2015 Volume 79 Issue 6 Pages 1145-1155
2015 Volume 79 Issue 6 Pages 1145-1155
Endothelial function is largely dictated by its ability to rapidly sense environmental cues and adapt to these stimuli through changes in vascular tone, inflammation/immune recruitment, and angiogenesis. When any one of these abilities is compromised, the endothelium becomes dysfunctional, which ultimately leads to disease. Reactive oxygen species (ROS) have been established at the forefront of endothelial dysfunction; however, more careful examination has demonstrated that ROS are fundamental to each of the sensing/signaling roles of the endothelium. The purpose of this review is to document endothelial ROS production in both disease and physiological adaptation. Through understanding new endothelial signaling paradigms, we will gain insight into more targeted therapeutic strategies for vascular diseases. (Circ J 2015; 79: 1145–1155)
The endothelium is a monolayer of cells that lines the lumen of blood vessels. Until the mid-1980 s this layer was thought to be largely passive and without functionality. However, Furchgott and Zawadzki made the seminal discovery that the endothelium was the key determinant of the response of intact blood vessels to acetylcholine. Without the endothelium, vessels contracted to acetylcholine, whereas an intact endothelial layer produced vasodilation, prompting speculation of an “endothelium-derived relaxing factor (EDRF)”.1 Subsequent investigation over the past 3 decades has demonstrated that the endothelium serves as an important regulator of most blood vessel functions that determine the vascular state in both health and disease.
As endothelial cells form a direct interface between the blood and the rest of the vessel wall, they have a fundamental role in vascular homeostasis and vascular adaptation to environmental changes. As one might expect, diverse phenomena such as blood flow regulation, vascular permeability, inflammatory cell recruitment, thrombosis, and angiogenesis are all under the control of the endothelium. In disease states, dysfunction of the endothelium has lead to a variety of phenotypes, such as spontaneous hemorrhage,2 vasospasm3 and hypertension.4 In patients, endothelial dysfunction has been linked to the precipitation of clinical atherosclerotic events.5 Thus, impairment of endothelial function has important consequences for vascular homeostasis.
Endothelial cells dictate vascular phenotype through the release of a number of small molecule species that act in both a paracrine and autocrine fashion. Many have been discussed at length, including nitric oxide (NO•), prostacyclin, heparins, and membrane-bound molecules such as ephrins.6 In this review, we will focus on the vascular production and consequences of reactive oxygen species (ROS) generation. Classically, ROS have been thought of as harmful byproducts of oxidative metabolism. However, over the past 2 decades, these species have been found to be specifically responsible for transducing signals to a wide variety of environmental cues. In this review, we will focus specifically on endothelial ROS production with an emphasis on NADPH oxidase-derived ROS and how they dictate the phenotype of the vascular endothelium in terms of both disease and reparative conditions.
Among the first demonstrations that the endothelium exerted control over the vasculature was the discovery of EDRF by Furchgott and Zawakski,1 as mentioned above. Ultimately Ignarro et al discovered that the EDRF was NO•.7,8 The production of NO• involves cleavage of the terminal guanidino nitrogen of L-arginine by nitric oxide synthases (NOS) to form NO• and L-citrulline. Endothelium-derived NO• is produced by the endothelial isoform of NOS (eNOS) in response to various stimuli such as increased shear stress or activation of G-protein coupled receptors by thrombin or acetylcholine, among others. The produced NO• diffuses out of the endothelial cells and interacts with the heme moiety of soluble guanylate cyclase to generate the second messenger, cyclic GMP (cGMP), from guanosine triphosphate (GTP). The soluble cGMP then activates cyclic nucleotide-dependent protein kinase G (PKG or cGKI), which then phosphorylates multiple proteins, ultimately leading to alterations in actin filament and myosin dynamics, resulting in smooth muscle relaxation.9 Similar mechanisms have been implicated in the ability of NO• to affect platelet aggregation and immune cell trafficking in the vasculature.
eNOS is regulated at multiple levels, including gene transcription, protein modification, and degradation.10 Among the more important regulators of eNOS NO• production and NO• bioactivity are ROS. For example, eNOS catalytic activity requires the cofactor tetrahydrobiopterin (BH4), which is readily oxidized to dihydrobiopterin (BH2). Although BH2 may still support eNOS enzymatic activity, it does so in an “uncoupled” manner whereby NADPH oxidation can result in superoxide (O2•−) production rather than NO•.11 This characteristic of eNOS is quite important when considering the bioactivity of NO•. Superoxide reacts with NO• in a diffusion-limited reaction to produce peroxynitrite (ONOO−), which has considerably less vascular homeostatic activity than authentic NO•. Thus, ONOO− production in the vasculature tends to be associated with disease states and vascular dysfunction (discussed later). It is important to note, however, that not all ROS have similar implications for NO• bioactivity. For example, hydrogen peroxide (H2O2), a non-radical species, has been shown to increase both eNOS expression and activity,12,13 thereby promoting NO• bioavailability.14,15 In this way the consequences of ROS for the vasculature are contextual, suggesting some knowledge of specific ROS is important to understand how ROS and NO• bioactivity interact.
As oxygen-breathing organisms, human cellular systems have adapted to utilize the benefits of this electronegative molecule to transfer electrons, resulting in highly reactive intermediates or ROS. Figure 1 shows the serial reduction of molecular oxygen to water (H2O) whereby the single-electron reduction of oxygen (a feature of many enzymatic systems and the mitochondria) yields O2•−, which is short-lived and rapidly dismutates to H2O2 either spontaneously or by superoxide dismutase (SOD). Indeed, the production of O2•− appears to be ubiquitous, as SOD is found in virtually every tissue in vivo16 and animals lacking specific SOD isoforms (eg, mitochondrial SOD) are not viable.17 Catalases and peroxidases then catalyze the final step in this sequence leading to production of H2O.
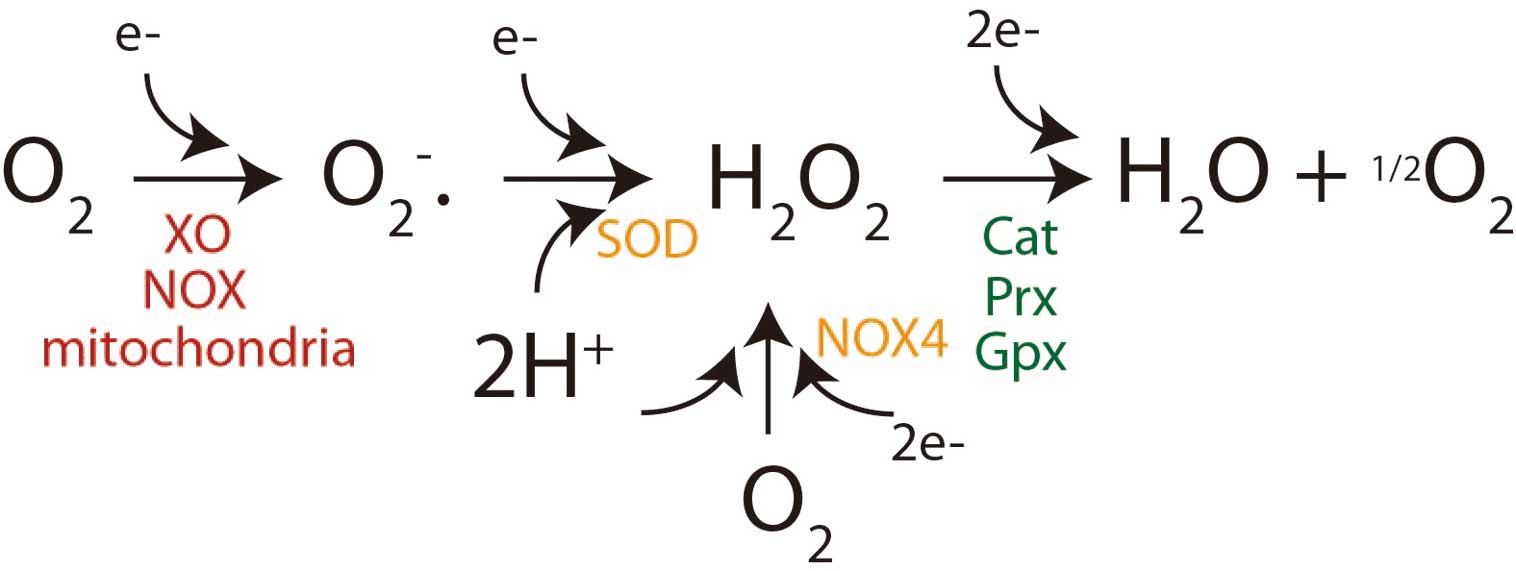
Reduction of oxygen. A single-electron transfer (by Nox, NADPH oxidase; XO, xanthine oxidase or mitochondria) converts molecular oxygen to the superoxide anion (O2•−), which rapidly dismutates to hydrogen peroxide (H2O2) either spontaneously or through superoxide dismutase (SOD). Recent data also demonstrate NADPH oxidase 4 (Nox4) can directly form H2O2 as its end product. Finally, H2O2 is converted to water and oxygen by catalase (Cat), peroxiredoxin (Prx) and glutathione peroxidase (Gpx).
The notion that ROS production has implications for organ function is not new. It was noted more than 60 years ago that antioxidant supplementation of model organisms improved maximum lifespan. From this observation Denman Harman developed the “oxidative theory of aging”, which suggests ROS-mediated damage to macromolecules (eg, DNA, RNA, protein) over one’s lifetime leads to the manifestations of aging.18 Many other conditions are known to involve oxidative stress, broadly defined as an excess of oxidants over the species required to detoxify them.19 These earlier concepts concerning ROS biology typically focused on their potential for damaging cellular components.
Because oxygen is a ubiquitous component of higher order biologic systems, it is not surprising that ROS can be generated by diverse cellular processes such as mitochondrial electron transfer, drug detoxification by cytochrome p450s, uncoupling of eNOS, and an array of oxidase enzymes such as xanthine oxidase and NADPH oxidases, among others. The most facile ROS produced in biological systems is O2•−, as it requires only a single-electron reduction of molecular oxygen (Figure 1). The most common fates for O2•− (based upon rate constants) are dismutation to H2O2 or its reaction with NO• to form ONOO−. In specific circumstances that involve accessible redox-active metal ions (which are rare in vivo), O2•− can also undergo sequential reduction.
In addition to their role in mediating cellular damage (typically at high concentrations), low level ROS production has been implicated in cellular signaling.20 In this context it is important to consider the specific properties of distinct ROS that may dictate the types of signaling to which they are best suited. Superoxide has been implicated in signaling, but it is a charged species that cannot cross membranes, suggesting it is best suited for very local signal propagation. Moreover, O2•− is subject to both spontaneous (rate constant=8×104 M−1s−1), and enzymatic (rate constant=2×109 M−1s−1) dismutation to H2O2. Given that most cells and cellular compartments have abundantly available SOD, one must consider that O2•− is likely to have a very short half-life in biological systems. Conversely, H2O2 is a relatively stable 2-electron oxidant that is able to cross membranes. It is ideal for regulating signaling cascades because it can react with protein thiol moieties to produce post-translational modifications that may modulate protein function in an autocrine and/or paracrine manner.21,22 Although H2O2 is thought to be largely formed through the general dismutation of O2•−, recent data has demonstrated that a specific NADPH oxidase member, Nox4, may directly produce H2O2.23,24 In the endothelium there are multiple reactions that produce ROS, but this review will focus primarily on NADPH oxidase production of ROS.
The NADPH oxidases (Nox) are a family of multicomponent transmembrane proteins that transfer electrons across membranes to O2 as the principal electron acceptor, thereby forming ROS. The Nox proteins incorporate 7 family members (Nox1–5, Duox1/2) and multiple accessory proteins (p22phox, p47phox, etc.; for review see Bedard et al25). Nox1–4 contain 6 transmembrane domains and have an NADPH and FAD-binding domain at the cytoplasmic C-terminus. In addition to this basic structure, Nox5 has an additional N-terminal calmodulin-like Ca2+ binding domain on an additional transmembrane segment. There are 2 related oxidases, Duox1 and 2, that contain a longer N-terminal extension with a peroxidase homology domain and an additional transmembrane segment.
The originally described or prototypical Nox family member is Nox2, which requires the cytosolic proteins p47phox, p67phox, and p40phox, as well as the membrane-associated p22phox, and gp91phox (now named Nox2) for activity. The activation of Nox1 and 3 is similar to this paradigm, involving a core catalytic subunit that is activated upon the membrane recruitment of a number of regulatory subunits. In contrast, Nox4 only requires the membrane protein p22phox and its activity seems to be constitutive and regulated by expression. Nox5 is Ca2+ sensitive, but does not require recruitment of other accessory proteins for activation. Nox catalytic activity requires the binding of NADPH to the C-terminus followed by the transfer of electrons through FAD to the 2-heme residues that ultimately transfer the electrons to O2.
Nox1, 2, 4, and 5 are expressed in vascular tissue with varied expression levels between cell types. In blood vessels Nox4 is the most abundant Nox transcript and for endothelial cells specifically, Nox4 mRNA is much higher (10–1,000-fold increased copy number) than that of Nox1 or 2.26–28 Proportionally, smooth muscle cells express the least amount of Nox4, with higher levels observed in endothelial cells and fibroblasts.15,26,28 Much information now highlights that, collectively, vascular Nox-produced ROS affect vascular phenotype.
The endothelium, in large part, controls vascular homeostasis, including the vascular tone of both conduit and resistance vessels. The ability of the endothelium to influence vascular tone is fundamental to the regulation of blood flow in response to many physiologic conditions. For example, muscular exercise produces an acute requirement for increased delivery of oxygen and nutrients that is largely achieved via dilation of resistance vessels to increase blood flow. Sustained increases in blood flow also increase the shear forces on the endothelium, thus prompting the release of vasodilators that result in conduit vessel dilation and normalization of endothelial shear. Conversely, tissue injury can prompt vasoconstriction as a means of stemming blood loss, a response that is, in part, due to the endothelium. These changes in vascular tone occur through the paracrine action of endothelial-derived vasorelaxants (eg, NO•, prostacyclin, and endothelial-derived hyperpolarizing factor [EDHF]) or vasoconstrictors (eg, leukotrienes and endothelin-1).29 In most cases, vascular production of ROS has proven important in the regulation of vascular tone (for reviews see Chatterjee et al,30 Brandes et al31) (Figure 2).
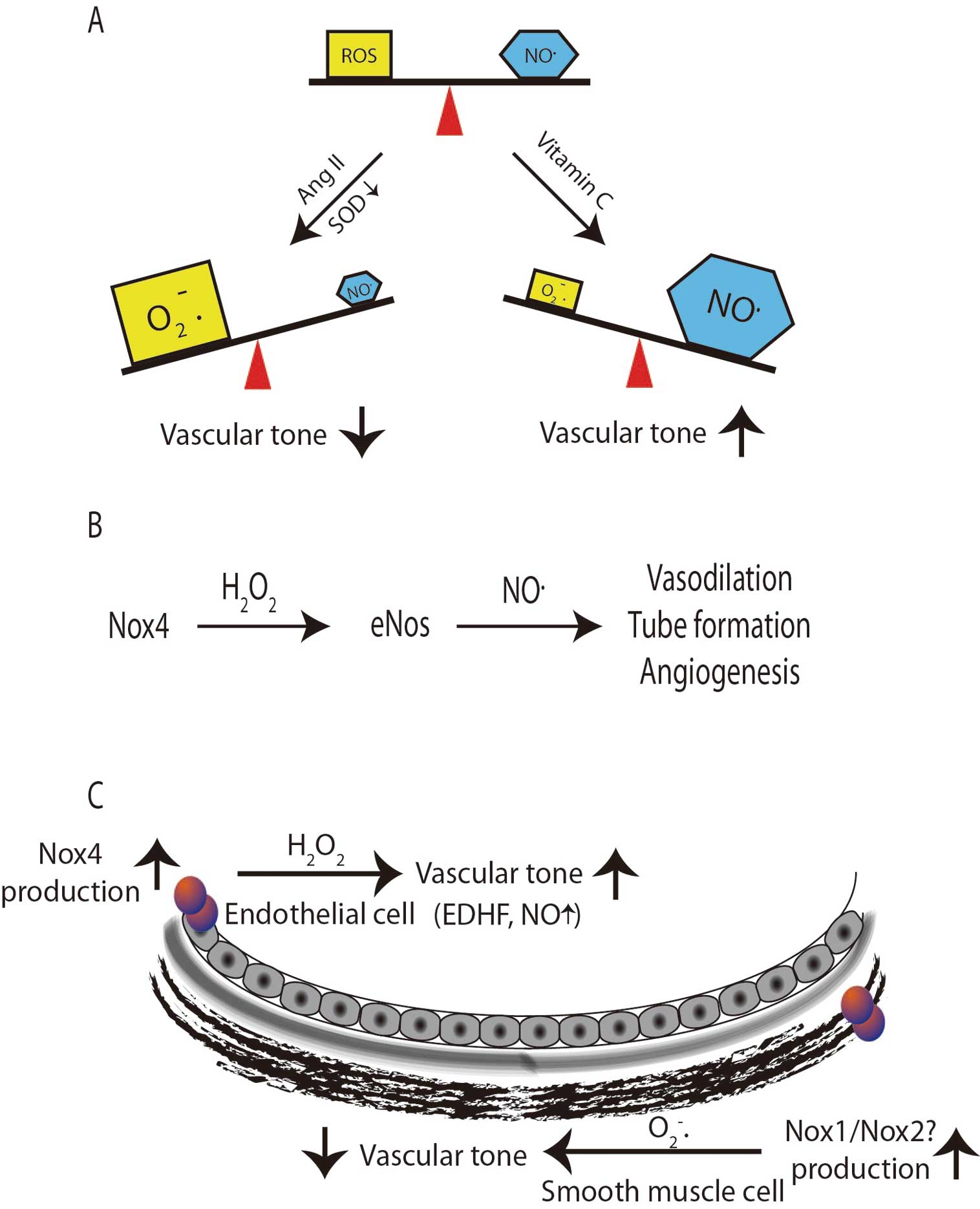
Schematic of nitric oxide (NO•) bioavailability. (A) NO• rapidly reacts with superoxide (O2•−) which decreases bioavailable NO•, leading to decreased vascular tone, whereas reducing O2•− improves vascular tone. (B) NO• bioavailability increases with an increase in Nox4 activity, because it can activate endothelial nitric oxide synthase (eNOS), which in turn produces NO•. (C) The site and species production of reactive oxygen species (ROS) lead to differing effects on vascular tone. AngII, angiotensin II; EDHF, endothelial-derived hyperpolarizing factor; SOD, superoxide dismutase.
The effect of ROS on the control of vascular tone has been examined extensively in both human subjects and model animal systems. Human studies have largely focused on small molecules that affect ROS such as vitamin C. The studies used flow-mediated dilation as an index of endothelial NO• regulation of vascular tone, and targeting ROS with vitamin C improved vascular function in a number of conditions known to be associated with excess oxidative stress (eg, type II diabetes and coronary artery disease).32–34 These data, and others, were interpreted to indicate that O2•− production in disease states modifies vascular tone. More direct support for this idea came from animal studies in which SOD was inactivated and resulted in decreased vessel relaxation35 and endothelial dysfunction (Figure 2A).
Another consequence of vascular tone is the regulation of total peripheral resistance, which partially dictates blood pressure. Impaired control of resistance vessel function has been linked to hypertension and there is ample evidence that ROS play a role in this disorder. Understanding the potential mechanism(s) of hypertension is clearly important because it is present in up to 30% of the US population and affected individuals are at increased risk for stroke, aneurysm, coronary artery disease, peripheral artery disease and chronic kidney disease. Of interest, a role for ROS in hypertension was first introduced in the 1960 s36 and since this first demonstration, multiple cell, animal, and clinical studies have demonstrated that hypertension is associated with an increase in oxidative stress in part via NADPH oxidases. Studies in humans indicate that acute administration of vitamin C results in blood pressure reduction,37 suggesting an important role for ROS and endothelial dysfunction in this context. In model systems of hypertension, a strong link between blood pressure regulation and NADPH oxidase activity has been established. For example, angiotensin II (AngII) infusion produces hypertension and this process is linked to increased Nox-derived O2•− production throughout the vascular wall in rats, leading to endothelial dysfunction.38 The infusion of AngII has been shown to increase the expression of multiple Nox proteins including Nox1, Nox2, Nox4, and p22phox.38 Over the ensuing decades, subsequent studies in humans and animal models of hypertension have been consistent in providing a link between Nox and hypertension.39
This link between NADPH oxidases and hypertension has been studied via genetic manipulation of Nox isoform expression and these studies have demonstrated that maladaptive effects of excess Nox activity are likely related to specific tissues and specific Nox family members. For example, Nox1 overexpression in vascular smooth muscle cells leads to an increase in hypertension and endothelial dysfunction in response to AngII.40,41 Conversely, deletion of Nox1 appears to prevent AngII-induced hypertension and also limits the development of endothelial dysfunction and oxidative stress.42–44 Infusion of AngII into Nox2-deficient animals had a minor effect on vascular hypertrophy, but very little effect on the development of hypertension.45,46 Examination of mice lacking Nox4 revealed them to be normotensive.
Most of the studies were focused on the Nox isoforms that generate O2•−, which is known to impair responses that are dependent on NO• bioactivity. Perhaps focusing on specific reactive species could yield additional insight into how ROS affect vascular tone. In particular, H2O2 is distinct from O2•− by not directly interacting with NO•. Moreover, it is known to stimulate guanylyl cyclase directly and induce vasodilation.47,48 These previous observations suggest that H2O2 can act as a vasodilator compound and have led many to conclude it is an EDHF as well.49 This has been substantiated in animal and in vitro models that involve the overexpression of Nox4 (considered a H2O2 generator) in the endothelium. In those studies, excess peroxide production was associated with an enhanced endothelial response to acetylcholine and a reduction in basal blood pressure that was normalized by supplementation with antioxidants.50 In light of these observations, coupled with the in vitro data demonstrating that H2O2 enhances eNOS expression and NO• bioactivity,12,13 it is likely that H2O2 acts not only as an EDHF, but may also promote bioavailable NO• in vivo. This notion is supported by studies in which Nox4 overexpression increased bioavailable NO•, whereas AngII infusion in animals lacking Nox4 resulted in decreased eNOS expression and NO• production14,15 (Figure 2B). This further emphasizes that not only the location of ROS production, but also the precise species formed, is contextually important. Thus, we might consider that O2•− produced within the smooth muscle cells leads to a hypertrophic, hypertensive phenotype, but H2O2 formed and excreted from endothelial cells enhances vascular tone (Figure 2C).
Inflammation/Immune FunctionIn addition to vascular tone, endothelial cells are key regulators of the local inflammatory and immune responses in both the vasculature and the surrounding tissues. Over the past 3 decades, atherosclerosis research has highlighted a deleterious role for ROS in the pathogenesis of the disease, which serves as a general model of inflammation. A paradigm generally agreed upon in the literature is depicted in Figure 3A, which begins with an initial endothelial activation that can be precipitated by consuming an atherogenic diet for as little as a few days.51–54 In this model, endothelial activation is manifest, given the increased expression of leukocyte adhesion molecules and inflammatory cytokines that are responsible for the rolling, firm attachment, and diapedesis of inflammatory cells into the vascular wall. This is followed by the promotion of lipoprotein deposits in the subintimal space, retention of inflammatory cells (including monocytes), and ultimately foam cell formation. Continued cycles of lipid deposition, inflammatory cell infiltration and activation, and foam cell formation, lead to progressive formation of more complex atherosclerotic plaques that often involve a necrotic core covered by a thin fibrous cap separating the lesion from the circulation. Eventually, continued inflammation can weaken the fibrous cap, leading to its rupture and the formation of an occlusive thrombus that can precipitate acute events such as myocardial infarction, ventricular fibrillation, or stroke.
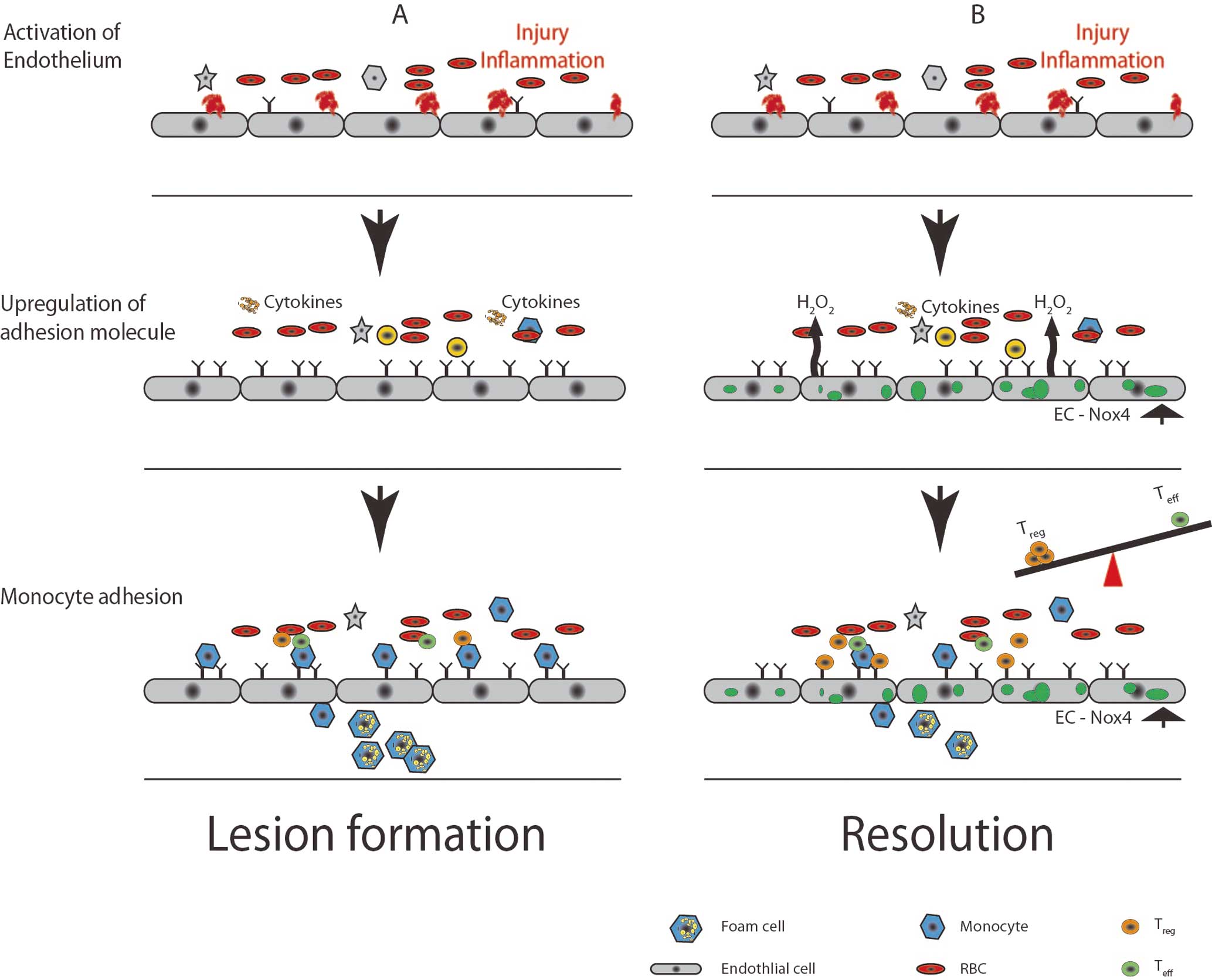
Oxidative modification and inflammation during atherosclerosis. Vascular endothelial cells (EC) sense injury and inflammation and respond through activation and expression of leukocyte adhesion molecules. Monocytes become tethered to the endothelium and then activated monocytes (macrophages) extravasate and accumulate lipids, finally forming foam cells (A). This process can be altered by local T-cell populations where the balance between Teffector (Teff) and Tregulatory (Treg) cells can lead to disease promotion or resolution (B). RBC, red blood cell.
Multiple studies have documented increased Nox in the vasculature with atherosclerosis.28,55–59 It follows that atherosclerotic lesions in coronary arteries contain abundant p22phox and Nox2, which correlates with the severity of atherosclerosis.28,60 Similarly, vascular tissue from bypass patients with diabetes (a risk factor for atherosclerosis) demonstrated increased expression of p22phox, p47phox, and p67phox (all Nox binding partners) compared with nondiabetic subjects.61,62 In primates, the onset and progression of atherosclerosis is associated with increased Nox expression, whereas atherosclerosis regression is associated with a decrease in vascular Nox expression.59 Moreover, statins, which are known to decrease Nox expression, also decrease atherosclerosis,63–66 suggesting that Nox proteins may be involved in disease onset and progression.
ROS are important in endothelial activation, a phenomenon that is characterized by upregulation of adhesion molecules, increased vascular permeability, and reduced bioavailable NO•.67,68 Endothelial treatment with the cytokine tumor necrosis factor α (TNFα) produces increased adhesion molecules (ICAM1 and VCAM1) and chemokine (MCP1) expression via activation of Nox2.69–71 Endothelial lipopolysaccharide (LPS) exposure induces increases in adhesion molecules through Nox4 activation of TLR4, which leads to increased monocyte adhesion and transmigration.72 As mentioned before, AngII, a potent stimulator of Nox-derived ROS and AngII, also increases the expression of adhesion molecules on the endothelium through ROS production.73,74 Furthermore, aldosterone may involve Nox4 activation by the mineral corticoid receptor in its impairment of endothelial function.75 Oscillatory shear stress leads to endothelial activation, increased adhesion molecules, and matrix metalloproteinase (MMP) secretion through Nox.76 Bone morphogenic proteins (BMPs) also activates endothelial cells through ROS-dependent mechanisms,77 although the precise pathways remain unclear.
Although there is an abundance of data implicating Nox in the pathogenesis of atherosclerosis, data demonstrating a clear causal role for NADPH oxidases in atherosclerosis has proven difficult because studies in genetic mouse models have produced inconsistent results. For example, mice lacking Nox2 did not exhibit a significant difference in atherosclerotic lesion formation when looking exclusively at the aortic sinus.78 However, a more recent study examining the length of the aorta from the arch to the iliac bifurcation demonstrated that Nox2 expression precedes lesion development and that deletion of Nox2 protects from lesion deposits.56 In mice lacking the Nox accessory protein, p47phox, one study documented no difference in aortic root atherosclerosis,79 but another study demonstrated a significant protection in the descending aorta.80 The data for Nox1 are a little more consistent; Nox1-deficient mice mice crossed onto an ApoE−/− model demonstrated decreased lesion formation57 and administration of the Nox1/4 inhibitor (GKT137831) in diabetic ApoE−/− mice produced decreased lesion formation compared with controls, which was caused by Nox1 inhibition. Together these data indicate that more comprehensive studies are needed regarding the effect of Nox on location-specific promotion and progression of atherosclerosis.
More recent atherosclerosis research has focused on the later stages of the disease, which can involve not only the promotion of inflammation, but also its resolution. Among the regulators of inflammation that have garnered attention are regulatory T cells (Tregs). Tregs are an important subset of T cells that lend themselves to immune tolerance, and data indicate that Tregs are relevant for atherosclerosis. For example, Tregs are important in several processes: they inhibit pro-atherogenic T cells (Th1 and Th17; T effector) via cell-cell contact and cytokine secretion (IL10, transforming growth factor [TGF]β, IL35); they inhibit foam cell formation via downregulation of CD36; they suppress endothelial cell activation through cytokine secretion or reducing leukocyte adhesiveness; and they inhibit inflammatory macrophages by promoting polarization towards the M2 (ie, reparative) macrophage variety. Indeed, multiple studies have demonstrated an inverse correlation between FoxP3 (a main transcription factor in determining Treg fate) and atherosclerosis.81–86 There are recent data suggesting a link between ROS and Treg cells wherein commitment of the Treg lineage is dependent on localized production of ROS and that scavenging of ROS decreases the Tregs/Teffector balance. The majority of these studies have focused on intracellular T-cell ROS, but it has also been demonstrated that localized macrophage ROS production can determine T-cell fate, thus potentiating Treg cell production.87–90 As the endothelium is a major regulator of local inflammatory and immune responses, and predominantly secretes ROS into the extracellular space, one might speculate that endothelial-derived ROS could influence T-cell fate by potentiating Treg differentiation (Figure 3B). Definitive answers will require investigation with atherosclerosis models that involve manipulation of endothelial ROS sources.
AngiogenesisAnother major function of the endothelium is the initiation and promotion of angiogenesis, the process of new blood vessel formation. In the postnatal state, angiogenesis is largely in response to inadequate tissue blood flow resulting in localized tissue hypoxia. During ischemic conditions, decreased blood flow leads to decreased nutrient availability (eg, oxygen and glucose). These metabolic perturbations trigger the activation of HIF-1α and other transcription factors to coordinate the release of cytokines and growth factors such as vascular endothelial growth factor (VEGF), PDGF, angiopoietins, and TGFβ. These transcriptional changes are known to occur in multiple tissue types (eg, macrophages, fibroblasts, endothelial cells, keratinocytes).91–94 Both physiological angiogenesis (wound healing, vessel damage, ischemic repair) and pathological angiogenesis (tumors, diabetic retinopathy, age-related macular degeneration) involve the same initial signaling paradigms, all of which involve ROS.
Antioxidant-mediated ROS scavenging results in decreased angiogenesis in multiple models95–99 that could be considered in the setting of pathological angiogenesis. However, many studies have now demonstrated that physiological angiogenesis also requires ROS production. Utilizing a model of hindlimb ischemia for peripheral vascular repair, multiple groups have demonstrated that endothelial Nox-derived H2O2 production is an important component of angiogenesis and tissue repair.14,49,100,101 These data suggest that under certain circumstances, H2O2 is not a mediator of tissue injury, but rather an important component of the resolution of tissue damage.
Endothelial cells typically reside in tissues in a quiescent state and become activated in the setting of angiogenesis. One factor critically responsible for endothelial cell activation is VEGF and this process is, in part, ROS-dependent. For example, ROS upregulate VEGF expression via activation of HIF-1α.102–104 VEGF has multiple receptors, but binding to VEGF receptor 2 (VEGFR2) is of particular interest because it induces ROS production that appears critical for key angiogenesis processes such as basement membrane degradation, migration, proliferation, and tube formation (Figure 4).
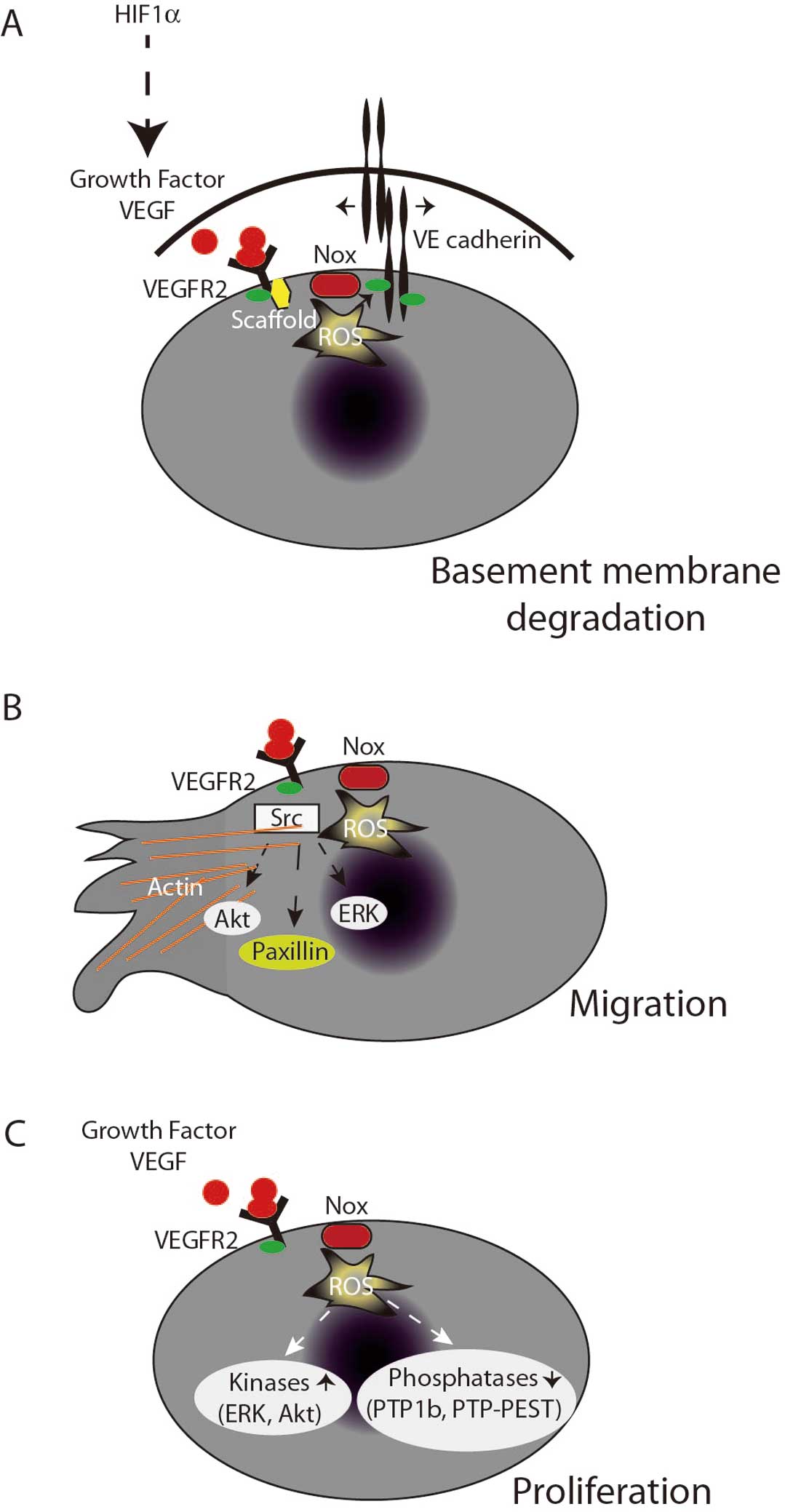
Endothelial angiogenesis. Under basal conditions, VE-cadherin creates tight junctions between endothelial cells. Vascular endothelial growth factor (VEGF) binding to receptor 2 (VEGFR2) induces reactive oxygen species (ROS) production. leading to VE-cadherin phosphorylation, thus dismantling the extracellular matrix (A). Activation of scaffold production of ROS at the leading edge of endothelial cells leads to localized phosphatase inhibition and kinase activation, ultimately resulting in actin remodeling and migration (B). ROS also promote Akt and ERK activation, leading to proliferation (C).
The process of endothelial cell migration and early tube formation requires initial detachment of the endothelial cell from its basement membrane followed by actin cytoskeletal rearrangements to facilitate movement, which also requires clearing out of extracellular matrix.105,106 These processes are tightly linked to signaling events in response to VEGF and angiopoietin-1.107–109 In quiescent endothelial cells, VE-cadherin dimers bind catenins anchoring the extracellular matrix to the actin cytoskeleton. Binding of VEGF to the VEGFR2 (flk-1/kdr) initiates binding of the scaffold IQGAP1 to actin, β-catenin, and Rac1, which then tightly associates with Ve-cadherin and the VEGFR.107,110,111 This process is critical for endothelial cell detachment and is characterized by localized ROS production that facilitates the tyrosine phosphorylation of VE-cadherin and β-catenin that is critical for disassembly of the VE-cadherin-catenin complex and junctional breakdown of the endothelial monolayer (Figure 4A).112
Endothelial cells must also reorganize the cytoskeleton to prepare for forward movement. Migrating endothelial cells create focal complexes transiently at their leading edge, which is critical for the cytoskeletal reorganization needed to produce filopodia or lamellipodia. In 2000, Moldovan et al describe discrete ROS formation at the leading edge of these membrane ruffles.113 Mechanistically, this occurs through IQGAP1 tethering Nox2 to actin at the leading edge110 where WAVE1 then recruits p47phox and binds Rac1, leading to membrane ruffle formation.114 This process also involves TRAF4, a protein known to directly bind p47phox,115 which directs oxidant production to the protein tyrosine phosphatase PTP-PEST and enhances focal complex phosphorylation,116 leading to directional migration. Thus, many of the steps involved in endothelial cell migration are ROS-dependent (Figure 4B).
During the process of angiogenesis, endothelial cells need to rapidly proliferate, and proliferating endothelial cells have increased ROS production as compared with quiescent cells.117 Moreover, Nox4-generated H2O2 is linked to the proliferative rate of endothelial cells.118 When exposed to growth factors (ie, serum), endothelial cell proliferation and activation downstream kinases (p38, ERK, and Akt) appear to be ROS-dependent and both Nox2 and Nox4 have been implicated in this process (Figure 4C).14,15,117,119,120
Finally, the migration and proliferation of endothelial cells ultimately results in tube formation, the earliest stage of new vessel formation. A recent observation indicates that autophagy, a process known to require ROS,121 is important in endothelial behavior relevant to tube formation. For example, ROS-driven autophagy results in endothelial survival in response to stressors such as caloric restriction.122 In addition, ROS-mediated autophagy is a key element in phenotypic responses of the endothelium critical for tube formation.123,124
Another important component of the tissue response to hypoxia is the dilation and recruitment of preformed capillaries to increase tissue blood flow. This process appears to be critical for the early responses to tissue ischemia and is enhanced by repeated metabolic stress such as exercise. Emerging data suggest that tissue ROS production is required for adaptation to exercise. For instance, exercise training increases blood flow, in part through increased expression of eNOS125 that can be recapitulated in vitro by H2O2. Exercise also increases tissue capillary density126–128 and tissue capillary density appears to be ROS-dependent,129,130 with at least one study specifically implicating Nox4.131
The role of ROS in pathologic and physiologic processes has undergone considerable evolution over the past 3 decades. Initial studies on ROS tended to focus on their role in tissue injury, inflammation and host defense. Under those circumstances, ROS were typically produced in relatively high concentrations for short periods of time. The interest in ROS-mediated tissue injury was instrumental in characterizing the variety of ROS produced in vivo and in identifying specific sources of ROS and their precise product(s). An important change in perspective was brought about by the discovery that certain growth factor responses were also dependent on ROS production, albeit at considerably lower levels than seen with inflammation. These observations have lead to the discovery of new sources (eg, NADPH oxidases) of ROS in tissues and better characterization of the molecular targets (ie, protein thiols) that mediate physiological responses. A pattern is now emerging that low level ROS production is an important feature of tissue adaptation to injury and stress (Figure 5), and further study will be needed to specifically understand how these reactive species generate specific responses. With regards to NADPH oxidases specifically, more studies will be needed with genetic models to clearly elucidate how this family of ROS- producing enzymes fits into the greater context of biological responses.
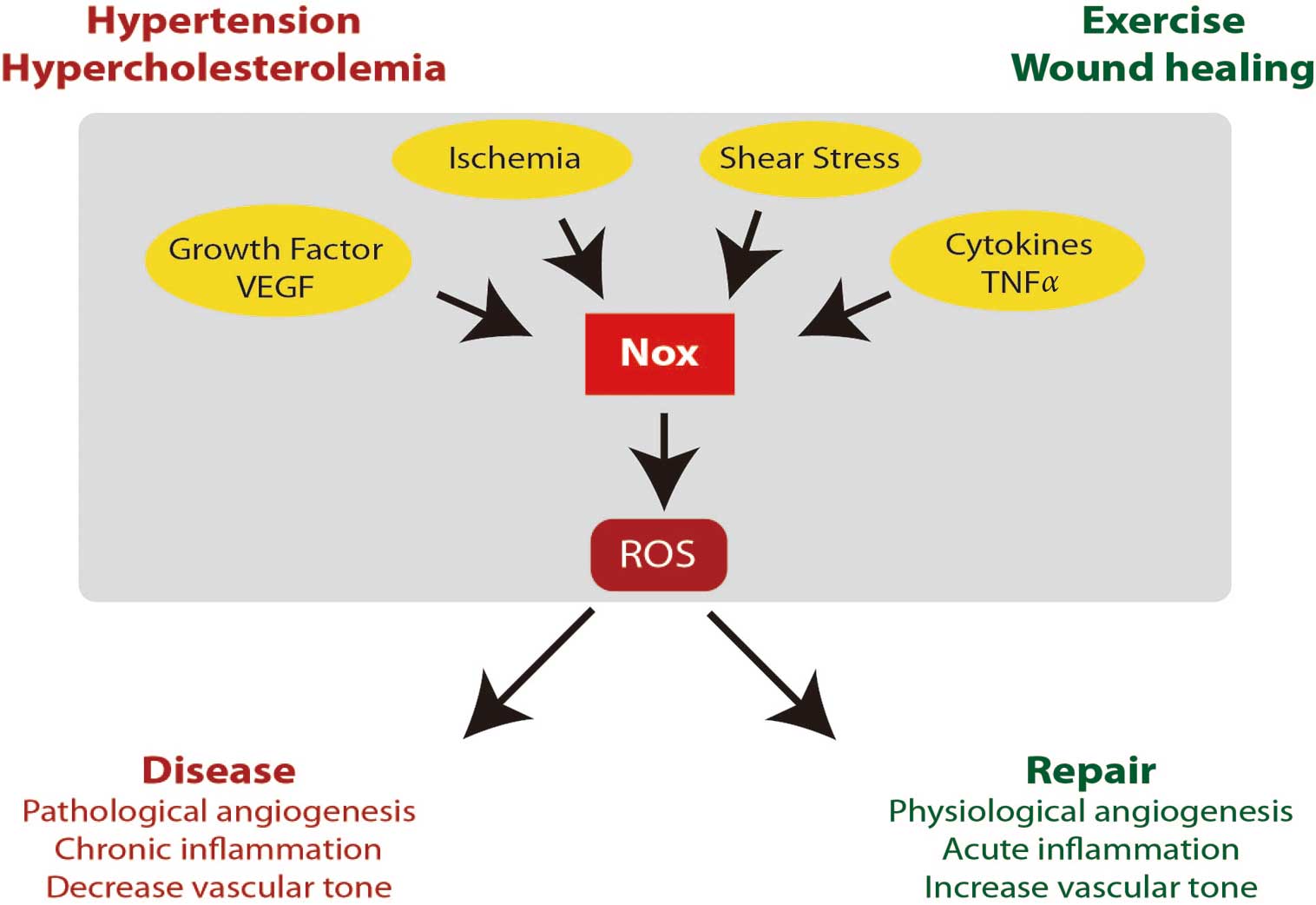
Nox pathway summary. A schematic representation of ROS-mediated effects shows the different activators and their biological outcomes. The Nox pathway can be activated by many stimuli, including ischemia, growth factors, inflammatory cytokines, and shear stress. Nox-produced ROS can activate different downstream targets, which can lead to either disease or repair. ROS, reactive oxygen species; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
The authors thank Michaella Reif for careful and thoughtful reading of this manuscript.
This work was supported by the National Heart Lung and Blood Institute grants to JFK (5R01HL092122-07; 2R01HL098407-05).