2024 Volume 47 Issue 3 Pages 660-668
2024 Volume 47 Issue 3 Pages 660-668
Flopropione (Flo) has been used for gallstone and urolithiasis as a spasmolytic agent almost exclusively in Japan. According to the package insert, its main mechanism is catechol-O-methyltransferase (COMT) inhibition and anti-serotonergic effect. This is obviously contrary to pharmacological common sense, but it is described that way in pharmacology textbooks and occurs in questions in the National Examination for Pharmacists in Japan. As this is a serious problem in education, we re-examined the action of Flo. The guinea pig ureter was hardly contracted by serotonin, but noradrenaline (NA) elicited repetitive twitch contraction, which was inhibited by Flo. The sphincter of Oddi (SO) exhibited a spontaneous repetitive twitch contraction, which was inhibited by NA and Flo. The inhibitory effect of NA was reversed by α- and β-blockers, whereas that of Flo was not. Entacapone, a representative COMT inhibitor, did not affect the movement of the ureter and the SO. Nifedipine suppressed carbachol-induced contraction of the taenia coli, spontaneous movement of the SO, and NA-induced contraction of the ureter to almost the same extent, whereas Flo did not inhibit the taenia coli, but inhibited the contraction of the SO and the ureter. The inhibitory pattern of Flo resembled that of the ryanodine receptor agonist 4-chloro-m-cresol and the inositol 1,4,5-trisphosphate (IP3) receptor antagonist 2-aminoethoxydiphenyl borate. It is concluded that COMT inhibition or serotonin inhibition is not involved in the spasmolytic action of Flo. Flo might act on ryanodine receptors and/or IP3 receptors, which are responsible for periodic Ca release from Ca stores, to disrupt coordinated Ca dynamics.
Flopropione (Flo; 2,4,6-trihydroxy-1-propiophenone) has been used for gallstone and urolithiasis as a spasmolytic agent almost exclusively in Japan. According to the package insert1) and the interview form,2) its main mechanism of action is catechol-O-methyltransferase (COMT) inhibition and it also has anti-serotonergic effects exclusively based on a few reports using experimental animals. However, this mechanism is clearly contrary to pharmacological common sense, i.e.,
As long as Flo does not interact with other drugs and is clinically useful, whether its primary efficacy is COMT inhibition or anti-serotonergic activity, this is not a major therapeutic issue. However, this point has caused a very serious problem in pharmacology education. In the National Examination for Pharmacists, care has been taken to ensure that questions are not biased toward specific disease or organs, but there are only a few drugs used in the field of the biliary system. Flo belongs to that class of drugs. Therefore, the frequency of questions involving Flo, with its mechanism of action, inevitably increases because there are few alternatives. Four questions have been asked in the last eight years, including those that do not directly touch on Flo but include the option of “involvement of COMT in the biliary system.” For the exam, Flo is always described in Japanese pharmacology textbooks. As authoritative overseas pharmacology textbooks do not include Flo,4–6) Japanese textbook authors have no choice but to reprint the “COMT inhibition and anti-serotonin theory” described in the package insert, which is an official document.
As described later, the papers cited in the package insert1) and the interview form2) are totally unreliable, so it is necessary to correct the descriptions in domestic textbooks as soon as possible. To do this, revision of the package insert of Flo is a priority. In the present study, experiments were performed to clarify the irrationality of “COMT inhibition and anti-serotonin theory.”
Hartley male guinea pigs weighing approximately 350 g were purchased from Shimizu Laboratory Supplies (Kyoto, Japan) and the organs were obtained under the isoflurane anesthesia. The animal experimentation was conducted according to the protocol approved by the president of Doshisha Women’s College of Liberal Arts after the review by the Institutional Animal Care and Use Committee (Permit Nos. Y21-003 and Y22-013).
DrugsIsoflurane was obtained from FUJIFILM-Wako Pure Chemical Corporation (Osaka, Japan). Tyramine, carbamylcholine chloride (CCh), prazosin hydrochloride, and serotonin hydrochloride were purchased from Tokyo Chemical Industry (Tokyo, Japan) and dissolved in H2O. Flo, Ent, nifedipine (Nif), 4-chloro-m-cresol (4CmC), 2-aminoethoxydiphenyl borate (2APB) were from Tokyo Chemical Industry and dissolved in dimethyl sulfoxide (DMSO). When added to the Magnus tube, the final concentration of DMSO was not to exceed 0.3%. Tetrodotoxin from FUJIFILM-Wako Pure Chemical Corporation were dissolved according to the manufacturer’s manual. Noradrenaline (NA; Alfresa Pharma, Osaka, Japan), phentolamine mesylate (Regitin; Novartis Pharma, Tokyo, Japan), and propranolol (Inderal; Astra-Zeneca, Osaka, Japan) were used as a dilution of injection.
Isolated vas DeferensUnder the isoflurane anesthesia, the vas deferens was isolated from guinea pig, and set to a rod with a pair of platinum ring wire electrodes. The sample was then immersed in 20 mL of Magnus tube filled with N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) buffered saline (HBS; 132.6 mM NaCl, 5 mM Na2HPO4, 1 mM NaH2PO4, 5.4 mM KCl, 1.2 mM MgCl2, 1.0 mM, CaCl2, 10 mM HEPES-Na (pH = 7.4), 11.1 mM glucose) aerated with 100% O2. The initial tension was adjusted to 0.5 g and after the equilibrium, electrical stimulation (50 Hz, 1.5 ms, 5 s duration) was applied every 1 min (SEN-2201, Nihon Koden, Tokyo, Japan). The isometric tension was monitored by a force displacement transducer (LoadCell, Biotex, Kyoto, Japan), and recorded on a pen-writing oscilloscope (Unicorder U-228, Pantos, Kyoto, Japan). The Magnus tube was kept at 35 °C by a constant temperature water circulator (NCB-1200, EYELA, Tokyo, Japan).
Measurement of Perfusion Pressure of Isolated UreterUnder isoflurane anesthesia, the kidney of guinea pig was incised, and a 22-gauge plastic cannula was inserted several millimeters into the ureter through the renal pelvis and ligated. After resection of the kidney, the entire ureter was removed just before the bladder and placed in a 20 mL Magnus tube filled with HBS and aerated with 100% O2. The ureter was perfused with HBS via the cannula connected to a 5 mL syringe set to a syringe pump (Legato 111, Kd Scientific, Holliston, MA, U.S.A.) at a rate of 1 mL/h. The initial hydrostatic pressure was set at 20 cm H2O. The perfusion pressure was monitored with a pressure transducer (PA-100/PAS-101, Star Medical, Tokyo, Japan) via a three-way cock installed in the middle of the flow and recorded on a pen-writing oscilloscope. The Magnus tube was kept at 35 °C.
Isometric Tension of Isolated UreterThe ureter of guinea pig was isolated under isoflurane anesthesia and immersed in a 10 mL Magnus tube filled with HBS and aerated with 100% O2. Isometric tension was monitored as described for the vas deferens.
Measurement of Perfusion Pressure of SOIn guinea pigs, the part where the common bile duct enters the duodenum is enlarged toward outside by about 5 mm length and opens into the duodenal lumen at the papilla. Under isoflurane anesthesia, the gallbladder, the bile duct, and the duodenum wall were removed from the guinea pig. The gallbladder was then incised, a 22-gauge plastic cannula was penetrated through the bile duct, the tip was inserted into the bulge. After the bile duct was ligated just before the bulge, the gallbladder, the bile duct, and the duodenal wall around the papilla were resected out. This preparation was immersed in a 20 mL Magnus tube filled with HBS and aerated with 100% O2, and the perfusion pressure was recorded the same as the ureter.
Isolated Ring Preparation of SOThe bulge part of SO on the duodenal wall was dissected and a ring shaped preparation was made. A pair of triangular stainless wires was inserted into the lumen and the isometric tension in the direction of circular muscle was recorded in a 10 mL Magnus tube.
In the case of SO, for both perfusion and ring preparations, spontaneous movements emerged in vitro after about 30 min. As this was inhibited by either 3 µM tetrodotoxin or 10 µM atropine, the involvement of endogenous cholinergic nerve was suggested. However, after about two hours of incubation, the spontaneous movements always became a tetrodotoxin/atropine resistant myogenic contraction. In the present study, experiments were performed on the preparations after 2 h and confirmed its atropine resistance.
Taenia ColiThe isometric contraction of isolated taenia coli was recorded as above. In some experiments, HBS was replaced with Ca-free, high K solution (HKB; 140 mM KCl, 1.2 mM MgCl2, 10 mM HEPES-Na, pH = 7.4). After complete relaxation, CaCl2 was cumulatively added to cause Ca-contraction under which the effects of Flo and Nif were examined.
In separate experiments, carbachol (CCh)-induced Ca release from intracellular Ca store was assessed according to the report of trepibutone, a similar spasmolytic drug.13) After equilibrium in HKB, the contraction by 1 mM Ca was recorded (CA1). After washing out, the contraction by 10 µM CCh (CCH1) was recorded. After the taenia was washed again with HKB, either DMSO (control) or Flo 100 µM was added and the contraction by 1 mM Ca was recorded (CA2). After washing out, the contraction by 10 µM CCh (CCH2) was recorded. Here, CA2/CA1 reflects the change of the contraction caused by Ca-influx, whereas CCH2/CCH1 reflects the change of the amount of Ca that stored during Ca-contraction and released by CCh. Data were expressed as % of CA1.
Data AnalysisFor quantification of the spontaneous movement of the SO, the trace was analyzed by ImageJ to obtain the area under the curve (AUC). Statistical analysis was performed by means of Student’s t-test, or ANOVA followed by Dunnet’s post hoc test, as appropriate, with p < 0.05 considered significant using EZR software.14) Summarized data are shown as means ± standard error of the mean (S.E.M.).
First, we examined whether the COMT inhibitory effect of Flo could be observed at an organ level using vas deferens, which is widely used as an organ with dense sympathetic innervation. In fact, a catecholamine releasing agent, tyramine 100 µM always induced contraction (data not shown). The vas deferens contracted by the application of electrical stimulation every 1 min, and this was abolished by 3 µM tetrodotoxin (data not shown) and inhibited by 10 µM prazosin (Fig. 1A), confirming that the contraction was mediated by endogenous NA at least in part. To this contraction, 100 µM Flo, approximately IC50 for COMT,15) showed clear inhibition (Fig. 1B). Considering the possibility that COMT inhibitory effect of Flo was masked by its spasmolytic effect, a representative COMT inhibitor, Ent 10 µM, hundreds of times more than the IC50 for COMT,16) showed practically no effect (Fig. 1C). These results indicate that COMT inhibition did not enhance the effects of endogenous NA even in organs with dense sympathetic innervation such as vas deferens.

The twitch response was elicited by electrical stimulation (50 Hz, 1.5 ms, 5 s duration, every 1 min). A: Inhibitory effect of 10 µM prazosin. B: Inhibitory effect of 100 µM Flo. C: Ent 10 µM does not affect the twitch response. D: Data are summarized as mean ± S.E.M. of 3 to 5 experiments. Both prazosin and Flo significantly inhibited the response, whereas Ent did not. ** p < 0.01.
Next, we investigated the effect of Flo on the ureter, one of the main therapeutic targets of Flo. The ureter showed a marked pressure increase by high K+ (90 mM) and CCh 10 µM, while serotonin 1 to 10 µM caused practically no change (data not shown). When the concentration of serotonin was increased to 100 µM, a transient, small increase in pressure was occasionally observed, but it was indistinguishable from the occasional spontaneous movement, since the interval between the administration and the increase in pressure was inconsistent. Therefore, it was impossible to verify the anti-serotonergic effect of Flo described in the previous report.17) Addition of 10 µM NA to specimens, that did not respond to 10 µM serotonin, caused a repetitive pressure increase on the transient tonic increase (Fig. 2A). To this pressure increase, Flo higher than 10 µM showed an inhibitory effect and almost complete inhibition was attained at a level of 100 µM (Fig. 2B). Although this preparation is close to the physiology of the ureter in vivo, there are some difficulties in handling the specimen and quantifying the contraction, so we performed a detailed examination by measuring isometric tension of isolated ureter.

A: Addition of 10 µM serotonin had no effect on ureteral perfusion pressure. When 10 µM NA was given 13 min later, a sustained pressure rise lasting 2 to 3 min with spike-like repeated pressure rises were observed. B: An increase in perfusion pressure was induced by 10 µM NA. When 30 µM Flo was added, the spike-like pressure rise did not change in magnitude but the frequency of the rise decreased. When the amount of Flo was increased to 100 µM, the pressure increase completely stopped.
This sample also showed no contractile response to serotonin, like the perfusion sample (data not shown). It showed only periodical large twitch responses without tonic contraction by the addition of NA (Fig. 3A). These twitches continued for more than 15 min, while their magnitude gradually increased for the first 3 min, then became constant but their interval was gradually increased. In order to assess the drug effects, the following protocol was employed.

A: Quantification of NA-induced contraction. The contraction height for 5 min before drug administration was integrated (S1), and the contraction height for 1 to 6 min after drug administration was integrated (S2), and the ratio R (%) = S2/S1 × 100 was obtained. The ratio (Rd/Rc) was calculated from the R values of the control (Rc) and the drug-administered (Rd), and used to estimate the drug effect. B: Effects of Flo (30, 100 µM) and Ent (10 µM) on NA (10 µM)-induced contraction in isolated guinea pig ureter. Values are calculated by Rd/Rc × 100% and expressed as mean ± S.E.M. (N = 4). Statistically significant vs. control at ** p < 0.01, *** p < 0.001.
After administration of NA 10 µM, when the magnitude of the twitch almost reached a maximum, it was observed for 5 min, followed by administration of a vehicle (control) or drug, and observation was continued for 6 min or more. The contraction height for 5 min before drug administration was integrated (S1), and the contraction height for 1 to 6 min after drug administration was integrated (S2), and the ratio R (%) = S2/S1 × 100 was obtained (Fig. 3A). R in the control group was approximately 70%. The ratio (Rd/Rc × 100) was calculated from the R values of the control (Rc) and the drug-administered (Rd), and used for estimation of the drug effect.
Figure 3B shows the effects of Flo 30, 100 µM and Ent 10 µM. Flo significantly inhibited NA-induced twitch contraction, and at 100 µM, almost stopped the contraction. On the other hand, Ent, a representative COMT inhibitor, did not show any inhibition or enhancement. As mentioned above, the COMT inhibitory effect of Ent administered here is several hundred times stronger than that of Flo.
Perfusion Pressure of SOSpontaneous and periodic pressure changes in the SO perfusion preparation were evaluated over a relatively short period of time, because the time-dependent fluctuations of the changes were not negligible when observed on a scale of 20 min or longer. When drug effects were evaluated, AUC for 2 min before drug was set to 100% (control) and the AUC for 2 to 4 min after drug administration was calculated and expressed as % of control. As shown in Fig. 4, Flo 3 to 300 µM showed a dose-dependent and significant inhibitory effect on the periodic pressure increase in the SO, whereas Ent 10 µM did not show an effect.
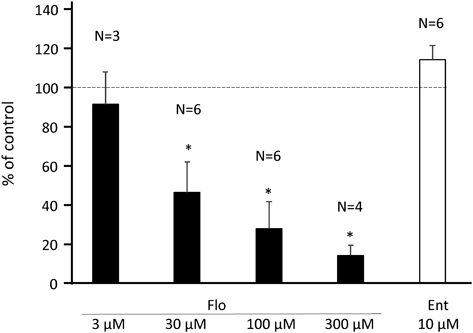
For the trace of pressure changes, the area under the curve (AUC) for 2 min before drug was set to 100% (control) and the AUC for 2 to 4 min after drug administration was calculated and expressed as % of control (N = 3–6). Statistically significant vs. control at * p < 0.05, ** p < 0.01, *** p < 0.001.
In contrast to ureter, NA 10 µM strongly inhibited the spontaneous pressure increase in the SO. When propranolol 10 µM was added, the pressure increase was partially recovered and further addition of phentolamine 10 µM caused a large recovery, resulting in higher activity than that observed before addition of NA (Fig. 5A). It appeared that the inhibitory effect of NA involved both alpha and beta receptors.

A: NA 10 µM inhibited the spontaneous pressure increase. The pressure increase was recovered by the addition of propranolol 10 µM (Prop) and phentolamine 10 µM (Phent). B: Inhibitory effects of NA (10 µM) and Flo (100 µM) on the spontaneous pressure increase of guinea pig SO in the presence and absence of propranolol (Prop, 10 µM) + phentolamine (Phent, 10 µM). Statistically significant vs. NA alone at ** p < 0.01 vs. NA alone.
In the next experiments, NA 10 µM and Flo 100 µM both reduced the spontaneous pressure increase down to about 10%. Pretreatment with phentolamine + propranolol abolished the inhibitory effect of NA, whereas that of Flo was unaffected (Fig. 5B). Therefore, it was reconfirmed that endogenous NA is not involved in the spasmolytic action of Flo, although NA suppresses the spontaneous movement of the SO via α and βreceptors. In the present experiments, when phentolamine was administered under NA-suppressed conditions, spontaneous activity was often enhanced more than before NA administration. We did not pursue it further because it was far from the present purpose. The lack of involvement of COMT inhibition in the SO relaxant effect of Flo was clearly demonstrated by the fact that α/β-blockers were ineffective and Ent did not exert a muscle relaxant effect.
Mechanism of Spasmolytic Effect of FloCalcium Contraction in the Depolarized Taenia ColiBased on the results presented so far, it is reasonable to assume that the molecular target of Flo is not related to neurotransmitters such as COMT or serotonin, but directly affects smooth muscle. In order to elucidate the mechanism of action, we first examined its effect on Ca-induced contraction in the depolarized taenia coli.
Trepibutone, which has a similar structure to Flo and has similar indications, is postulated to have a smooth muscle relaxant effect by promoting Ca uptake into sarcoplasmic reticulum.13) Thus, we performed a similar experiment for comparison.
As described in Materials and Methods, the contractions CA1 and CA2 reflect the amount of Ca influx, and the contractions CCH1 and CCH2 reflect the amount of Ca incorporated into the CCh sensitive store. The results are shown in Fig. 6, where Flo treatment had no effect on the respective contractions. Therefore, unlike trepibutone, Flo does not appear to induce muscle relaxation by promoting Ca uptake into Ca stores.

In a high K, Ca-free solution, the muscle was sequentially contracted with 1 mM Ca (CA1) and 10 µM CCh (CCH1), and repeated once more (CA2, CCH2). DMSO (control) or Flo was treated before the second contraction. Contraction was expressed as CA1 as 100%.
The above results also indicate that Flo has little inhibitory activity on the Ca influx. To confirm this, Flo was compared with a Ca-blocker, Nif. Figure 7 shows the results of Ca contraction induced by cumulative administration of Ca to the taenia coli in HKB. Nif 0.1 µM shifted the Ca concentration-response curve more than 10-fold to the right (Fig. 7A). On the other hand, the effect of 100 µM Flo was weak, suppressing only about 25% of the maximum response (Fig. 7B).

Contraction is expressed as % of the maximal contraction in the control at 10 mM. Data are mean ± S.E.M. of 6 pairs of strips. Statistically significant vs. control at * p < 0.05, ** p < 0.01.
Experimental and clinical data have shown that Flo has a relatively selective spasmolytic effect on the SO and the ureter, both of which are characterized by their periodic and rhythmic contractions. Since this type of contraction requires coordinated activation of inositol 1,4,5-trisphosphate (IP3) receptors and ryanodine receptors,18) we used drugs that act on these receptors, and compared them with smooth muscles. We selected the ureter, which undergoes periodic rhythmic contractions with NA, the SO, which produces spontaneous rhythmic contractions in the absence of agonist, and the taenia coli, which produces a single tonic contraction with CCh. We compared the effects of Flo on these smooth muscle contractions with the Ca antagonist Nif, the ryanodine receptor agonist 4CmC,19) and the IP3 receptor antagonist 2APB.20)
Figure 8A shows the effects of Flo 30, 100 µM on the three preparations. Flo hardly inhibited the CCh-induced contraction of the taenia coli, but strongly inhibited the spontaneous movement of the SO by 28 and 73%, and the ureter contraction by 38 and 98%, respectively, in a dose-dependent manner. For the comparison, the values in Fig. 3B are shown here again for the effect on the isolated ureter.

A: Effects of 30, 100 µM Flo on the three preparations. B: Effects of 0.1 µM Nif on the three preparations. C: Effects of 30, 100 µM 4CmC on the three preparations. D: Effects of 30, 100 µM 2APB on the three preparations. Data are expressed as % of vehicle control. The number of experiments is shown in parentheses on the top of each column. Statistically significant vs. control at * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 8B shows the effects of Nif 0.1 µM. It inhibited the taenia coli by 65%, the SO by 43%, and the ureter by 68%. Although Nif was slightly weaker in the SO, it inhibited all three preparations to almost the same degree.
As shown in Fig. 8C, 30 and 100 µM of the ryanodine receptor agonist 4CmC did not inhibit CCh-induced contraction of the taenia coli at all, but inhibited the SO by 30 and 60%, respectively, and showed a strong inhibition of NA-induced rhythmic contraction of the ureter by 40 and 90%, respectively. This inhibition profile closely resembles that of Flo.
As shown in Fig. 8D, 30 and 100 µM of the IP3 receptor antagonist 2APB weakly inhibited the taenia coli by 20 and 35%, respectively, while the SO was inhibited by 40 and 70%, respectively, and strongly inhibited the ureter by 90% in both concentrations.
In the present experiments, NA-induced rhythmic contraction in the guinea pig ureter was suppressed by Flo, but Ent, a standard COMT inhibitor, had no effect. Flo exhibited an inhibitory effect independent of α and β receptors on the spontaneous movement of guinea pig SO, whereas Ent had no inhibitory effect. Therefore, it is clear that COMT is not involved in the smooth muscle relaxing effect of Flo.
Why, then, has such a mechanism of action been proposed? According to the interview form,2) Flo originated from pharmacological analysis of phloroglucinol (2,4,6-trihydroxybenzene) by Cahen.21) He concluded that phloroglucinol exhibited papaverine-like direct action on the smooth muscle.
Flo was synthesized as a derivative of phloroglucinol in France22) and clinically developed in Japan. Up to this point, Flo was considered to be a papaverine-type, spasmolytic drug with unknown molecular targets.22) In fact, it had already been reported that pyrogallol (1, 2, 3-trihydroxybenzene) and catechol had a direct smooth muscle relaxing effect unrelated to COMT inhibition.23)
Subsequently, a paper on the structure–activity relationship of the COMT inhibitory action of trihydroxybenzene derivatives was published.15) It had long been known that pyrogallol inhibited COMT, and this article compared the COMT inhibitory effects of various hydroxybenzene derivatives, in view of the clinical application of the trihydroxybenzene derivative, Flo, as a spasmolytic drug. It is now established that COMT inhibitors commonly possess catechol structure24) such as Ent, and this is natural because COMT substrates are catecholamines. Looking at the inhibitory effects of various hydroxybenzenes in their paper,15) it is clear that the adjacent OH group on the benzene ring is essential for COMT inhibition, but for some reason the authors did not mention this point and concluded that Flo had COMT inhibitory activity. The IC50 of Flo for COMT read from the figure in the paper is more than 100 µM.15) According to the package insert,1) the maximum blood concentration of Flo in healthy volunteers is about 50 µM when the one-day dose (80 mg × 3) is taken at once. Considering this point, it is clear that the COMT inhibition theory has no validity.
However, based on the finding that Flo had a weak COMT inhibitory activity,15) a paper was published that the smooth muscle relaxant effect of Flo was attributed to COMT inhibition.25) It might be unavoidable in this era, but all the data were only raw data shown as typical examples, and in most cases, the results appeared as only qualitative descriptions in the text. The actual data on Flo were presented in only one case of bile secretion, and the author mentioned it as an apparent enhancement, but the traces never look like that. The author repeatedly mentioned that Flo augmented the effects of exogenous catecholamines, but no actual data were presented. The author claimed that the potency of trihydroxybenzenes were correlated with their COMT inhibitory activity, but the correlation cannot be discussed since there were no quantitative data in the first place. Therefore, there is no support for the theory that COMT inhibition is involved in the pharmacological effects of Flo.
Since it is clear that COMT inhibition alone cannot explain the spasmolytic effect on the ureter, we suppose that it was necessary to state in the package insert1) and interview form2) that there was another effect. A paper describing that the inhibitory effect of Flo on serotonin was strongest among three agonists was cited as evidence.17)
In order to verify this, we performed an experiment on the guinea pig ureter as they used, and found that no contraction was observed at a physiological concentration of serotonin (10 µM). When we conducted a literature search, we found that the most common examples of serotonin being applied to the urinary tract were humans26) and pigs,27) followed by dogs.28) In guinea pigs, research has only been conducted on serotonin receptors on the autonomic nerve terminals that innervate the detrusor muscle.29) Thus, guinea pig ureteral smooth muscle appears to have few serotonin receptors. The only paper describing the observation that serotonin directly contracts guinea pig ureteral smooth muscle is the one17) cited in the package insert for Flo. However, there are serious problems with this paper. Throughout the paper, dose-response relationships for all drugs were rarely examined, results were not quantified, and statistical analysis was completely absent. Serotonin applied to the guinea pig ureter had a nonphysiological concentration of 100 to 700 µM, and the contraction was inhibited by an extremely high concentration of Flo of 1.6 mM. In their experiment, there was no finding that Flo inhibited serotonin particularly well among the agonists. In guinea pig bile ducts, the authors expressed their impression that the effect of Flo on serotonin-induced spasm was stronger than that induced by acetylcholine or BaCl2. However, this is not acceptable since each agonist was administered at a single dose, and the results were not quantified. Although it was stated in the discussion that it could not be concluded that anti-serotonergic effects were involved in the spasmolytic effects of Flo, the conclusion and English summary stated that the effect of Flo on serotonin-induced spasm was stronger than that of acetylcholine or BaCl2. Therefore, it should be concluded that the “anti-serotonergic action” in the package insert and interview form is completely baseless, with only an inappropriate reference.
Although it has become clear that the mechanism of action of Flo is not based on COMT inhibition or anti-serotonergic action, the question remains as to what the mechanism of spasmolytic action of Flo is. It is clear that the site of action of Flo is on the post-receptor events, and it must explain the fact that it is relatively selective on the bile duct and the urinary tract, which was originally observed.21,22)
First, the effect to enhance Ca uptake into the store, which was proposed in a similar drug, trepibutone,13) was denied by the present experiments conducted with a similar protocol. Next, it was found that there was little inhibitory effect on L-type Ca channels based on the effect on Ca contraction in depolarized taenia coli.
Under the present experimental conditions, the guinea pig taenia coli exhibited a sustained contraction in response to CCh, whereas the spontaneous contraction of the SO in the direction of the circular muscle and the NA-induced contraction in the direction of the longitudinal muscle of the ureter showed a repetitive twitch contraction. The phenomenon of repeated contraction and relaxation of smooth muscle requires coordination between Ca influx and Ca release/reuptake in the store.30–32) We focused on the selectivity of Flo for smooth muscles that cause this rhythmic contraction, namely, Flo might affect the release from or uptake to the Ca store.
The Ca store of smooth muscle contains IP3 and ryanodine receptors; their ratio and localization differ among smooth muscles, and are known to contribute to unique contraction patterns.30) However, the pharmacological tool for analyzing these receptors in non-permeabilized cells is limited. Especially, there is practically no specific, cell permeable antagonist. 4CmC was reported to be a specific, cell permeable agonist for the ryanodine receptor,19) but it alone did not cause any contractions in these three preparations: the taenia coli, SO, and ureter, up to 5 mM in our preliminary experiments. However, we thought that a ryanodine receptor agonist could eventually disrupt the coordinated store gating, and could be useful. In fact, 4CmC showed relatively selective inhibition on the periodical twitch contraction of SO and ureter.
Due to the large negative charge of IP3, agonists and antagonists as their derivatives are known to be membrane impermeable.20) 2APB has been reported as a membrane-permeable IP3 receptor antagonist, but its specificity is insufficient, i.e., it inhibits not only IP3 receptor, but also store-operated calcium channels, TRPC6, and TRPM8, and activates TRPV1, V2, and V3.33) However, because of the scarcity of alternative drugs, 2APB was reluctantly used.
In the present experiments, the Ca antagonist Nif inhibited these three types of smooth muscle contraction to a similar extent, indicating that extracellular Ca influx through L-type channels is important for any type of contraction. On the other hand, Flo scarcely inhibited CCh-induced contraction of taenia coli, whereas it inhibited strongly and dose-dependently the rhythmic contractions in both the SO and ureter. The ryanodine receptor agonist 4CmC showed almost the same inhibitory profile as Flo. The IP3 receptor antagonist 2APB exhibited a similar inhibitory profile to Flo, but showed some inhibition of taenia coli contraction. It has been known that CCh contraction uses Ca influx from L-type channels and Ca release from IP3 receptors.34) Therefore, a simple interpretation of the effects of 2APB, 4CmC, and Nif suggests that L-type channels and IP3 receptors are largely involved in sustained single contraction of the taenia coli, and ryanodine receptors are hardly involved. On the other hand, NA-induced contraction of the ureter should also involve Gq-mediated IP3 production, whereas that of the SO is agonist-free myogenic contraction. Both of these are not sustained, but repeated twitch contractions, which occur when complete relaxation is achieved periodically, that is, a low Ca state, and the subsequent Ca rise reaches a certain threshold, the timing of positive feedback by Ca on the IP3 and ryanodine receptors coincides, and a large amount of Ca is released at once.35) Therefore, not only an antagonist but also an agonist of ryanodine or IP3 receptor could disrupt the coordinated store gating, subsequently, would disturb the positive feedback for causing a spike contraction. In fact, 4CmC was shown to cause a small sustained rise of Ca and to suppress Ca oscillation induced by bombesin in HIT-T15 cells.36) The pattern of inhibition by Flo, 2APB, and 4CmC in the ureter and the SO appeared to lengthen the interval between twitches, resulting in arrest, rather than reducing contraction height (e.g., Fig. 2B), supporting this mechanism. However, this scenario relies on the specificity of 2APB and 4CmC. In order to confirm this, it is necessary to measure intracellular Ca waves directly.18,30–32)
The above discussion indicates that Flo acts on IP3 and ryanodine receptors in cells and disrupts their coordination, thereby exhibiting a relatively selective spasmolytic effect, particularly on smooth muscle that cause periodic contractions. To confirm this, a new strategy is necessary as classical pharmacological methods have limitations. However, we have completed the main purpose of this research to prove that the description of the package insert, “the spasmolytic effect of Flo is based on COMT inhibition and also involves anti-serotonergic effects” is based on a lack of solid evidence. We hope that the package insert and pharmacology textbooks will be promptly revised.
The authors declare no conflict of interest.