2018 Volume 82 Issue 10 Pages 2493-2499
2018 Volume 82 Issue 10 Pages 2493-2499
Background: The incidence of subsequent need for permanent pacemaker implantation (PMI) after radiofrequency catheter ablation (RFCA) for atrial fibrillation (AF) in real world patients with and without pre-existing bradycardia has not yet been fully evaluated.
Methods and Results: A total of 1,131 consecutive patients undergoing first-time RFCA for AF who had no previous or planned device implantation, were enrolled in the present study. Of 799 paroxysmal AF (PAF) patients, 121 (15.1%) had sinus node dysfunction (SND). Of 332 non-PAF patients, 73 (22.0%) had slow ventricular response (VR), defined as heart rate <80 beats/min at rest without any rate-control drugs. The 5-year cumulative incidence of PMI after RFCA in PAF patients with and without SND was 14.8% and 1.7%, respectively (P<0.001). The 5-year cumulative incidence of PMI after RFCA in non-PAF patients with and without slow VR was 14.8% and 4.7%, respectively (P<0.001). SND and female gender in PAF patients, as well as slow VR and age ≥75 years in non-PAF patients, were independent and additive predictors of PMI. The 5-year cumulative incidence of PMI was 26.3% in female PAF patients with SND and 33.3% in elderly non-PAF patients with slow VR.
Conclusions: PMI was avoided in >85% of patients undergoing RFCA for PAF with pre-existing SND, although care should be taken for female patients. Decision-making regarding RFCA for non-PAF patients with slow VR, especially in the elderly, should be cautious.
Atrial fibrillation (AF) and bradycardia coexist and interact.1–4 Tachycardia-bradycardia syndrome, characterized as sinus arrest at the time of termination of paroxysmal AF (PAF), frequently requires pacemaker implantation (PMI).5,6 In previous studies with a relatively small number of patients, radiofrequency catheter ablation (RFCA) could eliminate AF and avoid PMI in the majority of PAF patients with sinus node dysfunction (SND).7,8 The clinical impact of AF ablation in PAF patients with pre-existing SND, however, has not been fully investigated in a real world patient group. Furthermore, few data are available on the risk of subsequent PMI after RFCA for persistent or long-lasting AF, so-called non-PAF, especially in those with pre-existing slow ventricular response (VR). Accordingly, we evaluated the subsequent need for PMI after AF ablation in patients with and without pre-existing bradycardia from a large single-center database during long-term follow-up.
Between February 2004 and March 2015, a total of 1,250 consecutive patients underwent first-time catheter ablation for AF at Kyoto University Hospital.9 The subjects consisted of 1,136 patients, excluding 43 patients who received cryoballoon ablation, 75 patients with prior or planned device implantation, and 1 patient who refused study participation (Figure 1). This study was conducted in accordance with the amended Declaration of Helsinki and the study protocol was approved by the institutional review board of Kyoto University Hospital.
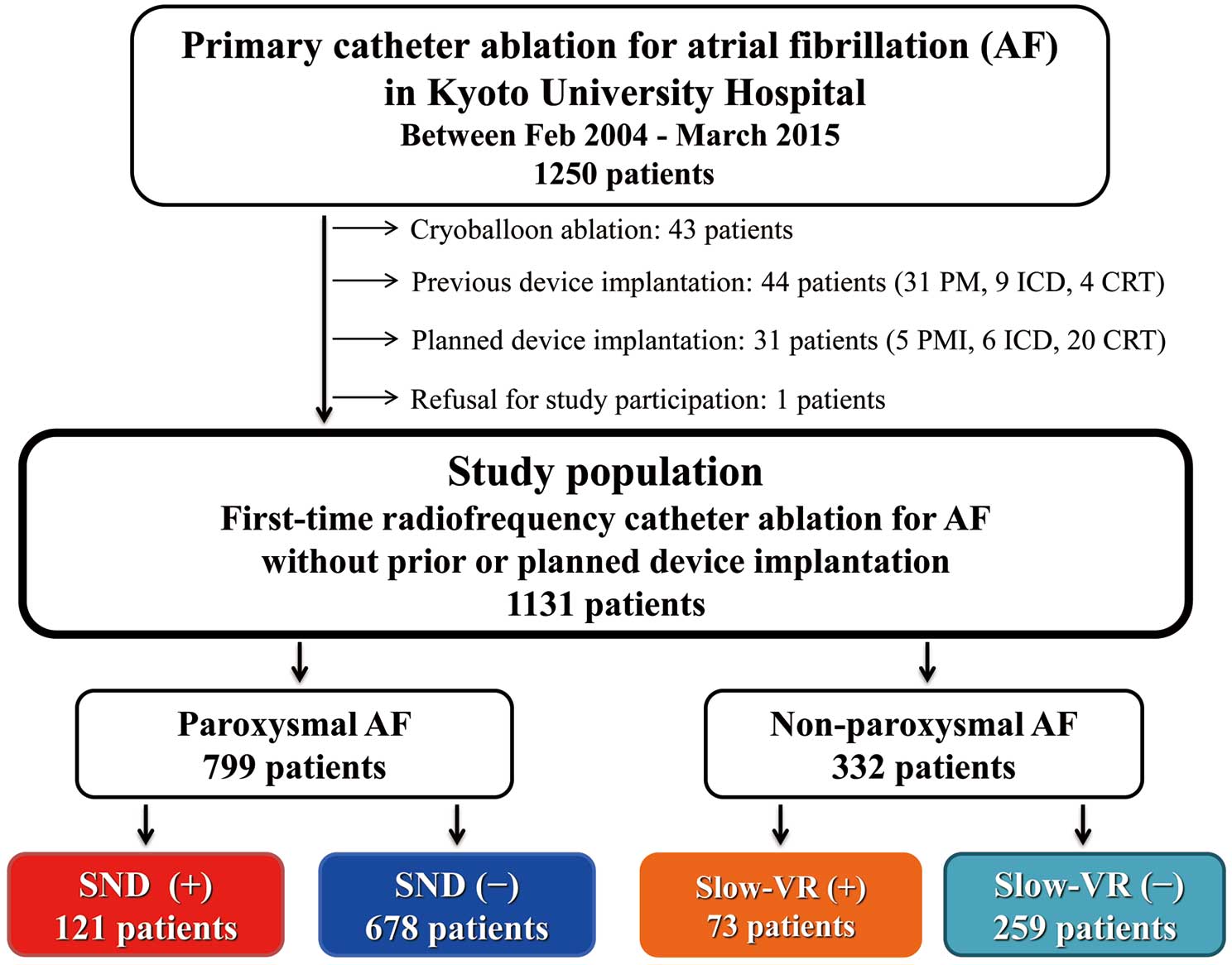
Study flow chart. AF, atrial fibrillation; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; PM, pacemaker; SND, sinus node dysfunction; VR, ventricular response.
PAF and non-PAF was defined as AF lasting for <7 days and ≥7 days, respectively. SND in PAF patients was defined as heart rate <50 beats/min at rest due to sustained sinus bradycardia (type I) or sinus arrest or sinoatrial block (type II), and sinus arrest ≥3 s at the time of AF termination (type III, bradycardia-tachycardia syndrome).9 Heart rate during sinus rhythm at rest was determined on the latest 12-lead electrocardiogram (ECG) during sinus rhythm before or at the time of hospital admission. Slow VR in non-PAF patients was defined as heart rate <80 beats/min at rest on 12-lead ECG at hospital admission. Patients treated with any atrioventricular (AV) node-blocking agents, such as β blockers, diltiazem, verapamil or digitalis were defined as not having slow VR, regardless of heart rate on 12-lead ECG. The primary end-point was the cumulative incidence of subsequent permanent PMI after the first ablation procedure. The secondary end-points were the event-free rate from recurrent atrial tachyarrhythmia after the first and last procedures with a blanking-period of 3 months. Recurrent atrial tachyarrhythmia was defined as documented AF or atrial tachycardia lasting >30 s or requiring repeat procedure.
AblationThe details of RFCA have been described elsewhere.10 In brief, the procedure was performed under conscious sedation with propofol and/or dexmedetomidine. Activated clotting time was kept >300 s with unfractionated heparin after trans-septal puncture. Extensive encircling pulmonary vein (PV) isolation (EEPVI) was performed with the double circular catheter method, placing 2 circular catheters in ipsilateral superior and inferior PVs. Continuous fractionated atrial electrocardiogram (CFAE) ablation and left atrial (LA) linear ablation such as mitral isthmus line and LA roof line were performed whenever appropriate. Tricuspid valve isthmus ablation was routinely performed regardless of the presence of previous typical atrial flutter. Superior vena cava (SVC) was isolated whenever necessary.
After the first procedure, oral anticoagulation was continued for ≥3 months. The use or discontinuation of anti-arrhythmic drugs and AV node-blocking agents after the procedure were left to the discretion of the attending physician. Repeat ablation was considered when recurrent atrial arrhythmia was detected after the blanking period of 3 months after ablation.
Data Collection and Follow-upBaseline patient characteristics and ablation data were collected from the hospital charts. Follow-up data were obtained from hospital charts or by contacting patients or referring physicians. Informed consent for the follow-up protocol was obtained by the attending physician for all patients, except 1 patient who refused during follow-up.
Statistical AnalysisCategorical variables are presented as number and percentage and were compared using the chi-square test or Fisher’s exact test. Continuous variables are presented as mean±SD or median (IQR) based on distribution, and were compared using Student’s t-test or Wilcoxon rank sum test. We used the Kaplan-Meier method to estimate the cumulative incidence of PMI and event-free rate from recurrent atrial tachyarrhythmia, and the difference was assessed with the log-rank test. Multivariable analysis using the Cox proportional hazard model was conducted to identify independent risk factors for the primary end-point of subsequent PMI. The clinical variables were age, gender, pre-existing bradycardia (SND in PAF patients and slow VR in non-PAF patients), AF duration, hypertension, diabetes mellitus, and LA dimension. Continuous variables were dichotomized by clinically meaningful reference values. Because of the limited number of events, only variables with P<0.1 on univariable analysis were included in the multivariable model. Statistical analysis was performed using JMP 10 (SAS Institute, Cary, NC, USA). All statistical analysis was 2-tailed, and P<0.05 was considered statistically significant.
Of the 1,131 patients, 799 (70.6%) had PAF and 332 (29.4%) had non-PAF. Baseline characteristics according to AF type are listed in Table 1. CHADS2 and CHA2DS2-VASc scores were not significantly different between the 2 groups. SVC isolation was more often performed in PAF patients, while CFAE and LA linear ablations were more often performed in non-PAF patients (Table 1).
| All (n=1,131) |
PAF (n=799) |
Non-PAF (n=332) |
P-value | |
|---|---|---|---|---|
| Age (years) | 64.1±9.5 | 64.4±9.8 | 63.5±8.9 | 0.14 |
| Female sex | 322 (28.5) | 258 (32.3) | 64 (19.3) | <0.001 |
| Hypertension | 671 (59.3) | 456 (57.1) | 215 (64.8) | 0.02 |
| DM | 182 (16.1) | 135 (16.9) | 47 (14.2) | 0.25 |
| Heart failure | 71 (6.3) | 37 (4.6) | 34 (10.2) | <0.001 |
| Ischemic stroke | 113 (10.0) | 73 (9.1) | 40 (12.1) | 0.14 |
| Vascular disease | 107 (9.5) | 79 (9.9) | 28 (8.4) | 0.44 |
| CHADS2 score | 1.1±1.0 | 1.1±1.0 | 1.2±1.1 | 0.05 |
| CHA2DS2-VASc score | 2.0±1.5 | 2.0±1.5 | 1.9±1.5 | 0.55 |
| ≥2 | 657 (58.1) | 472 (59.1) | 185 (55.7) | 0.30 |
| Echocardiography | ||||
| LVDD (mm) | 46.0±5.7 | 45.8±5.5 | 46.6±6.1 | 0.03 |
| LVEF (%) | 66.4±9.9 | 66.3±10.9 | 60.9±12.3 | <0.001 |
| LAD (mm) | 40.7±6.7 | 39.3±6.4 | 44.1±6.4 | <0.001 |
| Procedure characteristics | ||||
| PVI | 1,131 (100) | 799 (100) | 332 (100) | – |
| TVI ablation | 1,072 (9,482) | 753 (94.2) | 319 (96.1) | 0.19 |
| Additional ablation | ||||
| SVC isolation | 332 (29.4) | 255 (31.9) | 77 (23.2) | 0.003 |
| CFAE ablation | 284 (25.1) | 73 (9.1) | 211 (63.6) | <0.001 |
| Left atrial roof line | 32 (2.8) | 8 (1.0) | 24 (7.2) | <0.001 |
| Mitral isthmus line | 33 (2.9) | 7 (0.9) | 26 (7.8) | <0.001 |
Data given as mean±SD or n (%). AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; DM, diabetes mellitus; LAD, left atrial dimension; LVDD, left ventricular diastolic dimension; LVEF, left ventricular ejection fraction; PAF, paroxysmal atrial fibrillation; PVI, pulmonary vein isolation; SVC, superior vena cava; TVI, tricuspid valve isthmus.
A total of 121 PAF patients (15.1%) had pre-existing SND, which was classified as SND type I/II in 24 (3.0%) and type III in 97 patients (12.1%). Patients with SND were significantly older than those without, although LA diameter was not significantly different between the 2 groups. The prevalence of additional ablation to EEPVI and tricuspid valve isthmus ablation was not significantly different between the 2 groups (Table S1). Of 332 non-PAF patients, 73 (22.0%) had slow VR. The baseline characteristics did not differ significantly according to slow VR status, except for prevalence of female gender and left ventricular ejection fraction (Table S2). The prevalence of additional ablation to EEPVI and tricuspid valve isthmus ablation was not significantly different between the 2 groups (Table S2).
Subsequent PMIMean follow-up duration was 4.8±2.5 years. The cumulative 5-year incidence of subsequent PMI after AF ablation was generally low in the entire group (4.7%). The risk of PMI was not significantly different between PAF and non-PAF patients (3.7% vs. 7.1%, log-rank P=0.08; Figure S1).
The cumulative incidence of subsequent PMI at 1, 3, and 5 years in PAF patients without SND was 0.7%, 1.4%, and 1.7%. The corresponding incidence of PMI in PAF patients with SND was 9.9%, 11.7%, and 14.8%, respectively (log-rank P<0.001; Figure 2A). One PAF patient without pre-existing SND, who had a history of previous surgical resection of myxoma located at the atrial septum, developed intra-atrial block during tricuspid valve isthmus ablation and underwent PMI 3 days after ablation. No further patients developed bradyarrhythmia requiring PMI apparently due to the ablation procedure. Also, there was no significant difference in the incidence of subsequent PMI between patients with SND type I/II and those with SND type III, (14.3% vs. 16.7% at 5 years, respectively, P=0.79), although all patients with SND type I/II underwent PMI ≤1 year after RFCA, while the incidence of PMI in patients with SND type III gradually increased over time throughout the follow-up period (Figure S2).

Cumulative incidence of subsequent pacemaker implantation after radiofrequency catheter ablation in (A) paroxysmal AF (PAF) patients with and without SND, and (B) non-PAF patients with and without slow VR. Abbreviations as in Figure 1.
In 18 PAF patients with SND undergoing subsequent PMI after AF ablation, PMI was due to AF recurrence at ≤6 months in 10 (56%; Table S3). After 6 months, subsequent PMI was mostly due to progressive SND or AV block without AF recurrence. On multivariable analysis, SND (hazard ratio [HR], 7.44; 95% CI: 3.60–15.9, P<0.001) and female gender (HR, 3.49; 95% CI: 1.67–7.82, P<0.001) were independent predictors of subsequent PMI after RFCA for PAF (Table 2A). Female patents with SND had a 26.9% likelihood of PMI at 5 years (Figure 3A).
| Variables | Present | Absent | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|
| No. events/ no. patients (5-year cumulative incidence, %) |
No. events/ no. patients (5-year cumulative incidence, %) |
HR | 95% CI | P-value | HR | 95% CI | P-value | |
| A. PAF | ||||||||
| Age >75 years | 5/102 (3.9) | 25/697 (3.7) | 1.45 | 0.49–3.49 | 0.47 | |||
| Female | 20/258 (7.8) | 10/541 (1.8) | 4.25 | 2.04–9.47 | <0.001 | 3.49 | 1.67–7.82 | <0.001 |
| SND | 18/121 (14.8) | 12/678 (1.7) | 8.55 | 4.16–18.2 | <0.001 | 7.44 | 3.60–15.9 | <0.001 |
| AF duration >3 years | 14/353 (4.1) | 16/446 (3.3) | 1.06 | 0.51–2.18 | 0.87 | |||
| Hypertension | 20/456 (4.4) | 10/344 (2.8) | 1.51 | 0.73–3.38 | 0.27 | |||
| DM | 8/135 (5.3) | 22/664 (3.4) | 1.86 | 0.78–4.01 | 0.15 | |||
| LAD >45 mm | 5/129 (3.1) | 24/668 (3.7) | 1.08 | 0.36–2.61 | 0.87 | |||
| B. Non-PAF | ||||||||
| Age >75 years | 5/33 (18.5) | 16/299 (6.0) | 3.50 | 1.14–9.00 | 0.03 | 5.09 | 1.61–13.6 | 0.008 |
| Female | 4/64 (6.4) | 17/268 (4.7) | 1.05 | 0.30–2.85 | 0.92 | |||
| Slow VR | 11/73 (14.8) | 10/259 (1.7) | 3.87 | 1.63–9.30 | 0.003 | 4.83 | 1.98–12.0 | <0.001 |
| AF duration >3 years | 9/143 (7.1) | 12/189 (7.1) | 0.99 | 0.40–2.34 | 0.98 | |||
| Hypertension | 17/215 (8.7) | 4/117 (4.1) | 2.35 | 0.87–8.17 | 0.10 | |||
| DM | 2/47 (4.4) | 19/285 (7.5) | 0.64 | 0.10–2.20 | 0.52 | |||
| LAD >45 mm | 9/138 (6.8) | 12/193 (7.3) | 1.06 | 0.43–2.50 | 0.90 | |||
HR, hazard ratio; PMI, pacemaker implantation; RFCA, radiofrequency catheter ablation; SND, sinus node dysfunction; VR, ventricular response. Other abbreviations as in Table 1.

Additive impact of (A) SND and female gender in PAF patients, and (B) slow VR and age ≥75 years in non-PAF patients on the subsequent need for PM implantation. Abbreviations as in Figures 1,2.
The cumulative incidence of subsequent PMI at 1, 3, and 5 years in non-PAF patients with slow VR was high: 9.6%, 11.0%, and 14.8%. The corresponding incidence of PMI in non-PAF patients without slow VR was 1.6%, 3.4%, and 4.7%, respectively (log-rank P<0.001; Figure 2C). In 11 non-PAF patients with slow VR who underwent PMI after RFCA, the main bradyarrhythmias requiring PMI were SND in 7 (64%) and AV block in 4 (36%; Table S3). On multivariable analysis, slow VR (HR, 4.83; 95% CI: 1.98–12.0, P<0.001) and age ≥75 years (HR, 5.09; 95% CI: 1.61–13.6, P=0.008) were independent predictors of subsequent PMI after RFCA for non-PAF (Table 2B). Elderly patients (≥75 years) with slow VR had an extremely high incidence of PMI: 33.3% at 5 years (Figure 3B).
Recurrent Atrial TachyarrhythmiaThe 5-year event-free rate from recurrent atrial tachyarrhythmia after the first and last procedures were lower in non-PAF as compared with PAF patients (42.9% vs. 61.6%, P<0.001; and 71.1% vs. 87.5%, P<0.001; Figure S3). There was no significant difference, however, in the arrhythmia-free rate between patients with and without pre-existing bradycardia. The 5-year arrhythmia-free rate after the first and last procedures in PAF patients with and without SND were 59.5% vs. 61.9% (P=0.34) and 91.3% vs. 86.7% (P=0.40), respectively. The corresponding rates in non-PAF patients with and without slow VR were 43.9% vs. 42.7% (P=0.90) and 68.3% vs. 72.1%, (P=0.52), respectively.
In the present study, we found that (1) the need for subsequent PMI after RFCA for AF was generally low in the overall patient group; (2) PAF patients with SND and non-PAF patients with slow VR had an approximately 15% risk of PMI at 5 years; (3) conversely, PMI was avoided in approximately 85% of PAF patients with SND following successful elimination of AF by RFCA; (4) SND and female gender in PAF patients as well as slow VR and age ≥75 years in non-PAF patients were independent predictors of PMI; (5) in PAF patients with SND, the risk of PMI was approximately 4.5-fold higher in women than in men; (6) in non-PAF patients with slow VR, the risk of PMI was doubled in elderly patients; and (7) the arrhythmia-free rate after the first and last RFCA procedures was not significantly different in AF patients according to pre-existing bradycardia status.
Atrial arrhythmia, especially AF, is frequently complicated by SND, which is a common indication for PMI.11,12 Downregulation of the HCN channel in sinus node by high-rate atrial excitation is one of the mechanisms of SND with AF.13 Recovery of this downregulation by cessation of high-rate atrial pacing has been shown in an animal model.14 Also, in a human study involving 20 PAF patients with SND undergoing RFCA by Hocini et al, sinus node function gradually recovered over time during the 1-year follow-up period, with increasing heart rate and decreasing sinus node recovery time, resulting in avoidance of PMI in 95% of patients.7 In contrast, in the Inada et al study, although PMI was avoided in 32 of 37 PAF patients with SND undergoing RFCA (86%), there was no improvement in average heart rate and maximum RR interval during follow-up.8 In the present study with a larger number of patients, the event-free rate from PMI in 121 PAF patients with SND was 90.1% at 1 year and 85.2% at 5 years, concordant with the previous 2 studies.
Not only pre-existing SND but also female gender appeared to be an independent predictor of subsequent PMI after RFCA for PAF. In PAF patients with pre-existing SND, the risk of PMI after RFCA was approximately 4.5-fold higher in female than in male patients. The possible reasons for the higher risk of PMI in female patients include a higher rate of arrhythmia recurrence after RFCA and the poorer prognostic nature of female gender itself in AF patients, which are probably associated with extensive atrial degeneration.9,15 This gender difference should be taken into consideration in the decision-making for RFCA for PAF patients with SND.
In the present study, we also focused on the risk of subsequent PMI after RFCA in non-PAF patients, comparing those with and without slow VR. Non-PAF is usually associated with higher extent of atrial fibrosis and more frequent arrhythmia recurrence after RFCA compared with PAF.16,17 Because VR during AF is regulated by the AV node, slow VR in non-PAF is considered to suggest AV node dysfunction. At the same time, because compact AV node is located in the right atrium, extensive degeneration may also involve the sinus node, which is located at the junction of the right atrium and SVC.18 After restoration of sinus rhythm by RFCA, the need for subsequent PMI was much higher in non-PAF patients with slow VR as compared with those without slow VR (14.8% vs. 4.7% at 5 years, P<0.001). The main bradyarrhythmias requiring PMI in non-PAF patients with slow VR were AV block in 4 (36%) and SND in 7 (64%).
In addition to slow VR, age ≥75 years was also an independent predictor of subsequent PMI after AF ablation. The risk of subsequent PMI after RFCA in non-PAF patients with slow VR and age ≥75 years was 33% at 5 years. Thus, we should take into account the risk of subsequent PMI in the decision-making for RFCA in elderly non-PAF patients with slow VR, along with the risk of arrhythmia recurrence.
Study LimitationsThere were several important limitations in this study. First, the duration of enrollment in the present study was long, up to 11 years. The essential therapeutic strategy of AF ablation at Kyoto University Hospital, however, was consistent during the study period, characterized by EEPVI with the double circular catheter method along with tricuspid valve isthmus ablation. Second, we could not completely exclude the influence of anti-arrhythmic drugs and AV node-blocking agents on bradycardia or arrhythmia recurrence during follow-up because of the retrospective observational study design. Third, pre-existing bradycardia defined in the present study was not necessarily coincident with the indication criteria for PMI, and we had no control group of patients with AF and concomitant bradycardia who did not receive RFCA. As a result, we might have overestimated the impact of RFCA on PMI risk reduction. Fourth, there was a possibility of injury to the sinus or AV node during RFCA procedures such as SVC isolation and CFAE ablation. The risk of acute SND requiring PMI associated with SVC isolation, however, was low (1.1%) in a recent study with a large number of patients.19 In the present study, only 4 of 15 patients who required PMI ≤30 days after RFCA, had undergone SVC isolation. In addition, SVC isolation was not an independent predictor of subsequent PMI after RFCA in the additional multivariable analysis, and the sensitivity analysis excluding patients undergoing SVC isolation was consistent with the original analysis, identifying SND in PAF patients and slow VR in non-PAF patients as the independent predictors for subsequent PMI (data not shown). Also, CFAE ablation was not significantly associated with subsequent PMI after RFCA. Fifth, there was a possibility of unknown confounders in the multivariable analysis determining the independent predictors of subsequent PMI after RFCA. Finally, the heart rate cut-off of 80 beats/min for slow VR in non-PAF was not predetermined, but the results were consistent when the heart rate cut-off was set at 70 or 90 beats/min (Tables S4,S5).
RFCA could eliminate AF in the great majority of PAF patients, and PMI was avoided in >85% of patients with pre-existing SND. Care should be taken, however, for female patients with PAF plus SND, who required subsequent PMI in 26.3% of patients, in contrast to the 6.1% risk in male patients. In non-PAF patients, slow VR and age ≥75 years were the independent and additive predictors of subsequent PMI after RFCA. Considering the lower arrhythmia-free rate in non-PAF patients as compared with PAF patients, the decision-making for RFCA in non-PAF patients with slow VR, especially in the elderly, should be cautious.
We thank all the members of the cardiac catheterization laboratory, Graduate School of Cardiovascular Medicine, Kyoto University, for their contribution to this study.
The authors declare no conflicts of interest.
Supplementary File 1
Figure S1. Cumulative incidence of subsequent pacemaker implantation after radiofrequency catheter ablation according to paroxysmal atrial fibrillation (PAF) status.
Figure S2. Cumulative incidence of subsequent pacemaker implantation after radiofrequency catheter ablation in paroxysmal atrial fibrillation patients according to sinus node dysfunction (SND) type.
Figure S3. Recurrent atrial tachyarrhythmia event-free Kaplan-Meier curves after the (A,C,E) first and (B,D,F) last procedures according to (A,B) paroxysmal atrial fibrillation (PAF) status; in (C,D) PAF patients according to sinus node dysfunction (SND) status; and in (E,F) non-PAF patients according to slow ventricular response (slow VR) status.
Table S1. PAF patient characteristics comparing those with and without SND
Table S2. Non-PAF patient characteristics comparing those with and without slow VR
Table S3. Cause of subsequent PMI after RFCA
Table S4. Independent predictors of subsequent PMI after RFCA for non-PAF, using a slow VR cut-off of 70 beats/min
Table S5. Independent predictors of subsequent PMI after RFCA for non-PAF, using a slow VR cut-off of 90 beats/min
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-0214