2024 Volume 47 Issue 2 Pages 350-360
2024 Volume 47 Issue 2 Pages 350-360
Traumatic brain injury (TBI) is severe damage to the head caused by traffic accidents, falls, and sports. Because TBI-induced disruption of the blood–brain barrier (BBB) causes brain edema and neuroinflammation, which are major causes of death or serious disabilities, protection and recovery of BBB function may be beneficial therapeutic strategies for TBI. Astrocytes are key components of BBB integrity, and astrocyte-derived bioactive factors promote and suppress BBB disruption in TBI. Therefore, the regulation of astrocyte function is essential for BBB protection. In the injured cerebrum of TBI model mice, we found that the endothelin ETB receptor, histamine H2 receptor, and transient receptor potential vanilloid 4 (TRPV4) were predominantly expressed in reactive astrocytes. We also showed that repeated administration of an ETB receptor antagonist, H2 receptor agonist, and TRPV4 antagonist alleviated BBB disruption and brain edema in a TBI mouse model. Furthermore, these drugs decreased the expression levels of astrocyte-derived factors promoting BBB disruption and increased the expression levels of astrocyte-derived protective factors in the injured cerebrum after TBI. These results suggest that the ETB receptor, H2 receptor, and TRPV4 are molecules that regulate astrocyte function, and might be attractive candidates for the development of therapeutic drugs for TBI.
Traumatic brain injury (TBI) is severe damage to the head resulting from an external force caused by traffic accidents, falls, sports, etc. TBI is a major cause of unexpected death in young and old people worldwide and results in serious disabilities of motor, sensory, mental, and cognitive functions that cause an impressive decline in the QOL of surviving patients. Although emergent treatments are essential, current treatments such as decompressive craniotomy, hyperosmolar treatment, and sedation are extremely limited, and beneficial therapeutic drugs have not yet been developed. In the acute phase of TBI, the function of the blood–brain barrier (BBB) is substantially impaired by decreasing tight junction proteins and the loss of cells comprising the BBB,1,2) and disruption of the BBB causes brain edema and neuroinflammation, which are major causes of unexpected death or serious disabilities.3–5) Brain edema, especially vasogenic edema, results from the extravasation of intravascular water and serum proteins into the cerebral parenchyma following increased intracranial pressure and hernia, which causes critical impairment of the central nervous system and often results in the death of patients with TBI6,7) (Fig. 1). Neuroinflammation results from the infiltration of intravascular neutrophils and immune cells into the cerebral parenchyma, which exacerbates neuronal damage and causes various neurological dysfunctions in TBI patients.8,9) Therefore, the protection and recovery of BBB function are beneficial and essential therapeutic strategies for TBI.

TBI increases expression levels of vascular permeability-accelerating factors and cytokines that accelerate disruption of the BBB. BBB disruption promotes extravasation and accumulation of the intravascular fluid into the cerebral parenchyma following brain edema formation.
Astrocytes are the most abundant type of glial cell in the brain and are key components of the BBB, together with endothelial cells and pericytes. Astrocytic endfeet surround the cerebral microvessels, which strictly control the barrier function of the BBB. Additionally, astrocytes release various bioactive factors that regulate BBB permeability under both physiological and pathophysiological conditions.10) In response to brain damage, astrocytes transform from the resting to the reactive state, which is characterized by increased glial fibrillary acidic protein (GFAP) expression, hypertrophy, and ability to proliferate, resulting in astrogliosis.11,12) Reactive astrocytes exert protective and detrimental effects against brain damage through bioactive factors.10) Some studies have suggested a relationship between TBI and reactive astrocytes in both patients and experimental animal models. In patients with TBI, phenotypic conversion to reactive astrocytes was predominantly observed in the damaged areas.13) In experimental TBI animal models, the number of reactive astrocytes increased in the injured cerebrum.1,14,15) These findings suggest that astrocytes are involved in the pathogenesis of TBI. Therefore, we focused on the functional astrocytic molecules to identify novel therapeutic drugs for TBI.
FPI is an established experimental model of TBI performed by hydraulic impact on the dura mater of experimental animals using a fluid percussion device (AmScien Instruments, Richmond, VA, U.S.A.; model FP302) (Fig. 2A). An experimental animal is placed in a stereotactic device under anesthesia, and the skull is exposed. A 3-mm-diameter hole is drilled in the skull of the left hemisphere, and a lure fitting (ISIS Co., Ltd., Osaka, Japan; Cat. No. VRS306) (Fig. 2A) is fixed to the drilled area. The interior of the lure fitting is filled with sterilized saline, and the lure fitting is connected to a tube of the fluid percussion device, after which a hydraulic insult is applied in the range 1.2 to 1.4 atmospheres.1)

(A) FPI to the mouse cerebrum (in vivo FPI). FPI was performed using a fluid percussion device (A1). A lure fitting (A2) was fixed in the mouse cerebrum (A3) and the fixed lure fitting was connected to the fluid percussion device (A4). Arrowheads show the fixed lure fitting in the mouse cerebrum (A3, 4). (B) FPI to cultured astrocytes (in vitro FPI). A cell trauma chamber was used (B1). An astrocyte cultured dish was set to the chamber (B2, 3) and the chamber was connected to the fluid percussion device (B4). An arrowhead shows the culture dish (B2).
FPI is also performed on cultured cells using a cell trauma chamber and fluid percussion device (Fig. 2B). Cultured astrocytes are prepared from mouse cerebrum and incubated in 35-mm culture dishes (Corning, Corning, NY, U.S.A.; Cat. No. 430165). Most cultured cells are GFAP-positive (Fig. 3A). The culture dish is placed in the cell trauma chamber of the FPI device (AmScien Instruments, model FP302) (Fig. 2B). After the upper half of the chamber is filled with sterilized saline, the central tube of the chamber is connected to the fluid percussion device, and FPI is performed on cultured astrocytes.16)

(A) Immunocytochemistry for GFAP was performed in cultured cells. Scale bars = 20 µm. (B, C) Reactive astrocytes in the injured cerebrum after TBI. Immunohistochemistry for the GFAP was performed in mouse brain tissue. Scale bars = 200 µm (B) and 50 µm (C). Photographs (B) and (C) were obtained from different brain tissues.
BBB disruption is determined by extravasation of Evans blue (Sigma-Aldrich, St. Louis, MO, U.S.A.; Cat. No. E2129-10G) or FITC-dextran (Sigma-Aldrich, Cat. No. FD4-100MG) into the cerebral parenchyma.16) Saline containing 4% Evans blue or 4% FITC-dextran is administered via the tail vein at 3 mL/kg. Sixty minutes after administration, an aliquot of circulatory blood is collected to determine serum Evans blue or FITC-dextran content. The experimental animals are intracardially perfused with 50 mL of phosphate-buffered saline under anesthesia. The perfused cerebrum is collected, and a 5-mm thick coronal brain section (between 0 and 5 mm posterior to the bregma) is prepared using a microtome. The prepared brain tissues are weighed and immersed in 400 µL formamide (Wako Pure Chemical Corporation, Osaka, Japan; Cat. No. 065-00436) at 55 °C overnight. Evans blue content in the extract is determined by measuring the absorbance at 655 nm, and FITC-dextran content is determined by measuring the fluorescence intensity at 528 nm with 485 nm excitation. The percentage of Evans blue or FITC-dextran extravasation in brain tissue is calculated using the following formula:
 |
 |
Brain edema is determined based on increased brain water content.1) After FPI, the mouse brain tissue is collected and a 5-mm thick coronal section (between 0 and 5 mm posterior to the bregma) is prepared using a microtome. The prepared brain tissues are weighed (wet weight), dried at 70 °C overnight, and then dried tissues are weighed (dry weight). The percentage brain water content is calculated using the following formula:
 |
The brain water content of normal brain tissue is approximately 76 to 77%, whereas that of damaged brain tissue is over 80%.
In the damaged brain, reactive astrocytes produce various bioactive factors including vascular permeability-accelerating factors and protective factors that regulate permeability of the BBB.10) Factors that accelerate vascular permeability include endothelin-1 (ET-1), matrix metalloproteinase-9 (MMP-9), and vascular endothelial growth factor-A (VEGF-A), which promote BBB permeability and exacerbate BBB disruption in damaged brain.1,17–20) The vascular protective factors include angiopoietin-1 (Ang-1) and sonic hedgehog (Shh), which decrease BBB permeability and alleviate BBB disruption.16,21–26) In contrast, vascular permeability-accelerating factors decrease the expression levels of endothelial tight junction proteins, including claudin-5 and occludin, which are essential components for the barrier function of the BBB,27–31) whereas vascular protective factors increase these expression levels.16,22,23,32,33)
After FPI, the number of GFAP-positive reactive astrocytes increases in injured mouse cerebrum (Figs. 3B, C). Moreover, the expression of vascular permeability-accelerating factors, including MMP-9 and VEGF-A, are predominantly observed in GFAP-positive reactive astrocytes (Fig. 4A). In addition, the expression levels of MMP-9 and VEGF-A are increased in cultured astrocytes following in vitro FPI.34) Repeated intracerebroventricular (i.c.v.) administration of an MMP-9 inhibitor or VEGF-neutralizing antibody alleviates BBB disruption in experimental TBI model mice,1,31) and astrocyte-derived MMP-9 and VEGF-A promote TBI-induced BBB disruption.

(A) Immunohistochemistry for matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor-A (VEGF-A), and angiopoietin-1 (Ang-1) were performed in mouse brain tissues after TBI. Prepared brain sections were labeled by MMP-9, VEGF-A, and Ang-1 antibodies with GFAP antibody. Scale bars = 50 µm. (B) Expression of Shh in cultured astrocytes. At 3 h after FPI, cultured astrocytes were collected and mRNA expression level of sonic hedgehog (Shh) was determined by real-time PCR. The mRNA level of Shh was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Results are presented as the mean ± standard error of the mean (S.E.M.) of data from six different mRNA samples. ** p < 0.01 vs. control; Student’s t-test.
In the injured cerebrum after FPI, the expression of the vascular protective factor Ang-1 is predominantly observed in GFAP-positive reactive astrocytes in the injured cerebrum after FPI (Fig. 4A). Similarly, Shh expression is also observed in astrocytes in the injured cerebrum after FPI,26) and FPI increases the expression of Shh in cultured astrocytes (Fig. 4B). Repeated i.c.v. administration of recombinant Ang-1 or Shh reduces TBI-induced BBB disruption in experimental mice.16,26) These results suggest that astrocyte-derived Ang-1 and Shh alleviate TBI-induced BBB disruption. Therefore, astrocytes are key players in the regulation of BBB function, and astrocytic functional molecules may be attractive targets for the discovery of novel therapeutic drugs against TBI.
Endothelins (ETs), including ET-1, -2, and -3, were initially discovered as vasoconstrictor peptides responsible for various pathophysiological responses including ischemia, inflammation, and BBB disruption in the damaged brain.35,36) ET receptors are seven-transmembrane G protein-coupled receptors and are classified into two distinct types: ETA and ETB receptors. In the injured mouse cerebrum after FPI, expression of the ETB receptor, but not the ETA receptor, is predominantly observed in GFAP-positive reactive astrocytes (Fig. 5A). Repeated i.c.v. administration of BQ788, a selective ETB receptor antagonist (Fig. 5B), decreases the number of GFAP-positive reactive astrocytes after FPI.1) As shown in Fig. 5C, BQ788 reduces the extravasation of Evans blue and FITC-dextran in the injured cerebrum after FPI. The FPI-induced increase in brain water content can also be reduced by BQ788.1) Furthermore, BQ788 decreases the expression of astrocytic MMP-9 and VEGF-A in the cerebrum after FPI, whereas it increases the expression of astrocytic Ang-1 and Shh.1,16,26)

(A) Immunohistochemistry for ETB receptors in the mouse cerebrum after TBI. Prepared brain sections were labeled with ETB receptor antibody and GFAP antibody. Scale bar = 50 µm. (B) The schedule of ET receptor antagonist administration. Repeated intraventricular (i.c.v.) administration of BQ788 (15 nmol per day), a selective ETB receptor antagonist, was performed twice a day from 2 to 5 d after FPI. At 5 d after FPI, the brain tissues were collected and effects of BQ788 were evaluated (Left schedule). Repeated intravenous (i.v.) administration of bosentan (3 mg/kg per day), a non-selective ET receptor antagonist or ambrisentan (0.5 mg/kg per day), a selective ETA receptor antagonist, was performed from 2 to 5 d after FPI. At 5 d after FPI, the brain tissues were collected and effects of ET receptor antagonists were evaluated (Right schedule). (C) Effects of BQ788 in FPI-induced BBB disruption. At 5 d after FPI, the effects of BQ788 on BBB disruption were investigated by extravasation of Evans blue or FITC-dextran. (D) Effects of bosentan and ambrisentan on FPI-induced BBB disruption. At 5 d after FPI, the effects of bosentan and ambrisentan on BBB disruption were evaluated by the Evans blue extravasation.
Bosentan is a nonselective ET receptor antagonist used to treat pulmonary arterial hypertension. In the TBI mouse model, repeated intravenous (i.v.) administration of bosentan (Fig. 5B) reduces Evans blue extravasation into the injured cerebrum (Fig. 5D). The FPI-induced increase in brain water content is also reduced by bosentan.37) Furthermore, bosentan decreases the expression of ET-1, MMP-9, and VEGF-A in the injured cerebrum after FPI.37) In contrast, repeated i.v. administration of ambrisentan, a selective ETA antagonist (Fig. 5B), does not reduce FPI-induced Evans blue extravasation (Fig. 5D). Additionally, ambrisentan does not reduce brain water content in the injured cerebrum after FPI.37)
These results suggest that ETB receptor antagonists mitigate TBI-induced BBB disruption and brain edema by decreasing astrocytic vascular permeability-accelerating factors and increasing astrocytic vascular protective factors. Therefore, astrocytic ETB receptors may be attractive targets for developing therapeutic drugs for TBI.
4.2. Histamine H2 ReceptorHistamine is a major neurotransmitter that plays an essential role in multiple physiological reactions, including the sleep-wake cycle, learning, and memory in the central nervous system. Histamine receptors are classified into four types: H1, H2, H3, and H4. Some studies have suggested a relationship between H2 receptors and brain damage. H2 receptor antagonists reduced brain edema in experimental cerebral ischemia animal models.38) However, other studies have shown that H2 receptor antagonists aggravate ischemic damage in experimental cerebral ischemia model rats.39,40) Furthermore, H2 receptor agonists improve neuronal dysfunction by promoting astrocyte migration in cerebral ischemia rats.41,42) These findings imply that H2 receptors contribute to the pathological conditions of brain damage and regulate astrocyte functions. Mohanty et al.43) found that the increased brain water content as well as brain histamine levels were prevented by treatment with the histamine H2 receptor antagonist cimetidine in TBI model rats. Therefore, H2 receptors might be involved in TBI-induced pathological conditions.
In the injured mouse cerebrum after FPI, the expression level of the H2 receptor is increased,44) and GFAP-positive reactive astrocytes express H2 receptors (Fig. 6A). Repeated i.c.v. administration of selective H2 receptor antagonist ranitidine did not affect to Evans blue extravasation,44) whereas repeated i.c.v. administration of selective H2 receptor agonists, such as amthamine and dimaprit (Fig. 6B), reduce Evans blue extravasation in the injured cerebrum after FPI (Fig. 6C). A decrease in endothelial tight junction proteins causes BBB disruption following brain edema formation (Fig. 6D). Expression levels of tight junction proteins, such as claudin-5 and occludin, decrease in the injured cerebrum after FPI, whereas amthamine and dimaprit increase their expression levels (Fig. 6E). Furthermore, amthamine and dimaprit increase the expression levels of vascular protective factors, such as Ang-1 and Shh.44) In cultured astrocytes, treatment with amthamine or dimaprit increases the expression of Ang-1 and Shh.44) These results suggest that H2 receptor agonists reduce TBI-induced BBB disruption by increasing tight junction proteins and astrocyte-derived vascular protective factors. Therefore, the astrocytic H2 receptor might also be a target functional molecule for the development of therapeutic drugs for TBI.

(A) Immunohistochemistry for H2 receptors in the mouse cerebrum after TBI. Prepared brain sections were labeled with H2 receptor antibody and GFAP antibody. Scale bar = 50 µm. (B) The schedule of H2 receptor agonist administration. Repeated intraventricular administration of amthamine (100 nmol per day) or dimaprit (100 nmol per day), a selective H2 receptor agonist, was performed twice a day from 3 h to 3 d after FPI. At 3 d after FPI, the brain tissues were collected and effects of H2 receptor agonists were evaluated. (C) Effects of H2 receptor agonists on FPI-induced BBB disruption. At 3 d after FPI, the effects of amthamine and dimaprit for the BBB disruption were evaluated by Evans blue extravasation. (D) Decrease in endothelial tight junction proteins by TBI. TBI decreases expression levels of tight junction proteins including claudin-5, occludin, and zonula occludens-1 (ZO-1), which promote BBB disruption following brain edema formation. (E) Effects of H2 receptor agonists on expression level of tight junction proteins in the mouse cerebrum. Immunoblotting for claudin-5 and occludin was performed in mouse brain tissue. Typical immunoblots are shown. The quantitative data of immunoblotting was cited from Ref. 44. Results are presented as the mean ± S.E.M. (n = 4). * p < 0.05, ** p < 0.01 vs. sham (saline), #p < 0.05, ##p < 0.01 vs. FPI (saline) by one-way ANOVA with Tukey’s test.
TRPV4 is a calcium-permeable, nonselective cation channel sensitive to temperature and osmotic pressure. TRPV4 contributes to various physiological conditions, including the regulation of neuronal excitability and proliferation of brain cells,45,46) and is responsible for various pathological conditions of TBI, including neuronal death, inflammation, and brain edema.47–50) Lu et al.48) suggested that administration of the TRPV4 antagonist RN1734 significantly attenuated TBI-induced brain edema through decreasing the phosphorylation of mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK), extracellular signal-regulated kinase (ERK), and protein kinase B (Akt) proteins.
We previously found that astrocytes expressed TRPV4, and that the expression level of astrocytic TRPV4 was increased by FPI (Fig. 7A). In contrast, FPI did not increase TRPV4 expression in brain endothelial cells (bEnd.3 cells) (Fig. 7A). Repeated i.c.v. administration of selective TRPV4 antagonists, such as HC-067047 and RN-1734 (Fig. 7B), reduced Evans blue extravasation in the injured mouse cerebrum after FPI (Fig. 7C). HC-067047 and RN-1734 also reduced FPI-induced increases in brain water content.34) Furthermore, HC-067047 and RN-1734 decreased expression of ET-1, MMP-9, and VEGF-A in the injured cerebrum after FPI.34) In cultured astrocytes, treatment with HC-067047 or RN-1734 decreased expression of ET-1, MMP-9, and VEGF-A after FPI.34) These results suggest that TRPV4 antagonists alleviate TBI-induced BBB disruption and brain edema by decreasing the levels of astrocyte-derived vascular permeability-accelerating factors. Therefore, astrocytic TRPV4 may be a candidate functional molecule for the development of therapeutic drugs for TBI.
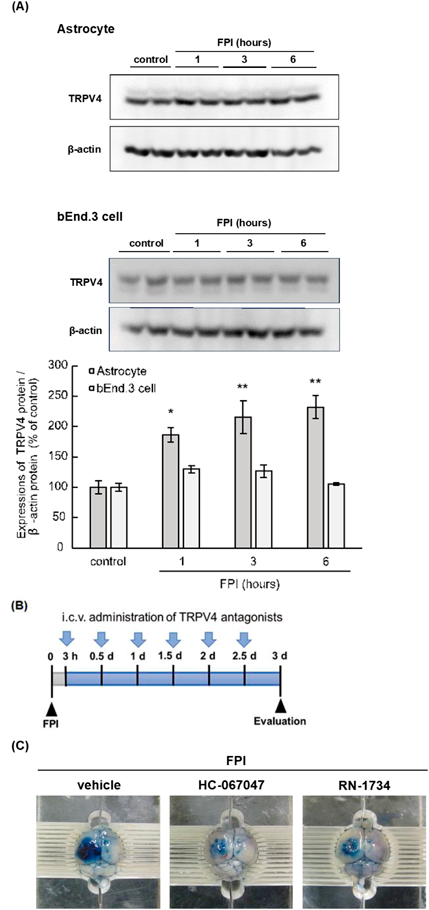
(A) Immunoblotting for TRPV4 in cultured astrocytes after FPI. At 1, 3, and 6 h after FPI, cultured astrocytes and bEnd.3 cells (brain endothelial cells) were collected. Typical immunoblots are shown. The quantitative data of immunoblotting was cited from Ref.34. Results are presented as the mean ± S.E.M. (n = 4). * p < 0.05, ** p < 0.01 vs. control by one-way ANOVA with Dunnett’s test. (B) The schedule of TRPV4 antagonist administration. Repeated intraventricular administration of HC-067047 (20 nmol per day) or RN-1734 (20 nmol per day), a selective TRPV4 receptor antagonist, was performed twice a day from 3 h to 3 d after FPI. At 3 d after FPI, the brain tissues were collected and effects of TRPV4 antagonists were evaluated. (C) Effects of TRPV4 antagonists on FPI-induced BBB disruption. At 3 d after FPI, the effects of HC-067047 and RN-1734 on BBB disruption were evaluated by Evans blue extravasation.
TBI-induced BBB disruption causes brain edema and neuroinflammation, resulting in unexpected death or serious disability. Therefore, the protection and recovery of the BBB may be a significant strategy for TBI. Since astrocyte-derived bioactive factors exert protective and detrimental actions against TBI-induced BBB disruption, the regulation of astrocyte function is essential for BBB protection. We have found that reactive astrocytes predominantly expressed endothelin ETB receptor, histamine H2 receptor, and TRPV4 in the injured cerebrum after TBI, and the administration of ETB receptor antagonists, H2 receptor agonists, and TRPV4 antagonists alleviated BBB disruption and brain edema in an experimental TBI model mice.1,34,44) Therefore, astrocytic functional molecules are important candidates for the development of therapeutic drugs against TBI.
Intravenous administration may be selected for introducing therapeutic drugs for TBI because emergent treatments are essential for TBI-induced BBB disruption and brain edema. We found that intravenous administration of BQ788 mitigated TBI-induced BBB disruption and brain edema in experimental model mice.1) However, delivery of BQ788 from the peripheral tissue into the central nervous system may be limited owing to its peptide-derived properties, which may require high doses of BQ788 for clinical use. Therefore, the development of alternative ETB receptor antagonists to facilitate brain transition may be essential in the future. Bosentan is a candidate therapeutic drug for treating TBI. Bosentan has already been used clinically as a therapeutic drug for pulmonary arterial hypertension, and we found that i.v. administration of bosentan alleviated BBB disruption and brain edema in experimental TBI model mice.37) Therefore, drug repositioning of bosentan for TBI is expected to occur in the future.
We showed that i.v. administration of H2 receptor agonists alleviate TBI-induced BBB disruption and increase expression levels of vascular protective factors in experimental mice.44) In contrast, intravenous administration of H2 receptor agonists decreases blood pressure in both sham and TBI model mice.44) Therefore, blood pressure management may be essential during the administration of H2 receptor agonists. Furthermore, H2 receptor agonists may only be administered for several days in the acute phase of TBI because persistent administration may cause gastrointestinal tract disturbances by accelerating gastric acid secretion. When these problems are resolved, H2 receptor agonists can be used as therapeutic drugs to treat TBI-induced BBB disruption.
We also found that i.c.v. administration of TRPV4 antagonists alleviates BBB disruption and brain edema in an experimental TBI model mice.34) However, we did not investigate the effects of i.v. administration on TBI-induced BBB disruption and brain edema. Liao et al.51) showed that intravenous administration of HC-067047 prevents shockwave-induced BBB disruption in experimental rats and that intravenous administration of TRPV4 antagonists might reduce TBI-induced BBB disruption, which should be investigated in the future.
Our studies suggest that astrocyte-targeting drugs can alleviate TBI-induced BBB disruption and brain edema by regulating the expression of astrocyte-derived bioactive factors (Fig. 8). Although appropriate administration routes, doses, side effects, and clinical trials should be investigated in the future, drugs targeting functional molecules in astrocytes are expected to become novel therapeutics for TBI.

TBI increases expression of astrocytic vascular permeability-accelerating factors and decreases expression of tight junction proteins. These processes cause BBB disruption following brain edema formation. Astrocyte targeting drugs decrease expression of astrocytic vascular permeability-accelerating factors and increase expression of astrocytic vascular protective factors following increase in endothelial tight junction proteins. These processes alleviate TBI-induced BBB disruption and brain edema.
I would like to acknowledge Dr. Yutaka Koyama of Kobe Pharmaceutical University, Dr. Shigeru Hishinuma of Meiji Pharmaceutical University, Dr. Hiroyuki Mizuguchi of Osaka-Ohtani University, Dr. Tomokazu Watano of Osaka-Ohtani University, Dr. Yasuhiro Ogawa of Meiji Pharmaceutical University, and Dr. Kahori Shimizu of Osaka-Ohtani University for their assistance. This work was supported by a Grant-in-Aid for Young Scientists (Grant Number: 20K16016) from the Japan Society for the Promotion of Science.
The author declares no conflict of interest.
This review of the author’s work was written by the author upon receiving the 2023 Pharmaceutical Society of Japan Award for Young Scientists.