2025 Volume 48 Issue 2 Pages 132-136
2025 Volume 48 Issue 2 Pages 132-136
In Japan, S-1 is used as adjuvant chemotherapy for pancreatic cancer. Neutropenia during S-1 chemotherapy is reported to be an independent predictor of prolonged survival in patients with advanced gastric cancer. However, this is unclear in pancreatic cancer. This study aimed to examine the effect of severe neutropenia caused by S-1 adjuvant chemotherapy on pancreatic cancer prognosis: overall survival (OS) and recurrence-free survival (RFS), and the potential effect of the duration from surgery to S-1 administration on OS and RFS. This single-center, retrospective, observational study included patients who newly received S-1 adjuvant chemotherapy after curative resection of pancreatic cancer at the Japanese Red Cross Kyoto Daini Hospital between January 1, 2016, and September 30, 2020. Of the 43 patients, 9 had grade 3 or higher neutropenia (G3 group) and had a significantly longer median OS than the other 34 (non-G3 group) did. The median RFS of the G3 group was longer than that of the non-G3 group. The median time from surgery to S-1 administration was significantly shorter in the G3 group than in the non-G3 group. Cox proportional hazards regression analysis revealed that duration from surgery to S-1 administration <51 d (hazard ratio: 0.375, 95% confidence interval: 0.154–0.914, p = 0.031) and occurrence of grade 3 neutropenia (hazard ratio: 0.198, 95% confidence interval: 0.046–0.860, p = 0.031) were significantly associated with prolonged OS. In conclusion, initiating S-1 adjuvant chemotherapy early after surgery and the occurrence of grade 3 neutropenia may improve pancreatic cancer prognosis.
Pancreatic cancer is a highly lethal malignancy. In most cases, a surgical resection is necessary for cure. Several phase 3 clinical trials have been conducted regarding adjuvant chemotherapy after curative resection. The European Study Group for Pancreatic Cancer (ESPAC)-4 trial showed that adjuvant chemotherapy with gemcitabine plus capecitabine significantly improved overall survival (OS), compared with gemcitabine alone after resection for pancreatic cancer.1) The use of modified FOLFIRINOX led to significantly longer survival than did gemcitabine use in France and Canada.2) In Japan, S-1, a fluorinated pyrimidine antineoplastic drug consisting of tegafur, gimeracil, and oteracil potassium, is used as adjuvant chemotherapy after curative resection based on the results of the Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC) 01 trial.3)
Several studies have been conducted to improve the antitumor effect of S-1 adjuvant chemotherapy in patients with pancreatic cancer. The studies verified the benefits of prolonging the duration of S-1 administration,4–6) investigated the dose intensity threshold that affects prognosis,7,8) and identified biomarkers for predicting chemosensitivity.9)
Several studies have also been conducted regarding the duration from surgery to S-1 administration in patients with pancreatic cancer.8,10) These studies have shown that patients who received delayed S-1 treatment (≥51 d) after surgery had worse survival outcomes8) and that initiating S-1 adjuvant chemotherapy within 10 weeks after surgery may be beneficial for survival, compared with late initiation.10) However, the effect of adverse events on the antitumor effect of S-1 has not been reported in patients with pancreatic cancer.
In general, severe adverse events result in treatment interruption or discontinuation and may have a negative effect on prognosis. In particular, severe neutropenia increases the risk of contracting life-threatening infections. Conversely, in patients with advanced gastric cancer, neutropenia during S-1 chemotherapy has been reported to be an independent predictor of increased survival.11) However, the effect of neutropenia on the antitumor effect of S-1 adjuvant chemotherapy in patients with pancreatic cancer has not been reported.
This study aimed to examine the effect of severe neutropenia caused by S-1 adjuvant chemotherapy on pancreatic cancer prognosis.
This retrospective, observational study included patients who newly received S-1 adjuvant chemotherapy after curative resection of pancreatic cancer at the Japanese Red Cross Kyoto Daini Hospital between January 1, 2016, and September 30, 2020. No exclusion criteria were applied.
OS was defined as the time from surgery to death and recurrence-free survival (RFS) as the time from surgery to recurrence or death. Furthermore, based on previous reports,8,10) the potential effects of duration from surgery to S-1 administration on OS and RFS were examined.
This study was conducted using existing records containing de-identified data and approved by the Clinical Research Review Board of the Japanese Red Cross Kyoto Daini Hospital (Approval Number: Sp2023-01). Patient consent was obtained using the opt-out method, in accordance with the Clinical Research Review Board guidelines. This study was conducted following the Ethical Guidelines for Medical and Biological Research Involving Human Subjects set forth by the Ministry of Education, Culture, Sports, Science and Technology; the Ministry of Health, Labour and Welfare; and the Ministry of Economy, Trade and Industry of Japan.
Data CollectionThe observation period spanned from January 1, 2016, to February 28, 2023, during which each case was followed for a minimum of 2 years. Data on patient characteristics, baseline laboratory measurements, S-1 dosage, and adverse events were retrospectively collected from medical records.
S-1 was orally administered at a dose of 40, 50, or 60 mg/m2 twice daily, depending on the patient’s body surface area: <1.25 m2, between ≥1.25 and <1.5 m2, or ≥1.5 m2. The treatment was administered for 4 weeks, followed by a 2-week withdrawal period, and then repeated every 6 weeks for 4 courses. Dose reduction and interruption based on the doctor’s judgment were deemed acceptable since this study was based on daily practice. Dose intensity, relative dose intensity (RDI), and cumulative dose, even if S-1 was administered for more than 6 months, were calculated up to 6 months. Adverse events were graded using the Common Terminology Criteria for Adverse Events version 5.0.
Statistical AnalysisThe patients were categorized into 2 groups: those with grade 3 or higher neutropenia (G3 group) and those without (non-G3 group). Fisher’s exact probability test was used to compare categorical data. Normally distributed continuous variables are presented as mean ± standard deviation, whereas non-normally distributed values are presented as median and interquartile range. The t-test and Mann–Whitney U test were used to compare normally and non-normally distributed variables, respectively, between groups. The Kaplan–Meier method was used to evaluate the distributions of OS and RFS, and the log-rank test was used to evaluate the statistical significance of differences. The hazard ratio (HR) and 95% confidence interval (CI) were calculated using the Cox proportional hazards model.
The cutoff value for the duration from surgery to S-1 administration was 51 d, based on previous reports, calculated using receiver operating characteristic (ROC) analysis.8) To investigate the effect of grade 3 or higher neutropenia and the duration from surgery to S-1 administration on OS and RFS, we conducted multivariate analyses using Cox proportional hazards regression.
Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan, https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html), a graphical user interface for R (The R Foundation for Statistical Computing, version 4.2.2). More precisely, it is a modified version of R commander (version 2.8-0) designed to add statistical functions frequently used in biostatistics.12) p-Values <0.05 were considered statistically significant.
This study included 43 patients aged 65–76 years (56% male). The median duration from surgery to S-1 administration was 51 d. The majority (n = 26; 60%) completed 6 months of adjuvant chemotherapy, whereas less than half the patients (n = 18; 42%) continued it for beyond 6 months.
Among the 43 patients, the most frequent adverse event reported was anemia (83.7%), followed by increased aspartate aminotransferase levels (53.5%) and skin disorders (48.8%). In addition, only a few (n = 9; 20.9%) experienced grade 3 neutropenia. Other grade 3 adverse events reported were diarrhea (3 patients, 7.0%), stomatitis (1 patient, 2.3%), and eye disorders (1 patient, 2.3%). No grade 4 or higher adverse events were observed.
Comparison of Patient Background with or without Grade 3 NeutropeniaThe duration from surgery to S-1 administration was significantly shorter in the G3 group than in the non-G3 group (39 vs. 55 d, p = 0.025, Mann–Whitney U-test) (Table 1). There were no significant differences between the 2 groups in terms of other factors.
| G3 group (n = 9) |
Non-G3 group (n = 34) |
p-Value | ||
|---|---|---|---|---|
| Patient characteristic | ||||
| Age | 69 (65–76) | 72 (65–76) | 0.580a) | |
| Sex | Male/female | 5/4 | 19/15 | 1.000b) |
| BSA (m2) | 1.51 (1.42–1.55) | 1.50 (1.36–1.58) | 0.893a) | |
| Operative procedure | PD/DP | 3/6 | 22/12 | 0.133b) |
| Duration from surgery to S-1 administration (d) | 39 (37–51) | 55 (45–61) | 0.025a) | |
| S-1 administration | ||||
| Initial dose reduction | Yes/no | 5/4 (56%) | 16/18 (47%) | 0.721b) |
| Dose intensityc) (mg/m2/week) | 282 (259–321) | 292 (267–323) | 1.000a) | |
| Relative dose intensityc) (%) | 83 (75–96) | 85 (80–100) | 0.857a) | |
| Cumulative dosec) (mg/m2) | 5572 (4781–7212) | 6667 (3318–7550) | 0.965a) | |
| Completed adjuvant chemotherapy | Yes/no | 5/4 (56%) | 21/13 (62%) | 1.000b) |
| Additional S-1 administrationd) | Yes/no | 4/5 (44%) | 14/20 (41%) | 1.000b) |
| Baseline laboratory data | ||||
| TP (g/dL) | 6.6 (6.4–7.3) | 6.6 (6.4–7.0) | 0.822a) | |
| Alb (g/dL) | 3.7 (3.6–4.1) | 3.7 (3.4–3.9) | 0.483a) | |
| T-bil (mg/dL) | 0.5 (0.4–0.8) | 0.5 (0.4–0.5) | 0.171a) | |
| ALP (U/L) | 258 (198–289) | 277 (222–342) | 0.387a) | |
| AST (U/L) | 21 (16–41) | 19 (15–25) | 0.301a) | |
| ALT (U/L) | 18 (12–31) | 16 (9–26) | 0.530a) | |
| LDH (U/L) | 190 (165–193) | 168 (137–184) | 0.117a) | |
| Amy (U/L) | 75 (63–89) | 64 (45–82) | 0.256a) | |
| CCr (mL/min) | 63 (55–84) | 70 (59–78) | 0.601a) | |
| Na (mEq/L) | 140 (139–141) | 140 (139–142) | 0.821a) | |
| K (mEq/L) | 4.1 (3.8–4.5) | 4.0 (3.7–4.3) | 0.436a) | |
| Cl (mEq/L) | 103 (101–103) | 104 (103–106) | 0.053a) | |
| WBC (/μL) | 5600 (4000–6000) | 5450 (4425–6725) | 0.601a) | |
| Hb (g/dL) | 11.5 (11.2–12.7) | 11.6 (10.8–12.4) | 0.964a) | |
| Plt (×104/μL) | 27.1 (19.9–34.9) | 26.4 (23.5–30.7) | 0.811a) | |
| Neutr (/μL) | 2454 (2052–3080) | 2903 (2367–4388) | 0.150a) |
BSA, body surface area; PD, pancreatoduodenectomy; DP, distal pancreatectomy; TP, serum total protein; Alb, serum albumin; T-bil, serum total bilirubin; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; Amy, serum amylase; CCr, creatinine clearance; Na, serum sodium; K, serum potassium; Cl, serum chloride; WBC, white blood cell; Hb, hemoglobin; Plt, platelets; Neutr, neutrophils. The patients were categorized into 2 groups: those with grade 3 or higher neutropenia (G3 group) and those without (non-G3 group). Continuous variables are presented as median and interquartile range. a) Mann–Whitney U-test, b) Fisher’s exact probability test, c) It was calculated up to 6 months. d) Refers to patients who continued treatment beyond the standard treatment period of 6 months.
Median OS was significantly longer in the G3 group than in the non-G3 group (63.8 vs. 26.9 months, p = 0.007, HR: 0.166, 95% CI: 0.038–0.713) (Fig. 1). Similar findings were observed for median RFS, although not significant (29.9 vs. 11.2 months, p = 0.128, HR: 0.507, 95% CI: 0.208–1.237) (Fig. 2).
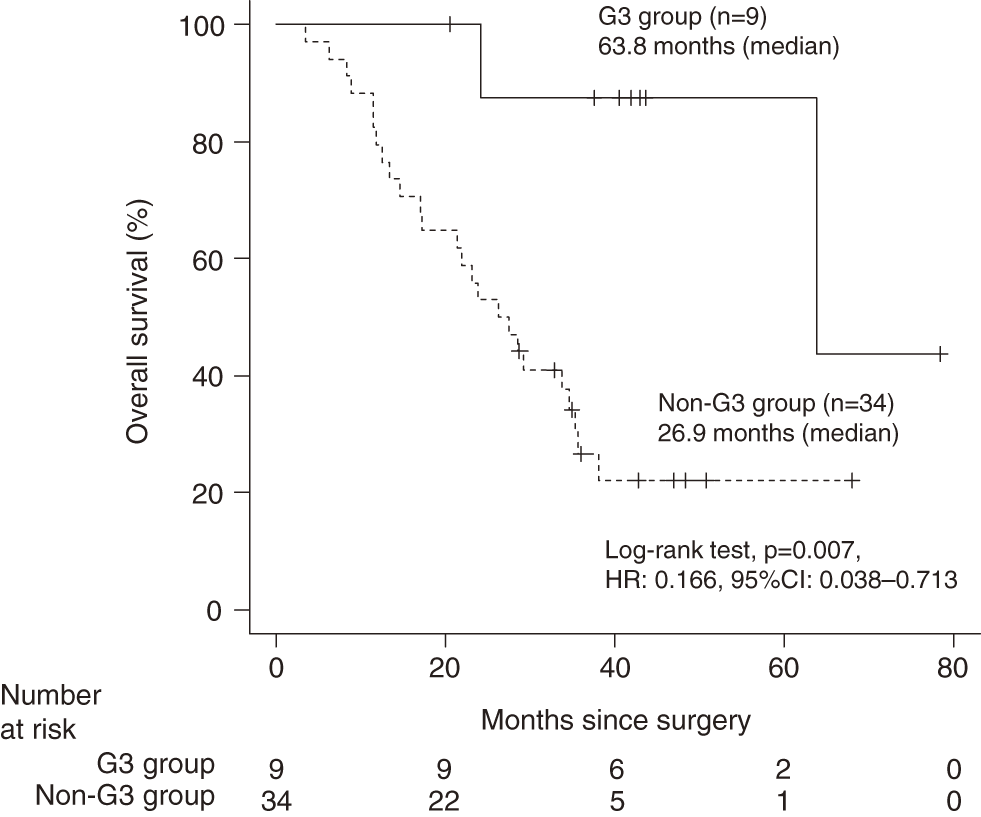
The patients were categorized into 2 groups: those with grade 3 or higher neutropenia (G3 group) and those without (non-G3 group). Overall survival distribution was estimated using the Kaplan–Meier method, and the log-rank test was used to evaluate the statistical significance of differences. The HR and 95% CI were calculated using the Cox proportional hazards model. HR, hazard ratio; CI, confidence interval.

The patients were categorized into 2 groups: those with grade 3 or higher neutropenia (G3 group) and those without (non-G3 group). Recurrence-free survival distribution was estimated using the Kaplan–Meier method, and the log-rank test was used to evaluate the statistical significance of differences. The HR and 95% CI were calculated using the Cox proportional hazards model. HR, hazard ratio; CI, confidence interval.
The Cox proportional hazards regression analysis (Table 2) revealed that patients with a duration from surgery to S-1 administration <51 d (HR: 0.375, 95% CI: 0.154–0.914, p = 0.031) and those who experienced grade 3 neutropenia (HR: 0.198, 95% CI: 0.046–0.860, p = 0.031) had significantly prolonged OS. Similar findings were observed for RFS, although not significant (HR: 0.606, 95% CI: 0.304–1.208, p = 0.155 and HR: 0.513, 95% CI: 0.209–1.258, p = 0.145, respectively).
| HR | 95% CI | p-Valuea) | |
|---|---|---|---|
| OS | |||
| Duration from surgery to S-1 administration <51 d (n = 20) | 0.375 | 0.154–0.914 | 0.031 |
| Grade 3 neutropenia (n = 9) | 0.198 | 0.046–0.860 | 0.031 |
| RFS | |||
| Duration from surgery to S-1 administration <51 d (n = 20) | 0.606 | 0.304–1.208 | 0.155 |
| Grade 3 neutropenia (n = 9) | 0.513 | 0.209–1.258 | 0.145 |
OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; 95% CI, 95% confidence interval. a) Cox proportional hazards regression analysis.
To our knowledge, no study has been conducted on the relationship between adverse events of S-1 adjuvant chemotherapy and pancreatic cancer prognosis. Cox proportional hazards regression analysis showed that the presence of grade 3 neutropenia and a duration from surgery to S-1 administration <51 d were significantly associated with prolonged OS in patients with pancreatic cancer after curative resection.
Median RFS did not show significant differences in the Cox proportional hazards regression analysis. The significant difference in OS but not RFS is thought to be due to the inclusion of some patients with early recurrence. Recurrence during adjuvant chemotherapy is common in pancreatic cancer. Reports have indicated that approximately one-third of patients (33%) were unable to continue S-1 for more than 150 d,5) and less than a quarter of patients (18%) relapsed or died within 6 months of starting S-1.8) Similarly, in the present study, less than a quarter of patients (23%, 10 out of 43) relapsed within 6 months of starting S-1 (data not shown). Since pancreatic cancer often recurs, there is a high probability that micrometastases are already present in the body even after curative resection. When these become apparent on imaging, the patient is diagnosed with recurrence even during treatment. In addition, this study was retrospective; thus, the date of confirmed recurrence depends on patient visits. This may be another reason for the variability in RFS data.
In patients with advanced gastric cancer, the presence of neutropenia during S-1 chemotherapy has been reported to be an independent predictor of increased survival.11) This suggests that the presence of chemotherapy-induced neutropenia may be a surrogate indicator of the efficacy of S-1 chemotherapy in patients with advanced gastric cancer.11) In this study, grade 3 neutropenia was significantly associated with prolonged OS in patients with pancreatic cancer after curative resection. Since this was a retrospective rather than a prospective study, the possibility of data bias influencing the results cannot be completely ruled out. Although results similar to those previously reported were obtained in our study, it may not be appropriate to evaluate the findings on the same level. Nevertheless, our study provides valuable insights into the management of pancreatic cancer, for which the efficacy of adjuvant chemotherapy is limited, neutropenia may also be used as a surrogate indicator of the efficacy of S-1 adjuvant chemotherapy.
On the other hand, grade 3 neutropenia being significantly associated with prolonged OS may have led to high treatment intensity in the patients. However, the present study revealed no significant difference in dose intensity, RDI, or cumulative dose between patients with or without grade 3 neutropenia. The results showing no difference in treatment intensity between the 2 groups support the hypothesis that the presence of grade 3 neutropenia can be a predictive indicator of the efficacy of S-1 adjuvant chemotherapy. Thus, patients who develop grade 3 neutropenia have no other adverse events and are in good general condition, continuation of S-1 treatment may be one of several options.
In the present study, in addition to investigating the incidence of grade 3 neutropenia, we examined time from surgery to S-1 administration and found that a duration <51 d was significantly associated with prolonged OS in patients with pancreatic cancer. Previous studies have shown the importance of initiating S-1 promptly after surgery,8,10) and the results of the present study support these previous findings.
This study has some limitations. It was a single-center retrospective study, which may have caused patient selection bias. In addition, the sample size was small. Therefore, the Cox proportional hazards regression analysis did not adjust for several factors influencing neutropenia, such as age, baseline bone marrow function, and baseline laboratory data indicative of nutritional status. However, to our knowledge, this study is the first to reveal that severe neutropenia during S-1 adjuvant chemotherapy may affect pancreatic cancer prognosis. This information may prove valuable for the treatment of pancreatic cancer, a highly lethal disease with limited effective therapies.
Additionally, the study results suggest the importance of pharmacist management of medication adherence and adverse events with oral anticancer drugs such as S-1. Pharmacists should fully monitor patient conditions, whether in the outpatient or inpatient setting, and should actively comment on the continuation or discontinuation of oral anticancer drugs. Consequently, maximum therapeutic effect may be achieved even in a highly lethal malignancy such as pancreatic cancer.
In conclusion, initiating S-1 adjuvant chemotherapy early after pancreatic surgery may improve the pancreatic cancer prognosis. In addition, patients who developed grade 3 neutropenia have no other adverse events and are in good general condition, continuation of S-1 treatment may contribute to improving the prognosis. The findings of this study should be validated by future multi-center or prospective studies using larger numbers of cases.
This study was supported by the pharmacists of the Japanese Red Cross Kyoto Daini Hospital. We also thank the doctors of the Department of Surgery of the Japanese Red Cross Kyoto Daini Hospital for providing us with valuable cases.
Yuichi Muraki has received funding for commissioned research from Kowa Company, Ltd., and for a medical education Grant from Pfizer Japan, but this study is not directly related to that funding. Ryo Inose has received funding for commissioned research from Kowa Company, Ltd. but this study is not directly related to that funding. Yusuke Noguchi, Tatsuya Ohtsubo, Daisuke Kobayashi, Yoshitaka Kato, and Kanji Tomogane declare no conflict of interest.