ESI-MS: m/z = 362.0 [M + H]+.
2-(4-Bromophenyl)-5-(4-fluorophenyl)thiazole-4-formaldehyde (5e): White solid, 88% yield; 1H-NMR (500 MHz, CDCl3) δ: 10.21 (s, 1H), 7.91 (d, J = 8.5 Hz, 2H), 7.84–7.79 (m, 2H), 7.62 (d, J = 8.5 Hz, 2H), 7.21–7.18 (m, 2H), ESI-MS: m/z = 362.0 [M + H]+.
2-(4-Bromophenyl)-5-(4-fluorophenyl)-2H-1,2,3-triazole-4-formaldehyde (5f): White solid, 92% yield; 1H-NMR (500 MHz, CDCl3) δ: 10.31 (s, 1H), 8.22–8.19 (m, 2H), 8.10 (d, J = 9.0 Hz, 2H), 7.69 (d, J = 9.0 Hz, 2H), 7.21 (t, J = 9.0 Hz, 2H); ESI-MS: m/z = 346.0 [M + H]+.
1-(4-Bromophenyl)-4-(4-fluorobophenyl)-1H-pyrazole-3-formaldehyde (5g): Yellow-white solid, 88% yield; 1H-NMR (500 MHz, CDCl3) δ: 10.16 (d, J = 0.5 Hz, 1H), 8.02 (s, 1H), 7.71–7.65 (m, 4H), 7.64–7.61 (m, 2H), 7.14 (t, J = 8.5 Hz, 2H); ESI-MS: m/z = 365 [M + H]+.
Synthesis of 2-Substituted-3-(4-nitrophenyl)oxazolididl-4-ketone Derivatives 6a–6gA mixture of the intermediate 5a (2.0 g, 5.5 mmol), intermediate 2 (1.3 g, 6.1 mmol), p-toluenic acid (28.7 mg, 0.1 mmol) and toluene (20 mL) was refluxed in a water separator until the raw material disappeared. The reaction mixture was concentrated in vacuum and the residue was purified by silica gel column chromatography to afford the intermediate 6a (90%, 2.83 g). Compounds 6b–6g were prepared using the same method.
2-(1-(4-Bromophenyl)-3-(4-fluorophenyl)-5-methyl-1H-pyrazole-4-)-3-(4-nitro-phenylethyl) oxazolidine-4-ketone (6a): White solid, 90% yield; 1H-NMR (500 MHz, CDCl3) δ: 8.03 (d, J = 8.5 Hz, 2H), 7.66 (d, J = 8.5 Hz, 2H), 7.51–7.48 (m, 2H), 7.36 (d, J = 8.5 Hz, 2H), 7.16–7.11 (m, 4H), 5.86 (s, 1H), 4.37–4.34 (m, 1H), 4.28–4.25 (m, 1H), 3.87–3.81 (m, 1H), 2.98–2.84 (m, 2H), 2.72–2.67 (m, 1H), 2.26 (s, 3H); ESI-MS: m/z = 565.0 [M + H]+.
2-(2-(4-Bromophenyl)-5-(4-fluorophenyl)oxazole-4-)-3-(4-nitrophenylethyl) oxazolidine-4-ketone (6b): White solid, 90% yield;1H-NMR (500 MHz, CDCl3) δ: 7.80 (d, J = 9.0 Hz, 2H), 7.94 (d, J = 9.0 Hz, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.55 (dd, J = 9.0, 5.0 Hz, 2H), 7.26–7.17 (m, 4H), 5.93 (d, J = 1.0 Hz, 1H), 4.54–4.51 (m, 1H), 4.36–4.33 (m, 1H), 3.98–3.93 (m, 1H), 3.19–3.13 (m, 1H), 3.00–2.87 (m, 2H); ESI-MS: m/z = 552.1 [M + H]+.
2-(2-(4-Bromophenyl)-4-(4-fluorophenyl)oxazole-5-group)-3-(4-nitrophenylethyl)oxazolidine-4-ketone (6c): White solid, 92% yield; 1H-NMR (500 MHz, CDCl3) δ: 7.98 (d, J = 9.0 Hz, 2H), 7.91 (d, J = 8.5 Hz, 2H), 7.63 (d, J = 8.5 Hz, 2H), 7.59–7.56 (m, 2H), 7.18–7.15 (m, 4H), 6.00 (s, 1H), 4.55–4.52 (m, 1H), 4.39–4.36 (m, 1H), 3.91–3.86 (m, 1H), 3.16–3.10 (m, 1H), 2.93–2.87 (m, 1H), 2.84–2.78 (m, 1H); ESI-MS: m/z = 552.1 [M + H]+.
2-(2-(4-Bromophenyl)-4-(4-fluorophenyl) thiazole-5-)-3-(4-nitrophenylethyl)oxazolidine-4-ketone (6d): White solid, 90% yield; 1H-NMR (500 MHz, CDCl3) δ: 7.99 (d, J = 8.5 Hz, 2H), 7.84 (d, J = 8.5 Hz, 2H), 7.63–7.59 (m, 4H), 7.20 (t, J = 8.5 Hz, 2H), 7.12 (d, J = 8.5 Hz, 2H), 5.95 (s, 1H), 4.49–4.46 (m, 1H), 4.33–4.30 (m, 1H), 3.95–3.90 (m, 1H), 3.07–3.01 (m, 1H), 2.89–2.83 (m, 1H), 2.81–2.75 (m, 1H); ESI-MS: m/z = 568.0 [M + H]+.
2-(2-(4-Bromophenyl)-5-(4-fluorophenyl)thiazole-4-)-3-(4-nitrophenylethyl)oxazolidine-4-ketone (6e): White solid, 86% yield; 1H-NMR (500 MHz, CDCl3) δ: 7.95 (d, J = 8.5 Hz, 2H), 7.82 (d, J = 8.5 Hz, 2H), 7.61–7.56 (m, 4H), 7.15 (t, J = 8.5 Hz, 2H), 7.10 (d, J = 8.5 Hz, 2H), 5.90 (s, 1H), 4.42–4.36 (m, 1H), 4.30–4.27 (m, 1H), 3.95–3.91 (m, 1H), 3.02–2.98 (m, 1H), 2.85–2.75 (m, 2H); ESI-MS: m/z = 568.0 [M + H]+.
2-(2-(4-Bromophenyl)-5-(4-fluorophenyl)-2H-1,2,3-triazole-4-)-3-(4-nitrophenylethyl)oxazolidine-4-ketone (6f): Yellow-white solid, 87.5% yield; 1H-NMR (500 MHz, CDCl3) δ: 8.05 (d, J = 9.0 Hz, 2H), 7.99 (d, J = 9.0 Hz, 2H), 7.66 (t, J = 3.0 Hz, 2H), 7.64 (t, J = 3.0 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H), 7.19 (t, J = 8.5 Hz, 2H), 6.14 (s, 1H), 4.35 (d, J = 1.0 Hz, 2H), 4.01–3.95 (m, 1H), 3.20–3.14 (m, 1H), 2.98–2.89 (m, 2H); ESI-MS: m/z = 552.1 [M + H]+.
2-(1-(4-Bromophenyl)-4-(4-fluorophenyl)-1H-pyrazole-3-)-3-(4-nitrophenylethyl)oxazolidine-4-ketone (6g): Yellow/white solid, 90.0% yield; 1H-NMR (500 MHz, CDCl3) δ: 8.06 (d, J = 9.0 Hz, 2H), 7.95 (s, 1H), 7.63–7.59 (m, 4H), 7.34–7.31 (m, 2H), 7.25 (d, J = 9.0 Hz, 2H), 7.12 (t, J = 8.5 Hz, 2H), 6.04 (s, 1H), 4.28–4.18 (m, 2H), 3.93–3.87 (m, 1H), 3.16–3.10 (m, 1H), 2.98–2.84 (m, 2H); ESI-MS: m/z = 551.1 [M + H]+.
Synthesis of 3-(4-Aminophenylethyl)-2-substituted Oxazolidine-4-ketone Derivatives 7a–7gA mixture of the intermediate 6a (2.0 g, 3.5 mmol), tin (II) chloride (4.0 g, 17.6 mmol) and ethanol (20 mL) was refluxed for 5 h. The solvent was removed under reduced pressure. Saturated NaHCO3 aqueous (100 mL) was added into the residue. The mixture was filtered by diatomaceous earth. The filtrate was extracted by dichloromethane (3 times). The organic layer was successively washed with water and saturated salt water, dried over anhydrous Na2SO4, filtered. The filtrate was concentrated in vacuum. The crude product was purified by silica gel column chromatography to afford the target compound 7a (90%, 1.7 g). Compounds 7b–7g were prepared by the same method.
3-(4-Aminophenylethyl)-2-(1-(4-bromophenyl)-3-(4-fluorophenyl)-5-methyl-1H-pyrazole-4-)oxazolidine-4-ketone (7a): White solid, 90% yield, m. p. >250 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 7.77 (d, J = 9.0 Hz, 2H), 7.59 (d, J = 9.0 Hz, 2H), 7.51–7.48 (m, 2H), 7.30 (t, J = 9.0 Hz, 2H), 6.65 (d, J = 8.5 Hz, 2H), 6.43 (d, J = 8.0 Hz, 2H), 5.97 (s, 1H), 4.91 (s, 2H), 4.26–4.17 (m, 2H), 3.43–3.39 (m, 1H), 2.74–2.69 (m, 1H), 2.48–2.45 (m, 1H), 2.35–2.29 (m, 1H), 2.24 (s, 3H); 13C-NMR (126 MHz, DMSO-d6) δ: 172.09, 163.78 (d, 1JCF = 245.7 Hz), 151.45, 149.01, 139.49, 138.82, 132.85, 130.73 (d, 3JCCCF = 8.8 Hz), 130.43, 129.41, 120.99, 119.63, 118.79, 116.43, 116.12 (d, 2JCCF = 21.4 Hz), 114.50, 112.66, 83.12, 73.78, 42.41, 33.34, 17.79; IR HR-MS (ESI) for C27H24BrFN4O2 [M +H]+, Calcd: 535.1139, Found: 535.1140.
3-(4-Aminophenylethyl)-2-(2-(4-bromophenyl)-5-(4-fluorophenyl)oxazole-4-)oxazolidine butyl-4-ketone (7b): White solid, 97% yield, m. p. 167–168 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 8.00 (d, J = 8.5 Hz, 2H), 7.78 (d, J = 8.5 Hz, 2H), 7.67–7.64 (m, 2H), 7.45 (t, J = 9.0 Hz, 2H), 6.73 (d, J = 8.0 Hz, 2H), 6.42 (d, J = 8.5 Hz, 2H), 6.17 (s, 1H), 4.97 (s, 2H), 4.41–4.38 (m, 1H), 4.32–4.30 (m, 1H), 3.67–3.62 (m, 1H), 2.90–2.84 (m, 1H), 2.61–2.55 (m, 1H), 2.48–2.45 (m, 1H); 13C-NMR (126 MHz, DMSO-d6) δ: 169.50, 163.68 (d, 1JCF = 249.5 Hz), 159.09, 148.86, 147.03, 133.29, 132.26, 129.10 (d, 3JCCCF = 8.8 Hz), 128.89, 128.27, 125.33, 125.28, 124.72, 122.99, 116.50 (d, 2JCCF = 21.4 Hz), 114.05, 84.54, 66.72, 41.76, 32.44; IR 3448.44, 3364.42, 3101.32, 3073.89, 2938.89, 2922.05, 2902.53, 2863.95, 1706.72, 1623.51, 1603.15, 1517.22, 1504.47, 1444.75, 1268.89, 1229.63, 1084.13, 1009.55, 838.72, 716.87, 588.57, 501.53; HR-MS (ESI) for C26H21BrFN3O3 [M + H]+, Calcd: 522.0823, Found: 522.0831.
3-(4-Aminophenylethyl)-2-(2-(4-bromophenyl)-4-(4-fluorophenyl)oxazole-5-)oxazolidine-4-ketone (7c): White solid, 95% yield, m. p. 180–181 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 7.96 (d, J = 9.0 Hz, 2H), 7.79 (d, J = 8.5 Hz, 2H), 7.64–7.62 (m, 2H), 7.39 (t, J = 9.0 Hz, 2H), 6.70 (d, J = 8.0 Hz, 2H), 6.41 (d, J = 8.0 Hz, 2H), 6.30 (s, 1H), 4.92 (s, 2H), 4.51–4.48 (m, 1H), 4.36–4.33 (m, 1H), 3.62–3.56 (m, 1H), 2.99–2.93 (m, 1H), 2.58–2.52 (m, 1H), 2.44–2.38 (m, 1H); 13C-NMR (126 MHz, DMSO-d6) δ: 169.76, 163.90 (d, 1JCF = 247.0 Hz), 160.43, 147.59, 142.50, 140.95, 132.81, 130.13 (d, 3JCCCF = 8.8 Hz), 129.33, 128.87, 126.70, 125.63, 125.55, 125.42, 116.60 (d, 2JCCF = 21.4 Hz), 114.53, 83.82, 67.09, 42.30, 32.89; HR-MS (ESI) for C26H21BrFN3O3 [M + H]+, Calcd: 522.0823, Found: 522.0824.
3-(4-Aminophenylethyl)-2-(2-(4-bromophenyl)-4-(4-fluorophenyl)thiazole-5-)oxazolidine-4-ketone (7d): White solid, 95% yield, m. p. 160–161 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 7.96 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H), 7.66–7.63 (m, 2H), 7.41 (t, J = 9.0 Hz, 2H), 6.63 (d, J = 8.0 Hz, 2H), 6.39 (d, J = 8.0 Hz, 2H), 6.19 (s, 1H), 4.88 (s, 2H), 4.41–4.38 (m, 1H), 4.29–4.27 (m, 1H), 3.60–3.55 (m, 1H), 2.94–2.88 (m, 1H), 2.44–2.39 (m, 1H); 13C-NMR (126 MHz, DMSO-d6) δ: 169.73, 168.06 (d, 1JCF = 248.2 Hz), 163.93, 161.96, 159.60, 155.13, 152.95, 147.56, 132.79, 132.34, 132.06, 131.37 (d, 3JCCCF = 8.8 Hz), 130.05, 129.30, 128.79, 125.18, 124.94, 116.41 (d, 2JCCF = 21.4 Hz), 114.48, 85.77, 66.87, 42.44, 32.39; HR-MS (ESI) for C26H21BrFN3O2S [M + H]+, Calcd: 538.0595, Found: 538.0596.
3-(4-Aminophenylethyl)-2-(2-(4-bromophenyl)-5-(4-fluorophenyl)thiazole-4-)oxazolidine-4-ketone (7e): White solid, 95% yield, m. p. 172–174 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 7.88 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H), 7.50–7.47 (m, 2H), 7.42 (t, J = 8.5 Hz, 2H), 6.64 (d, J = 8.5 Hz, 2H), 6.38 (d, J = 8.0 Hz, 2H), 5.90 (s, 1H), 4.86 (s, 2H), 4.38–4.25 (m, 2H), 3.64–3.59 (m, 1H), 2.80–2.75 (m, 1H), 2.47–2.45 (m, 1H), 2.37–2.32 (m, 1H); 13C-NMR (126 MHz, DMSO-d6) δ: 169.96, 165.27, 164.12 (d, 1JCF = 248.2 Hz), 148.69, 147.46, 138.44, 132.84, 132.10 (d, 3JCCCF = 8.8 Hz), 131.94, 129.17, 128.55, 125.49, 124.69, 116.91 (d, 2JCCF = 22.7 Hz), 114.47, 85.68, 67.15, 41.97, 32.74; HR-MS (ESI) for C26H21BrFN3O2S [M + H]+, Calcd: 538.0595, Found: 538.0600.
3-(4-Aminophenylethyl)-2-(2-(4-bromophenyl)-5-(4-fluorophenyl)-2H-1,2,3-triazole-4-)oxazolidine-4-ketone (7f): White solid, 83% yield, m. p. 200–202 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 8.01 (d, J = 9.0 Hz, 2H), 7.81 (d, J = 9.0 Hz, 2H), 7.73–7.70 (m, 2H), 7.41 (t, J = 9.0 Hz, 2H), 6.73 (d, J = 8.0 Hz, 2H), 6.41 (d, J = 8.5 Hz, 2H), 6.35 (s, 1H), 4.89 (s, 2H), 4.32–4.25 (m, 2H), 3.65–3.59 (m, 1H), 3.00–2.94 (m, 1H), 2.61–2.56 (m, 1H), 2.48–2.46 (m, 1H); 13C-NMR (126 MHz, DMSO-d6) δ: 169.89, 164.16 (d, 1JCF = 248.2 Hz), 147.51, 147.41, 143.60, 138.36, 133.22, 130.75 (d, 3JCCCF = 8.8 Hz), 129.32, 125.81, 125.55, 121.47, 121.14, 116.60 (d, 2JCCF = 22.7 Hz), 114.46, 84.79, 66.72, 42.33, 32.67; HR-MS (ESI) for C25H21BrFN5O2 [M + H]+, Calcd: 522.0935, Found: 522.0929.
3-(4-Aminophenylethyl)-2-(1-(4-bromophenyl)-4-(4-fluorophenyl)-1H-pyrazole-3-)oxazolidine-4-ketone (7g): White solid, 90% yield, m. p. 190–191 °C; 1H-NMR (500 MHz, DMSO-d6) δ: 8.83 (s, 1H), 7.86 (d, J = 8.9 Hz, 2H), 7.87 (d, J = 9.0 Hz, 2H), 7.76 (d, J = 9.0 Hz, 2H), 7.48 (dd, J = 8.5, 5.5 Hz, 2H), 7.30 (t, J = 8.5 Hz, 2H), 6.71 (d, J = 8.5 Hz, 2H), 6.42 (d, J = 8.5 Hz, 2H), 6.16 (s, 1H), 4.89 (s, 2H), 4.25–4.14 (m, 2H), 3.57–3.52 (m, 1H), 2.88–2.82 (m, 1H), 2.58–2.52 (m, 1H), 2.46–2.40 (m, 1H); 13C-NMR (126 MHz, CDCl3) δ: 170.00, 163.49 (d, 1JCF = 247.0 Hz), 147.26, 145.02, 138.61, 132.75, 130.44 (d, 3JCCCF = 7.6 Hz), 129.48, 128.09, 127.15 (d, 4JCCCCF = 2.5 Hz), 126.64, 124.03, 120.60, 120.54, 115.95 (d, 2JCCF = 22.7 Hz), 115.42, 86.78, 67.28, 42.06, 32.81; HR-MS (ESI) for C26H22BrFN4O2 [M + H]+, Calcd: 521.0983, Found: 521.0984.
Biological EvaluaitonIn Vitro Assay of HBV DNACell culture: HepG 2.2.15 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (gibco) medium with 10% inactivated fetal bovine serum (gibco) and 1/1000 (200 mg/mL) G 418.
Experimental procedures: After digesting HepG2.2.15 cells grown in logarithmic phase, blew them into single-cell suspension and inoculated in a 96 well cell culture plate, 1 × 104 cells per well, then incubated at 37 °C in a 5% CO2 incubator for twenty-four hours. After the cells adhere to the wall, added target compounds 7a–7g by gradient concentration. After four days incubation in the incubator with 5% CO2 at 37 °C, renewed the culture medium containing test compounds, and incubated for another 3 d. The cell supernatant was collected and the HBV DNA copy number was measured by fluorescence quantitative PCR according to the instructions of hepatitis B virus nucleic acid testing kit. The results were recorded. The inhibition rate was calculated by (blank group-experimental group)/blank group × 100%. EC50 values were obtained by fitting them with the Graphpad Prism 8.0.2 software.
Evaluation of Cell CytotoxicityCell Culture: Same as “In Vitro Assay of HBV DNA.”
Experimental Procedures: After digesting HepG2.2.15 cells grown in logarithmic phase, blew them into single-cell suspension and inoculated in a 96 well cell culture plate, 1 × 104 cells per well, then incubated at 37 °C in a 5% CO2 incubator for twenty-four hours. After the cells adhere to the wall, added target compounds 7a–7g by gradient concentration. After 4 d incubation in the incubator with 5% CO2 at 37 °C, renewed the culture medium containing test compounds, and incubated for another 3 d. After discarding the supernatant, 10% CCK 8 (Solarbio) medium was added to each well. After 1 h incubation in 37 °C. The absorbance value was detected at 450 nm by the microplate reader, and the results were recorded. The inhibition rate was calculated by: (blank group-experimental group)/blank group 100%. IC50 values were obtained by fitting them with the Graphpad Prism 8.0.2 software.
This study was financially supported by the National Natural Science Foundation of China (No.21877097), Zhejiang Province Teaching Reform Project of Higher Vocational Education (jg20230241), Cultivation Program of Taizhou Vocational and Technical College (2021 PY 04). The authors would like to thank Jianyang Pan (Research and Service Center, College of Pharmaceutical Sciences, Zhejiang University) for performing NMR spectrometry for structure elucidation.
Project supported by the National Natural Science Foundation of China (No.21877097), Zhejiang Province Teaching Reform Project of Higher Vocational Education (jg20230241), Cultivation Program of Taizhou Vocational and Technical College (2021 PY 04).
The authors declare no conflict of interest.
This article contains supplementary materials.