2016 Volume 80 Issue 3 Pages 696-702
2016 Volume 80 Issue 3 Pages 696-702
Background: In children with long QT syndrome (LQTS), risk factors for cardiac events have been reported, but age-, gender- and genotype-related differences in prognosis remain unknown in Asian countries.
Methods and Results: The study examined clinical prognosis at age between 1 and 20 years in 496 LQTS patients who were genotyped as either of LQT1–3 (male, n=206). Heterozygous mutations were observed in 3 major responsible genes: KCNQ1 in 271, KCNH2 in 192, and SCN5A in 33 patients. LQTS-associated events were classified into 3 categories: (1) syncope (n=133); (2) repetitive torsade de pointes (TdP, n=3); and (3) cardiopulmonary arrest (CPA, n=4). The risk of cardiac events was significantly lower in LQT1 girls than boys≤12 years (HR, 0.55), whereas LQT2 female patients ≥13 years had the higher risk of cardiac events than male patients (HR, 4.60). Patients in the repetitive TdP or CPA group included 1 LQT1 female patient, 1 LQT2 male patient, and 5 LQT2 female patients. All LQT2 patients in these groups had TdP repeatedly immediately after the antecedent event. In addition, all 5 female LQT2 patients in these groups had the event after or near puberty.
Conclusions: Female LQT2 children might have repeated TdP shortly after prior events, especially after puberty. (Circ J 2016; 80: 696–702)
Long QT syndrome (LQTS) is an inherited heart disease associated with increased propensity to syncope, torsade de pointes (TdP), and sudden arrhythmic death.1 It is an important cause of sudden cardiac death in children without structural heart disease.2 Syncope in LQTS is due to TdP, and death is usually due to ventricular fibrillation.3 Hundreds of mutations have been identified in >10 LQTS-susceptibility genes and approximately 75% of clinically diagnosed patients with LQTS are successfully genotyped. Most of them are found to carry a mutation in either one of 3 major genes: KCNQ1 (LQT1), KCNH2 (LQT2), or SCN5A (LQT3).4–6
Editorial p 598
Risk stratification depends on genotype, mutation type, and mutation location. Specifically, patients with cytoplasmic-loop (C-loop) missense KCNQ1 and S5-loop-S6 missense KCNH2 mutations have a greater risk for cardiac events.7–9 In neonates and infants, several previous studies have reported genetic background underlying fatal arrhythmias and indicated high prevalence of SCN5A and KCNH2 (S5-loop-S6 missense) mutations.10–13 Risk factors for cardiac events in children from birth to 20 years have been reported: LQT1 boy ≤15 years, LQT2 girl ≥16 years, corrected QT interval (QTc) prolongation >500 ms, and history of prior syncope.14–17 Age-, gender- and genotype-related differences in prognosis, however, remain unknown in Asian countries, although the presence of country-specific hot spots in LQTS mutations has been reported.18 In addition, differences in clinical course shortly after the prior event remain unknown. Therefore, the present study focused on patients with symptomatic LQTS who were identified as mutation carriers in any of the 3 major genes responsible for LQTS in children (from 1 to 20 years), and analyzed their distribution and severity of disease.
The study consisted of 496 LQTS patients (283 probands and 213 family members) who were genetically confirmed as either LQT1, LQT2, or LQT3. All but 1 Vietnamese boy were Japanese. They were referred to 2 institutes for genetic testing: Shiga University of Medical Science or Kyoto University Graduate School of Medicine between 1996 and December 2013. The study protocol was approved by the institutional review boards. Patients with compound or homozygous mutations were excluded from the study. We also excluded those with mutations in other LQTS-related genes. On enrollment, complete history was obtained retrospectively from birth to age of study entry. If someone was enrolled after reaching 20 years, we used only the clinical history from birth to 20 years. Patients who had received β-blockers before cardiac events were excluded during the analysis of the natural progress of the disease. No female patient was pregnant or in the postpartum phase when they had the first cardiac event. Thus, we assessed age- and genotype-related severity of LQTS children from 1 to 20 years.
LQTS-related events were classified into 3 categories: (1) syncope (transient and complete loss of consciousness); (2) repetitive TdP (documented within 24 h from the first syncope); and (3) cardiopulmonary arrest (CPA). The CPA group consisted of patients with aborted cardiac arrest requiring external defibrillation as part of resuscitation. In order to measure QTc (with Bazett correction19), 12-lead electrocardiograms (ECG) recorded at the enrolled age were used for asymptomatic patients. If asymptomatic patients were aged >20 years when enrolled, the first recorded ECG were used. For symptomatic patients, ECG recorded at onset were used. If there was no ECG available at onset, the ECG first recorded after onset was used. In patients with CPA, the ECG recorded before the cardiac event or ≥1 month after was used in order to avoid the influence of resuscitation.
Genetic AnalysisScreening for mutations was routinely performed for KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, and KCNJ2 using polymerase chain reaction (PCR) and denatured high-performance liquid chromatography (WAVE system; Transgenomic Omaha, NE, USA). For aberrant PCR products, subsequent direct DNA sequencing was conducted using a DNA sequencer (ABI 3130 DNA Sequencer, Perkin Elmer, Foster City, CA, USA). Genetic mutations were characterized by their location and coding effects on the amino acid sequence. We divided coding effects into 2 categories: (1) missense or (2) non-missense (putative splice sites, in-frame insertions/deletions, nonsense, and frameshift mutations). The C-loop region of KCNQ1 was defined as the coding sequence involving amino acid residues 171–195 (S2–S3) and 242–262 (S4–S5), and the S5-loop-S6 region of KCNH2 was defined as the amino acid region ranging from residue 552 to 657.
Statistical AnalysisStatistical analysis was performed using SPSS 22.0 (IBM, Armonk, NY, USA). Data are given as mean±SD for continuous variables and number (percentage) for categorical variables. Differences in characteristics were evaluated using unpaired Student’s t-test, Mann-Whitney U-test, or chi-squared test, as appropriate. Then, we analyzed patients with KCNQ1 and KCNH2 because of the limited number of the patients with SCN5A. We used the Kaplan-Meier method to estimate the distribution of time to first cardiac event before β-blocker therapy based on genotype-gender subsets (categorized as male with KCNQ1 mutation, female with KCNQ1 mutation, male with KCNH2 mutation, and female with KCNH2 mutation), using the log-rank test to compare differences between subsets. Cox proportional hazards models, allowing for separate baseline hazard function for each genotype, were used to assess the association of gender (female vs. male) with first cardiac event within each genotype and age group, adjusted for QTc.
The risk of cardiac events may be related to sex hormone factors.1 Puberty begins with the elevation of sex hormones, especially estrogen in girls. The mean age of puberty onset is 12.5±0.9 years in boys and 10.0±1.4 years in girls.20 In addition, menarche begins at the mean age of 12.4±1.0 years,21 after that time girls have higher estrogen to progesterone ratio around ovulation. Therefore, we conducted separate Cox models for 2 age groups: age 1–12 years and age 13–20 years. One year of age was used as the time origin in the analysis of mutation carriers age 1–12 years, whereas for the mutation carriers aged 13–20 years, 13 years of age was considered as the time origin. Differences were accepted as statistically significant for 2-tailed P<0.05.
Table 1 lists clinical characteristics according to genotype and gender. LQT1 or LQT2 patients had no gender-related difference in QTc in the 1–12-year age group (LQT1, P=0.73; LQT2, P=0.11). In contrast, in the ≥13-year age group, girls had significantly longer QTc than boys both in the LQT1 (male, 445±38 ms; female, 471±43 ms; P<0.001) and the LQT2 groups (male, 478±63 ms; female, 505±51 ms; P=0.002). Compared for age in gender-genotype, QTc was shorter in LQT1 male patients ≥13 years than in those ≤12 years (445±38 ms vs. 475±36 ms, P<0.001), and the QTc was longer in LQT2 girls ≥13 years than in those ≤12 years (505±51 ms vs. 474±35 ms, P<0.001), although there were no age-dependent differences in LQT1 girls (P=0.47) or LQT2 boys (P=0.23). LQT3 patients had no gender-related or age-dependent difference in QTc.
| KCNQ1 mutation carrier (n=271) | KCNH2 mutation carrier (n=192) | SCN5A mutation carrier (n=33) | ||||
|---|---|---|---|---|---|---|
| Male (n=104) |
Female (n=167) |
Male (n=81) |
Female (n=111) |
Male (n=21) |
Female (n=12) |
|
| Mean follow-up (years) | 10 (7–15) |
14 (9–20)** |
15 (10–20) |
16 (12–20) |
14 (12–20) |
13 (11–20) |
| ECG data (without β-blocker) | ||||||
| ECG recorded at ≤12 years | n=52 | n=47 | n=24 | n=24 | n=8 | n=4 |
| Age at ECG (years) | 8 (6.2–10.7) |
7 (7–10) |
9 (7.2–11) |
7.5 (6–9.7) |
11 (7.5–12) |
8.5 (6–11.7) |
| RR (ms) | 840 (730–979) |
821 (769–893) |
833 (713–985) |
783 (687–910) |
799 (777–951) |
751 (543–1,160) |
| QTc (ms) | 470 (452–493)††† |
475 (460–488) |
496 (454–533) |
472 (448–494) |
463 (416–499) |
438 (389–495) |
| QTc >500 ms | 12 (23) | 8 (17) | 11 (45) | 5 (20) | 2 (25) | 1 (25) |
| ECG recorded at ≥13 years | n=46 | n=103 | n=50 | n=77 | n=12 | n=7 |
| Age at ECG (years) | 30.5 (15–45) |
36 (17–42) |
29 (16–52.2) |
30 (17–40.5) |
32 (14–43) |
47 (16–58) |
| RR (ms) | 959 (880–1,057)†† |
967 (866–1,090)†† |
1,005 (905–1,152)*,†† |
921 (823–1,044)†† |
1,000 (843–1,091)† |
1,100 (846–1,170) |
| QTc (ms) | 448 (419–464) |
463 (444–484)*** |
476 (443–500) |
503 (469–526)**,††† |
422 (377–484) |
483 (427–495) |
| QTc >500 ms | 4 (8) | 18 (17) | 16 (28) | 45 (51)** | 2 (15) | 2 (25) |
| Cardiac events | ||||||
| Total | 30 (28) | 45 (26) | 16 (19) | 43 (38)** | 2 (9.5) | 4 (33) |
| Syncope | 30 (28) | 44 (26) | 15 (18) | 38 (34)* | 2 (9.5) | 4 (33) |
| Repetitive TdP | 0 | 0 | 0 | 3 (2) | 0 | 0 |
| CPA | 0 | 1 (0.5) | 1 (1) | 2 (1) | 0 | 0 |
Data given as median (IQR) or n (%). *P<0.05, **P<0.01, ***P<0.001 for comparison of male and female in the same genotype and age group. †P<0.05, ††P<0.01, †††P<0.001 for comparison of the groups ≤12 and ≥13 years in the same genotype and gender group. CPA, cardiopulmonary arrest; ECG, electrocardiogram; QTc, corrected QT interval; TdP, torsade de pointes.
In LQT2 patients, female subjects were more likely to have cardiac events between 1 and 20 years than male subjects (male, 16/81, 19%; female, 43/111, 38%; P=0.005), although there was no significant gender-related difference in the events rate in the LQT1 and LQT3 patients (LQT1, P=0.74; LQT3, P=0.16).
Age at first symptom onset and the respective number of genotyped patients are summarized in Table 2. A total of 140 patients (28%) had cardiac events between 1 and 20 years of age, and the mutation distribution was as follows: KCNQ1 mutations, n=75; KCNH2 mutations, n=59; and SCN5A mutations, n=6. LQT2 patients were more likely to have CPA or repetitive TdP than LQT1 patients (LQT1, 1/271, 0.3%; LQT2, 6/192, 3%; P=0.022). There was no significant difference in QTc between the CPA (515±55 ms), repetitive TdP (554±84 ms), and syncope (491±54 ms) groups (P=0.22), and the QTc in syncope group patients was longer than in asymptomatic patients (470±45 ms, P<0.001; Table 3).
| Total | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Onset age (years), median (IQR) |
n (%) | Onset age (years), median (IQR) |
n (%) | Onset age (years), median (IQR) |
||||
| Symptomatic/ Total |
CPA or repetitive TdP |
Symptomatic/ Total |
CPA or repetitive TdP |
Symptomatic/ Total |
CPA or repetitive TdP |
||||
| KCNQ1 | 75/271 (27) | 1 (0.3) | 10 (7–11) | 30/104 (28) | 0 (0) | 7 (6–10) | 45/167 (26) | 1 (0.5) | 10 (8–13)** |
| Missense | |||||||||
| C-loop | 11/41 (26) | 9 (7–13) | 6/15 (40) | 7 (5.7–9.7) | 5/26 (19) | 11 (9.5–14) | |||
| Non-C-loop | 44/169 (26) | 9 (7–11) | 16/62 (25) | 7 (6–10) | 28/107 (26) | 10 (7.2–11.7) | |||
| Non-missense | 20/61 (32) | 10 (7–14.5) | 8/27 (29) | 10 (5.2–10.7) | 12/34 (35) | 13 (7.7–16) | |||
| KCNH2 | 59/192 (30) | 6 (3)† | 13 (9–15)††† | 16/81 (19) | 1 (1.2) | 10 (7.2–12.7)† | 43/111 (38)** | 5 (4.5)† | 14 (12–16)**,†† |
| Missense | |||||||||
| S5-loop-S6 | 19 /46 (41) | 12 (8–15) | 6/21 (28) | 10.5 (6.7–15.5) | 13/25 (52) | 13 (9–15) | |||
| Non-S5-loop-S6 | 17/67 (25) | 13 (11–16) | 4/26 (15) | 10.5 (6.2–12.5) | 13 /41 (31) | 14 (12–17) | |||
| Non-missense | 23/79 (29) | 14 (8.5–16) | 6/34 (17) | 9 (6.5–11.5) | 17/45 (37) | 15 (13–18)** | |||
| SCN5A | 6/33 (18) | 0 (0) | 14 (9.7–16.2) | 2/21 (9) | 0 (0) | 16, 17 | 4/12 (33) | 0 (0) | 11.5 (7.2–15) |
| Missense | 5/25 (20) | 12 (8.5–16.5) | 1/15 (6) | 17 | 4/10 (40) | 11.5 (7.2–15) | |||
| Non-missense | 1/8 (12) | 16 | 1/6 (16) | 16 | 0/2 (0) | – | |||
| Total | 140/496 (28) | 7 (1.4) | 10.5 (7–14) | 48/206 (23) | 1 (0.4) | 9 (6–11) | 92/290 (31)* | 6 (2) | 12 (9–15)** |
*P<0.05, **P<0.01, ***P<0.001 for the comparison of male and female in the same genotype. †P<0.05, ††P<0.01, †††P<0.001 for the comparison of KCNQ1 and KCNH2 mutation carrier. C-loop, cytoplasmic loop. Other abbreviations as in Table 1.
| CPA (n=4) |
TdP (n=3) |
Syncope (n=102) |
Asymptomatic (n=345) |
|
|---|---|---|---|---|
| ECG data (without β-blocker) | ||||
| RR (ms) | 690, 869, 967, 1,304 | 882, 1,132, 1,176 | 920 (800–1,037) | 920 (800–1,024) |
| QTc (ms) | 464, 472, 557, 566 | 473, 549, 640* | 480 (461–518)*** | 466 (446–493) |
| QTc >500 ms | 2 (50) | 2 (66) | 39 (38)* | 74 (21) |
| Onset age (years) | 8, 12, 13, 15 | 9, 14, 15 | 11.0±4.6 | – |
Data given as n (%), mean±SD or median (IQR). *P<0.05, **P<0.01, ***P<0.001 compared with the asymptomatic group. Abbreviations as in Table 1.
All mutations were heterozygous and were classified into missense or non-missense. With regard to KCNQ1 mutations, there were 11 missense in the C-loop, 57 missense in the non-C-loop, and 7 non-missense. Among KCNH2 mutations, there were 19 missense in the S5-loop-S6, 17 missense in the non-S5-loop-S6, and 23 non-missense. There were 5 missense and 1 non-missense mutations in SCN5A (Table 2).
Genotype and Age at First Cardiac EventAs shown in Table 2, mean onset age in the KCNH2 group was significantly higher (12.5±4.7 years) than in the KCNQ1 group (9.7±3.8 years, P<0.001), and this propensity was similar in both boys (KCNQ1, 8.1±3.3 years; KCNH2, 9.8±3.9 years; P=0.028) and girls (KCNQ1, 10.7±3.8 years; KCNH2, 13.2±4.7 years; P=0.002). Boys became symptomatic at a significantly younger age than girls in the KCNQ1 (P=0.003) and KCNH2 (P=0.005) groups. There were no significant differences in mean patient age according to type and location of mutation in KCNQ1 and KCNH2, respectively. Furthermore, the same pattern was observed in both boys and girls.
Age at onset was not significantly different between the CPA (12.0±3.0 years), repetitive TdP (12.7±3.3 years), and syncope (11.0±4.6 years) groups (P=0.73; Table 3).
When comparing by genotype, the cumulative probability of a first cardiac event by age 20 years was 27% in LQT1, 30% in LQT2 and 18% in LQT3 (Figure 1).

Kaplan-Meier estimates of the cumulative probability of a first cardiac event by age 20 years according to genotype (data in parentheses are event rates).
Given that the number of KCNQ1 and of KCNH2 mutation carriers was relatively large, we examined the gender difference in these 2 groups (Figure 2). Figure 2A shows cumulative probability of a first cardiac event in patients with KCNQ1 mutation. On Cox regression analysis, LQT1 girls ≤12 years had significantly lower risk for cardiac events than boys (HR, 0.55; 95% CI: 0.33–0.93; P=0.024), whereas there was no significant gender difference in risk in LQT1 patients ≥13 years (HR, 1.65; 95% CI: 0.36–7.54; P=0.53; Table 4).
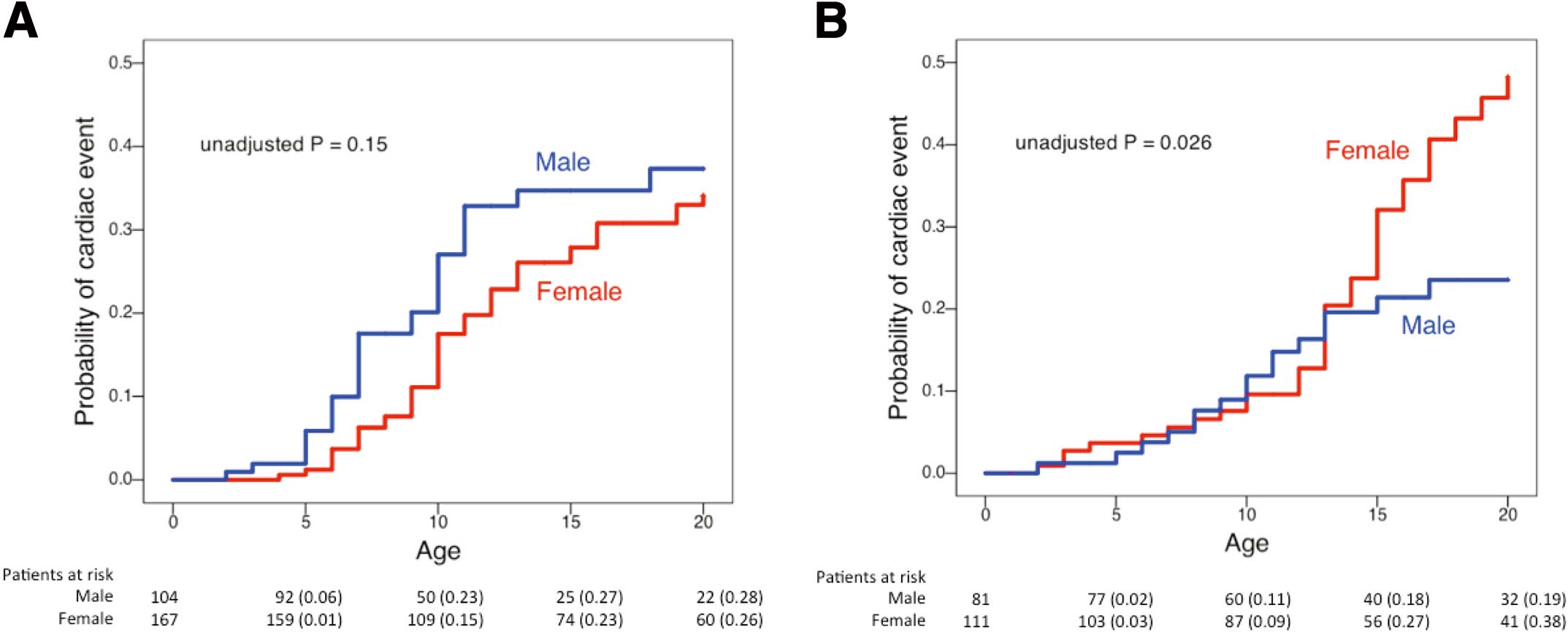
Kaplan-Meier estimates of the cumulative probability of a first cardiac event by age 20 years according to gender in (A) LQT1 (KCNQ1) and (B) LQT2 (KCNH2) mutation carriers (data in parentheses are event rates).
| LQTS mutation carriers age 1–12 years | LQTS mutation carriers age 13–20 years without prior events |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Female vs. Male | ||||||
| KCNQ1 | 0.55 | 0.33–0.93 | 0.024 | 1.65 | 0.36–7.54 | 0.53 |
| KCNH2 | 0.71 | 0.32–1.55 | 0.39 | 4.60 | 1.62–13.07 | 0.004 |
| QTc (10-ms increase) | 1.002 | 0.998–1.007 | 0.24 | 1.012 | 1.008–1.016 | <0.001 |
The Cox analysis involved 86 first cardiac events from 1 to 12 years in 463 KCNQ1 or KCNH2 mutation carriers, and 48 first cardiac events from 13 to 20 years in 263 KCNQ1 or KCNH2 mutation carriers without cardiac events before age 13. LQTS, long QT syndrome; QTc, corrected QT interval.
Figure 2B shows cumulative probability of a first cardiac event in LQT2 patients. The Kaplan-Meier curve clearly shows an exponential increase in symptomatic female patients after puberty, resulting in a cross-over of the 2 curves. Furthermore, LQT2 girls ≥13 years had a significant increase in the risk compared with boys of the same age range (HR, 4.60; 95% CI: 1.62–13.07; P=0.004; Table 4), although there was no significant gender difference in those ≤12 years (HR, 0.71; 95% CI: 0.32–1.55; P=0.39).
CPA and Repetitive TdPTable 5 summarizes the genetic background in 7 children with CPA or repetitive TdP. Interestingly, their mutations were either in the channel-pore region or frameshift/nonsense in the C-terminus. There were 6 patients with LQT2 (5 girls) and only 1 with LQT1 (12-year-old girl). All these 6 female patients with severe cardiac events had onset after or near puberty. The only male patient was an 8-year-old boy with CPA (CPA case 2) who had hypokalemia (<3.0 mEq/L) due to severe diarrhea at the cardiac event.
| ID no. | Gender | Age at event (years) |
Gene/Mutation | RR (ms) | QTc (ms) | TdP† |
|---|---|---|---|---|---|---|
| CPA | ||||||
| 1 | F | 12 | KCNQ1 S277L | 967 | 464 | (−) |
| 2 | M | 8 | KCNH2 K897fs+49X | 690 | 566 | (+) |
| 3 | F | 13 | KCNH2 A561V | 869 | 472 | (+) |
| 4 | F | 15 | KCNH2 K638del | 1,304 | 557 | (+) |
| Repetitive TdP | ||||||
| 1 | F | 9 | KCNH2 S871fs+31X | 1,176 | 473 | (+) |
| 2 | F | 14 | KCNH2 F640del | 882 | 640 | (+) |
| 3 | F | 15 | KCNH2 L908fs+30X | 1,132 | 549 | (+) |
†Documented within 24 h from the first cardiac event. F, female; M, male. Other abbreviations as in Table 1.
In the present study, we found that the phenotype in LQT2 was more severe than in LQT1 (Table 2), although the overall event rates were similar (Figure 1). We also showed that female LQT2 patients had TdP repeatedly within a short term after prior events, especially after puberty. In a study on 1,520 patients with LQTS before the age of 40 years, documented TdP was reportedly more common among female than male patients, particularly among LQT2 mutation carriers.22 The previous study, however, did not mention whether the TdP occurred repeatedly immediately after the event or not in LQT2 patients. The present study first reported that repetitive TdP or fatal events were more common in LQT2. If a patient were suspected of LQT2 based on ECG findings (QT prolongation, notched, or bifid T waves), he/she would have repetitive events after the first TdP attack. In such a condition, genetic testing would be of clinical importance because the genotype (eg, mutations in the channel-pore region) appears to influence disease severity.
Female LQT2 patients have their first cardiac event at a higher rate after the onset of adolescence.17,23 Consistent with previous data, the present study noted a significantly prolonged QTc and increased risk of first cardiac event after puberty in LQT2 female patients, although there was no significant age-related difference in LQT2 male patients. Therefore, sex hormones may contribute to QT interval shortening or prolongation and the occurrence of cardiac events. In addition, hormonal state varies with the menstrual cycle in female patients after puberty.
In the menstrual cycle, longer QT interval is observed during the follicular phase than during the luteal phase.24 Although electrophysiological experiments using a guinea pig model showed that estrogen prolongs the action potential duration (APD) through the inhibition of IKr,25 the effect of estrogen on QT interval in humans remains controversial.26 Progesterone shortens APD, which is mainly attributable to the enhancement of IKs under basal conditions and the inhibition of ICaL under cAMP stimulation, and a computer simulation model using a 50% IKr block indicated a protective effect of progesterone against arrhythmia.27 Furthermore, another study in an LQT2 rabbit model showed the proarrhythmic effect of estradiol and the anti-arrhythmic effect of progesterone.28
With regard to the 6 LQT2 patients in the CPA or repetitive TdP groups, all had TdP repeatedly shortly after the first cardiac event; moreover 4 of 6 were post-puberty girls. In the menstrual cycle, there are a number of days in which the estrogen:progesterone ratio is high, around ovulation.26 Although we were not able to check their menstrual phase, we suspected that LQT2 girls might have TdP repeatedly in the high estrogen:progesterone ratio phase. In contrast, an 8-year-old boy and a 9-year-old girl (Table 2) also had short-term TdP recurrence. Although the estrogen level might have begun to elevate in the 9-year-old girl, there may be other factors affecting the vulnerability to arrhythmias in LQT2 children.
In the CPA and repetitive TdP group, 1 patient had a missense mutation in the S5-loop-S6 region, 2 had a deletion mutation in the S5-loop-S6 region, and 3 had a frameshift/nonsense mutation in the C-terminus region. In line with the present findings, LQT2 patients with S5-loop-S6 missense mutations have been shown to have a high risk for cardiac events.8 In the KCNH2 C-terminus regions, nonsense mutations were also reportedly more malignant than missense mutations.8
In the present LQT1 male cohort, the Kaplan-Meier curve shows that cardiac events obviously decreased after puberty (Figure 2A). Testosterone has been shown to shorten APD mainly through the enhancement of IKs, in part due to ICaL suppression at higher concentrations.29 An adult cross-sectional study showed that the QTc interval was significantly shorter at high testosterone levels.30 Consistent with the previous report,17 increased testosterone may contribute to reduce the risk of cardiac event in male patients with KCNQ1 mutation.
One of the limitations of the present study was that, because we analyzed 3 major LQTS genotypes, the contribution of other minor LQTS-related mutations to cardiac events could not be entirely excluded. In addition, patient data were collected at only 2 institutes, therefore there might be a bias in patient entry and genetic background, and hence a larger collaborative study is needed.
To the best of our knowledge, we report for the first time that female LQT2 patients are likely to have TdP recurrence shortly after antecedent cardiac events, especially after puberty. Therefore, short-term recurrence of TdP is expected and these patients should be followed more closely. These findings will help in selecting an appropriate preventive strategy based on the age of onset and genotype.
The study was supported in part by MEXT KAKENHI Grant Number 24591575 (to S.O.), 25136705, 25460406 (to H.I.), and 25461054 (to T.M.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, grants from the Ministry of Health, Labor and Welfare of Japan for Clinical Research on intractable Disease (H26-040, H24-033 to M.H.) and a Translational Research grant from the Japanese Circulation Society (to M.H.)
The authors declare no conflicts of interest.