2024 Volume 47 Issue 4 Pages 827-839
2024 Volume 47 Issue 4 Pages 827-839
Parkinson’s disease (PD) is a common neurodegenerative disease with progressive loss of dopaminergic neurons in substantia nigra and the presence of α-synuclein-immunoreactive inclusions. Gaucher’s disease is caused by homozygous mutations in β-glucocerebrosidase gene (GBA). GBA mutation carriers have an increased risk of PD. Coptis chinensis (C. chinensis) rhizome extract is a major herb widely used to treat human diseases. This study examined the association of GBA L444P mutation with Taiwanese PD in 1016 cases and 539 controls. In addition, the protective effects of C. chinensis rhizome extract and its active constituents (berberine, coptisine, and palmatine) against PD were assayed using GBA reporter cells, LC3 reporter cells, and cells expressing mutated (A53T) α-synuclein. Case-control study revealed that GBA L444P carriers had a 3.93-fold increased risk of PD (95% confidence interval (CI): 1.37–11.24, p = 0.006) compared to normal controls. Both C. chinensis rhizome extract and its constituents exhibited chemical chaperone activity to reduce α-synuclein aggregation. Promoter reporter and endogenous GBA protein analyses revealed that C. chinensis rhizome extract and its constituents upregulated GBA expression in 293 cells. In addition, C. chinensis rhizome extract and its constituents induced autophagy in DsRed-LC3-expressing 293 cells. In SH-SY5Y cells expressing A53T α-synuclein, C. chinensis rhizome extract and its constituents reduced α-synuclein aggregation and associated neurotoxicity by upregulating GBA expression and activating autophagy. The results of reducing α-synuclein aggregation, enhancing GBA expression and autophagy, and protecting against α-synuclein neurotoxicity open up the therapeutic potentials of C. chinensis rhizome extract and constituents for PD.
Parkinson’s disease (PD) is the second most common neurodegenerative disease. It is caused by a combination of genetic changes and environmental factors that predispose individuals to PD. To date, studies on rare monogenic familial forms of PD have identified >20 risk genes that are associated with PD pathogenesis.1) The histopathological hallmark of PD is the presence of Lewy bodies in the substantia nigra and other subcortical nuclei. Lewy bodies comprise a heterogeneous mixture of proteins, mostly PD-linked α-synuclein and molecules implicated in autophagy.2)
Gaucher’s disease is an autosomal recessive lysosomal storage disease that is caused by mutations in the β-glucocerebrosidase gene (GBA). GBA mutation carriers are at increased risks of PD and dementia with Lewy bodies.3) For example, the GBA L444P mutation has been identified as a significant contributor to PD in Han-Chinese populations.4) This mutation appears to promote α-synuclein accumulation through impaired GBA activity and subsequent downregulation of autophagy signaling.5) Clinically, GBA-associated PD is indistinguishable from sporadic PD.6)
GBA is a 497-amino-acid-long lysosomal hydrolase that metabolizes glucosylceramide to ceramide and glucose.7) Defective autophagy and/or lysosomal depletion have been implicated in PD,8) and acceleration of the autophagy-lysosome pathway is a potential therapeutic target for PD.9) In brain autopsy studies of patients with PD carrying GBA mutations, reductions in GBA activity and expression were most pronounced in the substantia nigra.10) In induced pluripotent stem cell-derived neurons from patients with PD carrying GBA mutations, a reduction in GBA activity or expression was associated with an increase in α-synuclein level and the presence of autophagic and lysosomal defects.11) Ectopic expression of α-synuclein has been demonstrated to reduce GBA activity in cellular models.12) GBA overexpression in mice ameliorates α-synuclein pathology.13) Because GBA mutations result in reduced GBA activity, which influences the autophagic/lysosomal pathway,14) agents that enhance GBA expression may mitigate α-synuclein pathology.
Coptis chinensis rhizome extract (also called Huanglian in Chinese) is a widely used traditional Chinese medicine with anticancer, anti-inflammatory, and antibacterial properties; it also confers neuroprotection and ameliorates cardiovascular disease.15) The main bioactive alkaloids present in the rhizomes of C. chinensis are berberine, palmatine, and coptisine.16) C. chinensis rhizome extract has been reported to reduce dopaminergic neurodegeneration and improve motor function in mice with PD induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).17) Berberine, palmatine, and coptisine have been demonstrated to serve as potent autophagy inducers in Alzheimer’s disease (AD),18) PD,19) hepatocellular carcinoma,20) and alcoholic liver disease.21)
In the present study, we investigated the chemical chaperone activity of C. chinensis rhizome extract and its three constituents and their effects on GBA expression and autophagy in human neuroblastoma SH-SY5Y cells expressing mutated (A53T) α-synuclein. The reduced α-synuclein aggregation and neuronal vulnerability supporting the therapeutic potential of C. chinensis rhizome extract and its constituents (berberine, coptisine, and palmatine) against PD.
Patients diagnosed with PD (63.4 ± 10.9 years, n = 1016) (46.4% females) were recruited from the neurology clinics of Chang-Gung Memorial Hospital. The diagnosis of PD was based on the UK PD Society Brain Bank clinical diagnostic criteria.22) Unrelated healthy adult volunteers (62.1 ± 10.9 years, n = 539) (48.6% females) matched for age, gender, ethnic origin and area of residences were recruited as controls. The study was performed under a protocol approved by the institutional review boards of Chang Gung Memorial Hospital (Ethical License No: 201701458B0) and all examinations were performed after obtaining written informed consents in accordance with the Declaration of Helsinki 2000.
Genomic DNA was extracted from leukocytes using the standard protocols. The L444P (c.1331T > C) variation was examined by PCR using sense 5′-GGAGGACCCAATTGGGTGCGT-3′ and antisense 5′-ACGCTGTCTTCAGCCCACTTC-3′ primers (638 bp) and NciI (New England Biolabs, Ipswich, MA, U.S.A.) restriction analysis (CCCGG, polymorphic base underlined; gain of site). Chi square test was used to compare the frequencies of the genotypes and alleles in patients and controls (p-values <0.05: statistically significant). Odds ratio (OR) and 95% confidence interval (95% CI) were calculated to test association between genotype/allele and disease.
HPLC of C. chinensis Rhizome ExtractAqueous extract from C. chinensis rhizome was provided by Sun-Ten Pharmaceutical Co., LTD. (New Taipei City, Taiwan). HPLC was performed using the Waters 600 pump and Waters 2996 photodiode array detector (Waters, Milford, MA, U.S.A.). The chromatographic separation of C. chinensis rhizome extract (10 µL, 1 mg/mL) was performed using a mobile phase comprising (A) buffer/acetonitrile solution (60 : 40 (v/v); buffer composition: 50 mM sodium acetate, 2% acetic acid, and 5 mM sodium dodecyl sulfate (SDS)) and (B) water/acetonitrile/methanol solution (10 : 45 : 45 (v/v)). The following linear gradient elution was used: 100% A (0 min), 65% A and 35% B (0–15 min), 65% A and 35% B (15–30 min), and 100% A (30–35 min). The flow rate was 1.0 mL/min, the column was maintained at 35 °C, and the UV wavelength for detection was set at 345 nm. The reference compounds berberine, coptisine, and palmatine23) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Cell CultureHuman 293 derived Flp-In-293 cells (Invitrogen, Carlsbad, CA, U.S.A.) were cultivated in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, U.S.A.), 5 µg/mL blasticidin (InvivoGen, San Diego, CA, U.S.A.), and 100 µg/mL zeocin (InvivoGen). GBA fluorescent reporter cells, Flp-In-293-derived cells expressing green fluorescent protein (GFP) reporter driven by GBA promoter (described below), were maintained in DMEM with 10% FBS, 5 µg/mL blasticidin, and 100 µg/mL hygromycin B Gold (InvivoGen). DsRed-LC3 cells, Flp-In-293-derived cells expressing DsRed-LC3 fusion protein,24) were maintained in DMEM with 10% FBS, 5 µg/mL blasticidin, and 100 µg/mL hygromycin B Gold. A53T α-Syn-GFP SH-SY5Y cells, SH-SY5Y-derived cells expressing GFP-tagged A53T α-synuclein transduced by lentivirus,25) were maintained in DMEM/nutrient mixture F12 (DMEM/F12) with 10% FBS and 5 µg/mL blasticidin. Cells were cultured at 37 °C, 95% humidity, and 5% CO2.
Flp-In GBA Reporter Construct and 293 Reporter CellsThe GBA P1 promoter26) was PCR amplified using forward (5′-ATTAATGTGTGCCACTCCCGCTAAATC-3′, AseI site underlined) and reverse (5′-AGACCACAGGGGTTCCAGAGT-3′) primers and cloned into pGEM-T Easy vector (Promega, Madison, WI, U.S.A.) and sequenced. Then the 921-bp AseI/EcoRI (within the multiple cloning region of pGEM-T Easy) fragment containing GBA P1 promoter and the 772-bp EcoRI/NotI fragment containing enhanced green fluorescent protein (EGFP) (from pEGFP-N1; Clontech, Mountain View, CA, U.S.A.) were subcloned into AseI- and NotI-digested pcDNA5/FRT/TO vector (Invitrogen) to generate pcDNA5/FRT/TO-GBA-GFP; this recombinant plasmid was used to generate Flp-In 293 cells expressing GFP as a quantitative reporter of GBA promoter activity according to the supplier’s instruction.
GBA-GFP 293 Reporter Cell AssayTo perform reporter assay, the aforementioned cells were seeded onto a 24-well plate (density, 105 cells/well). After 48 h, the cells were treated with C. chinensis rhizome extract (1–20 µg/mL), berberine (1–10 µM), coptisine (1–10 µM), or palmatine (1–10 µM) for 24 h. Subsequently, their GFP fluorescence was analyzed through flow cytometry (Becton Dickinson, Franklin Lakes, NJ, U.S.A.) at an excitation/emission wavelength of 488/530 ± 15 nm. The concentration required to achieve 50% of the maximal effect (EC50) was calculated using the interpolation method. From cells cultured in 6-well plates (density, 5 × 105 cells/well), GBA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; loading control) proteins were analyzed as described below.
Autophagy AssayOn day 1, DsRed-LC3 cells were seeded onto a 24-well plate (density, 5 × 104 cells/well). On the next day, doxycycline (2 µg/mL; Sigma-Aldrich) was added to induce the expression of DsRed-LC3. After 24 h of incubation, rapamycin (1 µM; Sigma-Aldrich), C. chinensis rhizome extract (10 µg/mL), berberine (10 µM), coptisine (10 µM), or palmatine (10 µM) was added to the medium and further incubated for 1 d. A high content analysis (HCA) system (Micro Confocal High-Content Imaging System; Molecular Devices, San Jose, CA, U.S.A.) was used to automatically image and quantitatively analyze the DsRed-LC3-positive vacuoles (puncta) within cells at 543 ± 11/593 ± 20 nm. Puncta were determined on the basis of granule count, area, and integrated intensity per cell. In addition, from cells cultured in 6-well plates (density, 5 × 105 cells/well), microtubule associated protein 1 light chain 3 alpha (LC3) protein was examined as described below.
Examination and Quantification of Biochemical α-Synuclein AggregationHuman α-synuclein cDNA was amplified and cloned into pET-28a(+) (Novagen, Madison, WI, U.S.A.) as described.27) The expression of the resulting plasmid in Escherichia coli (E. coli) BL21(DE3) (Novagen) was induced after addition of 0.4 mM isopropyl-β-D-thiogalactopyranoside (IPTG; Sigma-Aldrich) for 3 h, and histidine (His)-tagged α-synuclein (α-Syn-His6) protein was purified using His-Bind resins (Novagen). Both bacterial cell lysates and purified α-Syn-His6 protein were analyzed using 10% polyacrylamide gels and visualized by Coomassie blue staining and anti-His immunostaining (1 : 1000; Acris Antibodies #AM00195PU-N, San Diego, CA, U.S.A.).
To induce the aggregation of α-synuclein, the purified α-Syn-His6 protein (1 µg/µL) was incubated in phosphate buffer saline (PBS) at 37 °C under continuous shaking for 3–6 d. The formed α-synuclein aggregates were diluted to 1 µM in distilled water, spotted onto a coverslip, and dried using a vacuum desiccator. Atomic force microscopy imaging was performed using the multimode Solver P47-PRO instrument (NT-MDT, Moscow, Russia). Topographic images were obtained in a semi-contact mode by using an NSG01 cantilever (NT-MDT); the scan rate and resolution were 1 Hz and 512 pixels×512 pixels, respectively.
To perform an α-synuclein aggregation assay by using thioflavin T, C. chinensis rhizome extract (10 µg/mL), curcumin (positive control28); Sigma-Aldrich) (1–10 µM), berberine (1–10 µM), coptisine (1–10 µM), or palmatine (1–10 µM) was added to the purified α-Syn-His6 protein solution (1 µg/µL) and incubated at 37 °C under continuous shaking for 3 d. To quantify α-synuclein aggregates, thioflavin T (10 µM; Sigma-Aldrich) was added and incubated for 5 min at room temperature. The intensity of the thioflavin T-conferred fluorescence of the obtained samples was recorded using the Bio-Tek FLx800 microplate reader (Winooski, VT, U.S.A.) at an excitation/emission wavelength of 420 ± 25/485 ± 10 nm.
To perform a filter trap assay to quantify α-synuclein aggregates, the purified α-Syn-His6 protein was incubated with the tested herb (10 µg/mL) or compounds (1 µM) at 37 °C for 3 d. Subsequently, the obtained samples (0.5 µg) were diluted in 2% SDS in PBS and filtered through a cellulose acetate membrane (pore size, 0.2-µm; Merck, Darmstadt, Germany) by using a dot-blot filtration unit (Bio-Rad Laboratories, Hercules, CA, U.S.A.). After washing, the membrane was blocked with PBS containing 5% nonfat dried milk and stained with anti-α-synuclein antibody (1 : 1000; BD Biosciences #610787, San Jose, CA, U.S.A.). The immune complex on the filter was detected per the procedure described below.
Assessment of Dopaminergic DifferentiationA53T α-Syn-GFP SH-SY5Y cells were treated with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA; 120 nM; Enzo Life Sciences, Farmingdale, NY, U.S.A.) for 14 d to promote dopaminergic differentiation.29) The cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 2% bovine serum albumin, and stained with primary anti-tyrosine hydroxylase (TH) antibody (1 : 500; Santa Cruz Biotechnology #sc-374047, Santa Cruz, CA, U.S.A.) at 4 °C overnight. After washing, the cells were incubated with Cy5-conjugated secondary antibody (Jackson ImmunoResearch #115-175-062, West Grove, PA, U.S.A.) at room temperature for 2 h. The nuclei were detected using 4′-6-diamidino-2-phenylindole (DAPI; 0.1 µg/mL; Sigma-Aldrich). Dopaminergic differentiation was assessed through the simultaneous fluorescent imaging of GFP (excitation/emission wavelength, 482 ± 17.5/536 ± 20 nm) and Cy5 (excitation/emission wavelength, 628 ± 20/692 ± 20 nm) fluorescence using the HCA system.
High Content α-Synuclein Aggregation and Neurite Outgrowth AnalysesA53T α-Syn-GFP SH-SY5Y cells (5 × 104 cells for aggregation analysis and 2 × 104 cells for neurite outgrowth analysis) were seeded onto 24-well plates. After 24 h of incubation, TPA (120 nM) was added to the cells. On day 8, the cells were treated with C. chinensis rhizome extract (100 µg/mL), berberine (10 µM), coptisine (10 µM), or palmatine (10 µM) for 8 h. Next, doxycycline (10 µg/mL) was added to induce A53T α-Syn-GFP expression. In addition, 0.1 µM preformed α-synuclein fibrils (obtained after 6 d of incubation) was added to facilitate the formation of α-synuclein aggregates in A53T α-Syn-GFP cells.30) After 6 d, the cells were fixed and permeated as per the aforementioned procedure and stained with the ProteoStat dye (1 : 5000; Enzo Life Sciences)31) at room temperature for 30 min. The nuclei were counterstained with DAPI (0.1 µg/mL). The aggregation percentage was evaluated using the HCA system; the excitation/emission wavelength was set to 482 ± 17.5/536 ± 20 nm for GFP and 543 ± 11/593 ± 20 nm for ProteoStat. In addition, the α-synuclein aggregates in the cell lysates were examined through a filter trap assay performed using GFP antibody (1 : 500; Santa Cruz Biotechnology #sc-9996).
To perform neurite outgrowth analysis, the fixed and permeated cells were stained with neuronal class III β-tubulin (TUBB3) antibody (1 : 1000; Covance #PRB-435P, Princeton, NJ, U.S.A.), followed by anti-rabbit Alexa Fluor 555 antibody (1 : 1000; Invitrogen #A31572). After nuclei staining (DAPI, 0.1 µg/mL) was completed, neuronal images were captured using the HCA system (excitation/emission wavelength, 543 ± 11/593 ± 20 nm). To quantify neurite outgrowth, microscopic images were segmented using a multi-colored mask to assign each outgrowth to its corresponding cell body. Neurite total length (µm) and numbers of process (originating from the cell body) and branch (secondary neurites extended from primary neurites) were measured using the Neurite Outgrowth Application Module (Molecular Devices).
Western BlottingTotal protein was extracted using lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (pH 8.0), 1 mM ethyleneglycoltetraacetic acid (pH 8.0), 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, and a protease inhibitor cocktail (Sigma-Aldrich). Protein samples (25 µg) were separated through 10% SDS-polyacrylamide gel electrophoresis. The resultant protein bands were transferred onto a polyvinylidene difluoride membrane (Sigma-Aldrich) through reverse electrophoresis. After blocking, the membrane was probed with a 1 : 1000 dilution of GBA (Abcam #ab55080, Cambridge, CB, U.K.), LC3 (Novus Biologicals #NB100-2220, Centennial, CO, U.S.A.), transcription factor EB (TFEB) (Cell Signaling #4240, Danvers, MA, U.S.A.), p62 (also known as SQSTM1/sequestosome 1) (Cell Signaling #5114), or GAPDH (MDBio #30000002, Taipei, Taiwan) antibody overnight at 4 °C. Thereafter, immune complexes were detected using horseradish peroxidase-conjugated goat anti-mouse (#GTX213111-01) or goat anti-rabbit (#GTX213110-01) immunoglobulin G (IgG) antibody (1 : 5000; GeneTex, Irvine, CA, U.S.A.) and a chemiluminescent substrate (Millipore, Temecula, CA, U.S.A.).
Lactate Dehydrogenase Release and Caspase-3 Activity AssaysTo perform a lactate dehydrogenase (LDH) release assay, culture media of A53T α-Syn-GFP SH-SY5Y cells were collected on day 14. LDH release was evaluated using an LDH cytotoxicity assay kit (Cayman Chemical, Ann Arbor, MI, U.S.A.). After a 30-min incubation of culture supernatant and reaction mixture, the absorbance of samples was measured at 490 nm by using the Multiskan GO microplate reader (Thermo Fisher Scientific). To perform the caspase-3 activity assay, the harvested cells were lysed in cold PBS by performing repeated cycles of freezing and thawing. In 10 µg of supernatant, caspase-3 activity was measured using the acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC), a synthetic fluorogenic tetrapeptide substrate (Sigma-Aldrich). The release of fluorescent AMC was monitored at excitation/emission wavelength of 360 ± 20/460 ± 20 nm (Bio-Tek FLx800).
Quantitative Real-Time PCR AssayTo examine the mRNA expression levels of GBA and TFEB, cells from the previously described cultures were harvested and RNA was extracted using TRIzol reagent (Invitrogen). Residual DNA was eliminated through deoxyribonuclease (DNase) I (Thermo Fisher Scientific) treatment, followed by cDNA synthesis using high capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA, U.S.A.). Expression of GBA (ID: Hs00164683_m1; NM_000157.3: exon boundary 10–11, context sequence TCGTGCTAAACCGCTCCTCTAAGGA, amplicon length 85 bp), TFEB (ID: Hs01065085_m1; NM_001167827.2: exon boundary 7–8, context sequence ATGACCTGGACGTGCGCTGGAACAA, amplicon length 56 bp), and endogenous control hypoxanthine phosphoribosyltransferase 1 (HPRT1, ID: 4326321E; NM_000194.2: exon boundary 6–7, context sequence TGGTCAAGGTCGCAAGCTTGCTGGT, amplicon length 100 bp) was determined using StepOnePlus™ Real-time PCR system (Applied Biosystems). Fold changes were calculated relative to the control group using the 2−ΔΔCt method, where ΔΔCt = ΔCt (target sample) − ΔCt (control sample) and ΔCt = Ct (target gene) −Ct (HPRT1 gene).
Statistical AnalysisThree independent experiments were performed for each outcome measure. Data are presented in terms of the mean ± standard deviation values. Between-group differences were analyzed using Student’s t or one-way ANOVA test with Tukey’s post hoc test (SigmaPlot 14.0) where appropriate. All p-values were two-tailed, and a p–value of <0.05 indicated statistical significance.
To elucidate the specific role of GBA mutations in the Taiwanese population, we investigated the prevalence of the GBA L444P variant, a known PD risk factor in the Han-Chinese population,4) among 1016 PD patients and 539 control subjects. The genotype and allele frequencies were in Hardy–Weinberg equilibrium in both patients and healthy controls (both p > 0.05). However, genotype analysis showed a significantly higher frequency of the heterozygous TC genotype (L444P carrier) in PD patients compared with control subjects (2.9 vs. 0.7%, p = 0.006). Compared to subjects with the TT genotype (wildtype), carriers of L444P mutation had a 3.93-fold increased risk of developing PD (95% CI: 1.37–11.24, p = 0.006). This association is also true for subjects carrying the minor C allele, 3.89-fold increased risk of PD (95% CI: 1.36–11.09, p = 0.006) (Table 1). This results strongly indicate the role of GBA and its downstream signaling in the pathogenesis of Taiwanese PD patients.
| PD (%) | Controls (%) | Odds ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Genotype | ||||
| Wildtype (TT) | 987 (97.1%) | 535 (99.3%) | 1.00 | |
| Heterozygote (TC) | 29 (2.9%) | 4 (0.7%) | 3.93 (1.37–11.24) | 0.006 |
| Allele | ||||
| Major (T) | 2003 (98.6%) | 1074 (99.6%) | 1.00 | |
| Minor (C) | 29 (1.4%) | 4 (0.4%) | 3.89 (1.36–11.09) | 0.006 |
To investigate the presence of berberine, coptisine, and palmatine (Fig. 1A) in the aqueous extract of C. chinensis rhizome, the extract was subjected to chemical profiling and full-spectrum analytical HPLC. The chromatograms exhibited peaks at 345 nm, corresponding to the retention times of 15.88, 18.32, and 19.37 min for coptisine, palmatine, and berberine, respectively (Fig. 1B). HPLC further revealed that the aqueous extract of C. chinensis rhizome contained 2.76% berberine, 0.72% coptisine, and 0.78% palmatine, which corresponded to the concentrations of 40.97, 11.27, and 11.02 mM, respectively, in the 0.5 g/mL aqueous extract (Fig. 1C). In a cell culture medium, berberine and palmatine were soluble at a concentration of up to 1 mM; however, coptisine precipitated at a concentration of 100 µM (Fig. 1D).

(A) Structure, formula, and molecular weight of berberine, coptisine, and palmatine. (B) Chemical profile of C. chinensis rhizome extract displaying chromatographic peaks compatible with berberine, coptisine, and palmatine. (C) Relative percentages of berberine, coptisine, and palmatine in C. chinensis rhizome extract. (D) Solubility of berberine, coptisine, and palmatine in cell culture medium.
To investigate the effects of C. chinensis rhizome extract and its constituents on GBA expression, we generated Flp-In 293 cells expressing GFP reporter (Fig. 2A). The cells were treated with the C. chinensis rhizome extract (1–20 µg/mL) or its constituents (1–10 µM) for 24 h, after which GFP fluorescence was measured (Fig. 2B). As shown in Fig. 2C, C. chinensis rhizome extract (127 ± 4.7–202 ± 7.1% in 10–20 µg/mL; p = 0.005–0.001), berberine (105 ± 0.7–194 ± 15.5% in 1–10 µM; p = 0.018–0.003), coptisine (110 ± 3.2% in 10 µM; p = 0.020), and palmatine (115 ± 3.0% in 10 µM; p = 0.004) exhibited potential for enhancing the activity of GBA promoter (EC50: C. Chinensis rhizome extract, 11.2 µg/mL; berberine, 5.1 µM; coptisine, 33.6 µM; palmatine, 29.3 µM). The effects of C. chinensis rhizome extract (10 µg/mL) and its constituents (10 µM) on GBA expression were further confirmed through Western blotting (121.8 ± 9.4–157.4 ± 10.7%; p = 0.039–0.005; Fig. 2D). These results suggest increased GBA expression induced by C. chinensis rhizome extract and its constituents in Flp-In 293 cells.
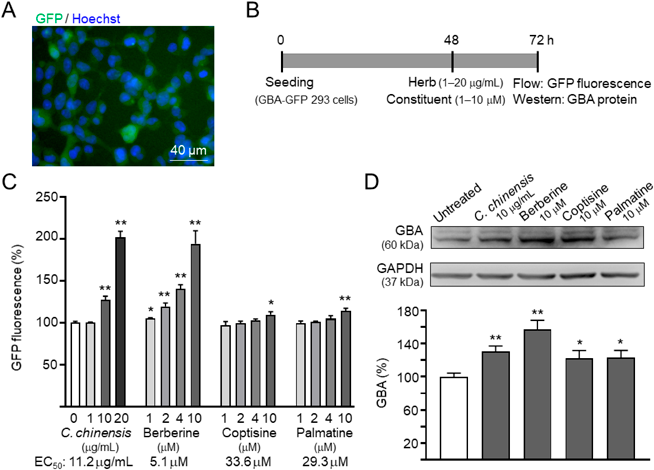
(A) Flp-In GBA-GFP 293 reporter cells. Nuclei were counterstained with Hoechst 33342 (blue). (B) Experimental flow chart. The cells were seeded on day 1, C. chinensis rhizome extract (1–20 µg/mL) or one of its constituents (1–10 µM) was added on day 3, and GFP fluorescence and GBA protein level were assessed on day 4. (C) GFP fluorescence level. Relative fluorescence level in untreated cells was set as 100%. Shown below were EC50 values of C. chinensis rhizome extract and its constituents. (D) Endogenous GBA protein level. Relative GBA level in untreated cells was set as 100%. Comparisons between with and without herb/compound treatment (* p < 0.05 and ** p < 0.01).
The expression of DsRed-tagged LC3 was induced in DsRed-LC3 293 cells24) to evaluate the potential of C. chinensis rhizome extract and its constituents for enhancing autophagy. A day after the induction of DsRed-LC3 expression, the cells were treated with C. chinensis rhizome extract (10 µg/mL) or one of its constituents (10 µM) for 24 h. DsRed-LC3-positive vacuoles (puncta) were quantified; these vacuoles indicated the formation of autophagosomes (Fig. 3A). Rapamycin (1 µM) was included in the experiment for comparison. Rapamycin significantly induced the recruitment of DsRed-LC3 to autophagic vacuoles (count/cell, 224.7 ± 21.6% [p = 0.006]; total area/cell, 235.5 ± 30.6% [p = 0.012]; integrated intensity/cell, 260.6 ± 33.5% [p = 0.011]; Fig. 3B). This phenomenon was also observed after treatments with C. chinensis rhizome extract and its constituents (count/cell, 115.9 ± 4.4–142.2 ± 7.6% [p = 0.039–0.002]; total area/cell, 123.4 ± 10.7–156.2 ± 11.6% [p = 0.044–0.003]; integrated intensity/cell, 143.9 ± 14.6–187.1 ± 22.2% [p = 0.025–0.009]) compared with no treatment (100.0 ± 6.9% for count/cell, 100.0 ± 8.6% for total area/cell, and 100.0 ± 8.2% for integrated intensity/cell).

(A) Experimental flow chart. DsRed-LC3 293 cells were seeded on day 1, doxycycline was added on day 2, treatment with C. chinensis rhizome extract (10 µg/mL)/constituents (10 µM) was performed on day 3, and puncta and LC3-II levels were assessed on day 4. (B) Fluorescent microscopy images of DsRed-LC3 cells with puncta (white, right row). Rapamycin (1 µM) was included as a positive control. Relative punctum level in untreated cells was set as 100%. (C) Endogenous LC3-II protein level. GAPDH was included as an internal loading control. Relative LC3-II level in untreated cells was set as 100%. Comparisons between with and without herb/compound treatment (* p < 0.05 and ** p < 0.01).
In addition to analyzing puncta, we compared the expression level of membrane-bound LC3-II between cells treated with C. chinensis rhizome extract (10 µg/mL) or one of its constituents (10 µM) and untreated cells. As shown in Fig. 3C, a significant increase was observed in the LC3-II level in rapamycin-treated cells (1 µM; 142.1 ± 16.2%; p = 0.037) compared with the level in untreated cells (100.0 ± 4.8%). Significant increases were also noted (141.8 ± 15.0–163.6 ± 13.8%; p = 0.031–0.003) in cells treated with C. chinensis rhizome extract (10 µg/mL) or one of its constituents (10 µM). These findings suggest that C. chinensis rhizome extract and its constituents increased autophagosome accumulation in Flp-In 293 cells.
Inhibition of α-Synuclein Aggregation by C. chinensis Rhizome Extract and Its ConstituentsE. coli-derived recombinant α-Syn-His6 protein was prepared (Fig. 4A) to enable a thioflavin T–based fluorescence assay for examining the fibrillation of α-synuclein in the presence or absence of C. chinensis rhizome extract and its constituents. After 3–6 d of incubation at 37 °C, the recombinant α-Syn-His6 protein exhibited a 9.3 ± 1.5–14.5 ± 1.2-fold increase in the fluorescence of thioflavin T, indicating the formation of strong aggregates, which exhibited irregular globular structures under an atomic force microscope (Fig. 4B). When the relative aggregation level was set to 100.0 ± 6.8%, the addition of curcumin significantly reduced α-synuclein aggregate formation (42.8 ± 3.0–28.0 ± 1.6% at 1–10 µM; p < 0.001) (Fig. 4C). Significant reductions in α-synuclein aggregation were also noted after the addition of C. chinensis rhizome extract (48.6 ± 5.0% at 10 µg/mL; p < 0.001) or one of its constituents (62.0 ± 7.2–25.8 ± 6.1% at 1–10 µM; p < 0.001) (Fig. 4C). When the protein samples were subjected to a filter trap assay and stained with α-synuclein antibody, significant reductions were noted in the levels of SDS-insoluble aggregates in samples treated with curcumin (1 µM), C. chinensis rhizome extract (10 µg/mL) or one of its constituents (1 µM) (65.6 ± 11.1–54.4 ± 10.2%; p = 0.042–0.006; Fig. 4D).

(A) Bacterial α-Syn-His6 evaluated using Coomassie blue (left) and anti-His antibody (right). (B) Results of thioflavin T-based fluorescence assay and atomic force microscopy examination performed to assess the formation of α-synuclein aggregates. Comparisons between days 3–6 and day 0 (** p < 0.01 and *** p < 0.001) and between day 6 and day 3 (&p < 0.05). (C) Results of the thioflavin T–binding assay. Relative fluorescence level of α-Syn-His6 was set as 100%. Comparisons between herb/compound-treated and untreated samples (*** p < 0.001) and between 10-µM compound-treated samples and 1-µM compound-treated samples (&p < 0.05). (D) Results of the filter trap assay. Relative level of trapped α-synuclein after 3 d of incubation was set as 100%. Comparisons between day 3 and day 0 (###p < 0.001) and between herb/compound-treated and untreated samples (* p < 0.05 and ** p < 0.01).
A53T α-Syn-GFP SH-SY5Y cells were used to evaluate the potential of C. chinensis rhizome extract and its constituents for reducing α-synuclein aggregation. The cells were seeded on day 1 and treated with TPA to promote dopaminergic differentiation on day 2. On day 8, the cells were treated with C. chinensis rhizome extract (100 µg/mL) or its constituents (10 µM) for 8 h, followed by doxycycline-inducing SNCA-GFP expression; then, preformed α-synuclein fibrils were added and incubated for 6 d to induce the formation of aggregates in A53T α-Syn-GFP cells. Fluorescent images were automatically recorded using the HCA system (Fig. 5A). After 13 d of TPA treatment, the cells displayed the properties of dopaminergic neurons, as revealed by increased TH immunofluorescence (14.9 ± 1.8-fold change; p = 0.003) and TH-positive cells (from 2.2 ± 0.3 to 96.1 ± 0.4%; p < 0.001) on day 14 (Fig. 5B). After the cells were stained with ProteoStat, the percentage of cells with α-synuclein aggregates significantly increased after the addition α-synuclein fibril (without preformed fibrils vs. with preformed fibrils, 8.5 ± 1.3 vs. 32.9 ± 1.2%, p < 0.001). Treatment with C. chinensis rhizome extract or one of its constituents significantly reduced the percentage of cells with α-synuclein aggregates (untreated cells vs. treated cells, 32.9 ± 1.2 vs. 24.4 ± 2.3–22.2 ± 1.4%, respectively; p = 0.001 to <0.001; Fig. 5C). When the protein samples from these cells were subjected to a filter trap assay, the amount of α-synuclein-containing insoluble aggregates was significantly reduced in cell lysates treated with C. chinensis rhizome extract or one of its constituents (67.1 ± 3.0 − 47.2 ± 16.3 vs. 100.0 ± 3.9%; p = 0.005 to <0.001; Fig. 5D).

(A) Experimental flow chart. Cells were seeded on day 1 and TPA was added on day 2. On day 8, C. chinensis rhizome extract (100 µg/mL) or one of its constituents (10 µM) was added to the cells and incubated for 8 h; this was followed by the induction of GFP-tagged α-synuclein expression and the addition of preformed α-synuclein fibrils (incubation for 6 d). On day 14, TH expression, α-synuclein aggregation, LC3-II, GBA and TFEB protein levels, as well as GBA and TFEB mRNA levels were analyzed. (B) Images of TH staining (red) on days 1 and 14 in SH-SY5Y cells expressing A53T α-Syn-GFP (green). Nuclei were counterstained with DAPI (blue). Shown right were quantitation of TH immunofluorescence and percentage of TH-positive cells. Comparisons between day 14 and day 1 (** p < 0.01 and *** p < 0.001). (C) Images of A53T α-Syn-GFP SH-SY5Y cells exhibiting α-Syn-GFP (green) and ProteoStat-stained aggregates (red). Nuclei were counterstained with DAPI (blue). Shown below were quantitation of percentage of aggregated cells. (D) Filter trap analysis of α-synuclein aggregation. Relative α-synuclein aggregation in the presence of additional preformed fibrils was set as 100%. (E) LC3-II, GBA, p62, and TFEB protein levels. Relative protein levels in uninduced cells were set as 100%. GAPDH was included as an internal loading control. (F) GBA and TFEB mRNA levels. Relative mRNA levels in uninduced cells were set as 100%. (C–F) Comparisons between doxycycline-treated and untreated cells (#p < 0.05, ##p < 0.01, and ###p < 0.001), between cells with and without additional fibrils (&p < 0.05 and &&&p < 0.001), and between herb/compound-treated and untreated cells (* p < 0.05, ** p < 0.01, and *** p < 0.001).
The expression levels of LC3-II and GBA were compared to evaluate the degree of autophagy induction in the study cells. As the selective autophagy substrate p62 protein level inversely correlates with overall autophagic activity,32) we included it as a marker to assess the impact of our intervention on autophagy. Additionally, we examined the expression of TFEB, a key transcription factor regulating lysosomal genes including GBA,33) to gain insights into potential downstream effects on lysosomal function. As shown in Fig. 5E, the induced expression of A53T α-Syn-GFP and addition of α-synuclein fibrils for 6 d reduced the LC3-II level (from 100.0 ± 6.3 to 74.5 ± 6.3%), although not to a significant level (p > 0.05). Significant increase in the LC3-II was observed when the cells were treated with C. chinensis rhizome extract or one of its constituents (untreated cells vs. treated cells, 74.5 ± 6.3 vs. 119.0 ± 17.0 − 125.0 ± 12.0%, respectively; p = 0.042 − 0.018). In addition, the induced expression of A53T α-Syn-GFP combined with the addition of preformed α-synuclein fibrils significantly downregulated the expression of GBA (induced cells with additional α-synuclein fibrils vs. uninduced cells/induced cells without additional α-synuclein fibrils, 56.9 ± 4.1 vs. 100.0 ± 3.9/80.4 ± 5.2%, respectively; p < 0.001/0.027). Significant increase in GBA expression was noted after the treatment of cells with C. chinensis rhizome extract or one of its constituents (79.0 ± 2.2 − 84.6 ± 7.9%; p = 0.046 − 0.009). Furthermore, p62 accumulation was significantly increased in induced cells with additional α-synuclein fibrils (from 100.0 ± 11.0 to 137.8 ± 14.6%; p = 0.033). C. chinensis rhizome extract and its constituents mitigated this effect by promoting p62 degradation via autophagy upregulation (94.6 ± 20.3 − 84.8 ± 10.9%; p = 0.013 − 0.002). A53T α-Syn-GFP expression and preformed fibril addition reduced TFEB protein levels (from 100.0 ± 8.3 to 60.9 ± 8.4%; p = 0.011). C. chinensis rhizome extract and berberine significantly restored TFEB levels (93.3 ± 14.4 − 96.6 ± 16.8%; p = 0.04 − 0.021). mRNA analysis in A53T α-Syn-GFP SH-SY5Y cells revealed that the extract and its constituents increased GBA expression (from 74.8 ± 3.9 to 94.3 ± 1.4 − 105.1 ± 3.2%; p = 0.002−<0.001). Notably, only the extract and berberine increased TFEB mRNA level (from 74.3 ± 2.9 to 97.0 ± 2.5 − 100.4 ± 11.3%; p = 0.001−<0.001) (Fig. 5F).
Effects of C. chinensis Rhizome Extract and Its Constituents on Neuronal Survival and Neurite Outgrowth in A53T α-Syn-GFP SH-SY5Y CellsThe effects of C. chinensis rhizome extract (100 µg/mL) and its constituents (10 µM) on neuronal survival, such as LDH release (Fig. 6A) and caspase-3 activity (Fig. 6B) in A53T α-Syn-GFP SH-SY5Y cells were investigated. The addition of doxycycline increased LDH release (110.8 ± 5.5%) and caspase-3 activity (109.1 ± 0.7%) but not to a significant level (p > 0.05). The addition of preformed α-synuclein fibrils significantly increased LDH release (136.0 ± 7.0%; p < 0.001) and caspase-3 activity (118.2 ± 1.7%; p = 0.037). Treatment with C. chinensis rhizome extract and its constituents reduced LDH release (106.3 ± 6.3 − 93.7 ± 9.1%; p = 0.003–<0.001) and caspase-3 activity 100.7 ± 8.0 − 95.2 ± 9.6%; p = 0.048–0.007) in the cells.

(A) Results of LDH release and (B) caspase-3 activity assays. To normalize, relative levels of LDH release and caspase-3 activity in uninduced cells were set as 100%. (C) Assessment of neurite length, process, and branch. Shown were TUBB3 (yellow)-stained images and images of cells outlined with multi-colored mask to assign each outgrowth to a cell body for neurite outgrowth quantification. Nuclei were counterstained with DAPI (blue). Comparisons between doxycycline-treated and untreated cells (#p < 0.05, ##p < 0.01, and ###p < 0.001), between cells with and without additional fibrils (&p < 0.05), and between herb/compound-treated and untreated cells (* p < 0.05, ** p < 0.01, and *** p < 0.001).
Finally, we examined the neurite morphology, including neurite length, process, and branch. As shown in Fig. 6C, the induction of α-synuclein expression combined with the addition of preformed α-synuclein fibrils significantly reduced neurite length (from 26.7 ± 0.6 to 21.8 ± 0.3 µm; p < 0.001), process (from 1.89 ± 0.02 to 1.63 ± 0.03; p < 0.001), and branch (from 0.61 ± 0.01 to 0.48 ± 0.01; p < 0.001) in A53T α-Syn-GFP SH-SY5Y cells. Pretreatment with C. chinensis rhizome extract (100 µg/mL) or one of its constituents (10 µM) significantly increased the neurite length (23.2 ± 0.2–23.6 ± 0.5 µm; p = 0.015–0.002), process (1.75 ± 0.03; p = 0.007–0.006), and branch (0.58 ± 0.02–0.59 ± 0.04; p = 0.002–0.001). Collectively, these results indicate that C. chinensis rhizome extract and its constituents promoted neurite outgrowth and conferred protection against cell death in A53T α-Syn-GFP SH-SY5Y cells.
An effective treatment for decelerating neurodegeneration in patients with PD remains to be developed. PD-associated α-synuclein aggregates are degraded mainly through the autophagy lysosomal pathway.34) The accumulation of α-synuclein aggregates leads to a reduction in GBA activity/expression and the development of autophagic/lysosomal defects.11) The level of autophagy in neurons has been suggested to be low because autophagy is rarely observed in healthy brains.35,36) Autophagy may be regulated differently in primary neurons and other cell types.37) Therefore, identifying the inducers of neuronal autophagy that can metabolize misfolded α-synuclein may be an effective therapeutic approach for PD.38) Because α-synuclein is prone to aggregation,39) potent chemical chaperones conferring protection against α-synuclein aggregation may also have therapeutic potential against PD. Through the thioflavin T–based fluorescence and filter trap assays, we discovered that C. chinensis rhizome extract and three of its constituents (i.e., berberine, coptisine, and palmatine) exhibited chemical chaperone activity, reducing the aggregation of α-synuclein (Fig. 4). A53T α-Syn-GFP SH-SY5Y cells treated with C. chinensis rhizome extract or one of its constituents exhibited upregulated GBA expression and increased autophagy, which resulted in reduced α-synuclein aggregation and LDH release, and enhanced neurite outgrowth (Figs. 5, 6). Notably, a previous study found that C. chinensis rhizome extract and its constituents did not improve cell viability in SH-SY5Y cells,40) suggesting a lack of neurotrophic effect. These findings outline the neuroprotective potential of C. chinensis rhizome extract and its constituents (i.e., berberine, coptisine, and palmatine) against α-synuclein toxicity.
Berberine is the most widely studied constituent of C. chinensis rhizome extract. In a study involving TgCRND8 transgenic mice, berberine exerted neuroprotective effects by targeting the generation of amyloid-β.41) Berberine can also regulate blood lipid levels42) and blood pressure,43) ameliorate atherosclerosis,44) and reduce oxidative stress and inflammation associated with diabetes mellitus.45) By promoting TFEB nuclear translocation and deacetylation, berberine triggers autophagy in peritoneal macrophages and inhibits apoptosis.46) Evidence also suggests that berberine inhibits neuroinflammation and oxidative and endoplasmic reticulum stress, further reducing neuronal damage and apoptosis.47) PD-related studies have revealed that berberine protects against the toxicity of 6- hydroxydopamine in human cells48) and that of MPTP in mice.17) Notably, berberine was demonstrated to significantly reduce the level of α-synuclein and increase the degree of LC3-II-associated autophagy in the substantia nigra of MPTP-treated mice.18) We found that berberine (also C. chinensis rhizome extract) can upregulate TFEB and GBA expression and enhance autophagy to reduce α-synuclein aggregation and protect dopaminergic neurons from degeneration in PD; these findings highlight the multidimensional therapeutic effects of berberine on human diseases. Given the diverse pharmacological effects and mechanisms of berberine in PD, including its role in autophagy, future studies should be conducted to comprehensively analyze the effects of berberine.
Coptisine modulates nuclear factor-kappaB (NF-κB), mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), and NOD-like receptor protein 3 (NLRP3) inflammasome signaling pathways, exerting anticancer, anti-inflammatory, antibacterial, and cardioprotective effects.49) As an inhibitor of indoleamine 2,3-dioxygenase, coptisine ameliorates cognitive impairment in a mouse model of AD.50) In contrast to berberine and palmatine, coptisine displays unique vascular smooth muscle cell-selective effects on multidrug resistance protein induction.51) Furthermore, coptisine protects SH-SY5Y cells against a tert-butyl hydroperoxide-induced decline in mitochondrial membrane potential and against apoptosis.52) Moreover, coptisine inhibits the PI3K/AKT/mammalian target of rapamycin (mTOR) signaling pathway and enhances reactive oxygen species-mediated mitochondrial dysfunction to induce the autophagy in Hep3B liver cancer cells.20) As the main active compound of C. chinensis rhizome extract, coptisine confers protection against neurotoxin-induced PD.16) In the present study, we demonstrated that coptisine exerts neuroprotective effects by upregulating GBA expression and enhancing autophagy to reduce α-synuclein aggregation. Because chronic neuroinflammation is associated with α-synuclein aggregation, further studies are required to investigate whether coptisine can protect against PD through the reported anti-inflammatory activity.53,54)
Palmatine is associated with various pharmacological activities, including neuroprotective, blood lipid-modulating, anticancer, antibacterial, antiviral, and anti-inflammatory activities.55) These pharmacological effects of palmatine suggest its potential for preventing and treating specific human diseases. For example, palmatine has value in the prevention and treatment of osteoporosis.56) In addition, palmatine exhibits therapeutic potential against AD mainly by inhibiting acetylcholinesterase activity,57) exerting antioxidative effects against peroxynitrite radicals,58) and inhibiting tau aggregation.59) In a relevant study, mice were subjected to chronic unpredictable mild stress; subsequent palmatine treatment exhibited antidepressive (by inhibiting monoamine oxidase A) and antioxidative effects.60) Another study reported that palmatine mitigated alcohol-induced hepatocyte injury by promoting autophagy through the AMPK/mTOR pathway.21) Palmatine also exhibits potent, concentration-dependent antioxidative activity, contributing to the management of oxidative stress-induced PD.61) In the α-synuclein-expressing cells used in the present study, palmatine reduced α-synuclein aggregation and promoted neurite outgrowth by upregulating GBA expression and enhancing autophagy; to the best of our knowledge, the present study is the first to report this finding. Among the three examined constituents of C. chinensis rhizome extract, palmatine was most effective in reducing caspase-3 activity (Fig. 6). Therefore, further animal studies are warranted to validate the therapeutic potential of palmatine against PD.
By using α-synuclein-expressing SH-SY5Y cells, we demonstrated that C. chinensis rhizome extract and its active constituents reduce α-synuclein aggregation through chemical chaperone activity and protect the cells against α-synuclein-induced neurotoxicity by upregulating GBA expression and enhancing autophagy. Our study may be the first to report that coptisine and palmatine can enhance neuronal autophagy. Given that multiple pathogenic pathways are involved in the development of PD, the ability of C. chinensis rhizome extract and its active constituents to target multiple pathways may offer important insights into the development of PD drugs. Nonetheless, our findings are limited to cellular models. Hence, further animal studies on PD should be conducted to explore the roles of C. chinensis rhizome extract and its active constituents (berberine, coptisine, and palmatine) as autophagy inducers. It remains unclear whether the benefits of C. chinensis rhizome extract and its active constituents are specific to GBA mutation carriers or extend to a broader PD population. Further clinical trials are needed to establish the safety and efficacy of this extract and its components in improving motor and cognitive symptoms in PD patients.
We thank the Instrumentation Center of National Taiwan Normal University (MOST 107-2731-M-003-001, MD ImageXpress Micro Confocal, BIO002000) for providing technical support. This manuscript was edited by Wallace Academic Editing. This work was supported by the Taiwanese Ministry of Science and Technology (MOST 104-2314-B-182A-025-MY3, 104-2811-B-182A-006, 105-2811-B-182A-008, and 106-2811-B-182A-013).
Y-RW and G-JL-C conceived and designed the experiments. Y-RW obtained funding. C-HL conducted the experiments. C-YC, W-LC, and P-NY helped with the experiments. C-HL and G-JL-C analyzed and interpreted the data. C-HL, Y-RW, and G-JL-C wrote the manuscript. C-MC and K-HC contributed to the study conception and design. All authors have read and approved the final manuscript.
The authors declare no conflict of interest.