2019 Volume 83 Issue 2 Pages 481-484
2019 Volume 83 Issue 2 Pages 481-484
Background: Although we and others have reported cases of patients with Anderson-Fabry disease (AFD) complicated by coronary spastic angina (CSA), the prevalence of CSA in these patients remains unknown.
Methods and Results: We performed the acetylcholine-induced provocation test, according to the Japanese guidelines for the diagnosis and treatment of patients with CSA, in 9 consecutive patients having 5 independent AFD pedigrees. Coronary spasms were provoked in conjunction with symptoms and ECG ischemic changes in 8 of 9 (89%) patients with AFD.
Conclusions: We found an unexpectedly high prevalence of CSA in patients with AFD.
Anderson-Fabry disease (AFD) is an X-linked lysosomal storage disorder caused by abnormalities in the α-galactosidase (Gal) A gene (GLA) (MIM:300644), which leads to reduced activity of the lysosomal enzyme, α-galactosidase A (α-Gal A),1 presenting as the classic early manifestations (acroparesthesias, clustered angiokeratoma, cornea verticillata, hypo-anhidrosis), and vascular disease of the heart, kidneys, and brain.1
Editorial p 283
Cardiac symptoms, including left ventricular (LV) hypertrophy, arrhythmia, angina, and dyspnea, are reported in approximately 40–60% of patients with AFD.2 Furthermore, coronary artery disease in patients with AFD is reportedly induced by deposits in endothelial cells and coronary smooth muscle cells, leading to stenosis.3 Although we and others have reported individual cases of patients with AFD complicated by coronary spastic angina (CSA), without coronary artery stenosis, following acetylcholine (ACh)-loaded coronary angiography,4,5 the prevalence of CSA in AFD remains unknown. Here, we investigated whether CSA can be induced using the ACh provocation test in patients diagnosed with AFD using GLA genetic analysis.
See Supplementary Methods.
The Table shows a summary of the clinical evaluations and genetic analyses for all patients. Between January 2008 and December 2017, 9 consecutive patients (2 male, 7 female; median age, 50 years), with 5 independent AFD pedigrees, participated in this study. Coronary angiography revealed the absence of stenotic lesions in all patients. However, coronary spasms were provoked, in conjunction with symptoms and ischemic ECG changes, in 8 of 9 patients (4 in the LCA; 1 in the RCA; 3 in the LCA and RCA) (Figure A–D, Table). The spasms resolved promptly following intracoronary administration of isosorbide dinitrate, indicating a CSA diagnosis in 8 of the 9 (89%) patients with AFD. We used calcium-channel blockers, nitrate, nicorandil and enzyme replacement therapy (ERT) to treat these 8 patients and of them, 6 had angina completely abolished and 2 had partially improved angina.
| Case no. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7* | 8* | 9* | |
| Age (years)/sex | 36/M | 67/F | 37/F | 63/M | 47/F | 45/F | 50/F | 66/F | 68/F |
| Phenotype | Classic | Classic | Classic | Classic | Classic | Classic | Late-onset | Late-onset | Late-onset |
| α-Gal A activity (AgalU) |
2.8 | 8.2 | 8.3 | 1.9 | 10.0 | 13.0 | 16.1 | 10.0 | 12.7 |
| Exon | 5 | 5 | 7 | 4 | 5 | 5 | 4 | 4 | 4 |
| GLA mutation | p.W236X | p.W236X | c.1072– 1074delGAG |
p.M187V | c.723dupT | c.723dupT | p.G195V | p.G195V | p.G195V |
| Hypertension | – | – | – | + | – | – | + | – | – |
| Diabetes mellitus |
– | – | – | – | – | – | – | – | – |
| Dyslipidemia | – | – | + | – | + | – | + | + | + |
| Smoking | ex | – | – | – | ex | – | ex | – | – |
| Angina at rest | + | + | + | + | + | + | + | + | + |
| BP (mmHg) | 110/65 | 121/73 | 84/51 | 106/58 | 113/73 | 122/69 | 123/87 | 106/75 | 89/62 |
| IVS/PW (mm) | 13/13 | 17/7 | 7/7 | 18/18 | 17/12 | 12/15 | 9/9 | 9/12 | 12/8 |
| Ejection fraction (%) |
66 | 59 | 60 | 61 | 65 | 60 | 65 | 65 | 28 |
| LDL-C (mg/dL) | 115 | 139 | 139 | 55 | 105 | 101 | 106 | 95 | 97 |
| Triglycerides (mg/dL) |
66 | 152 | 245 | 54 | 89 | 83 | 112 | 119 | 144 |
| BNP (pg/mL) | 8 | 385 | 8 | 133 | 47 | 66 | 11 | 41 | 1,140 |
| LGE on CMR | + | + | – | NA | + | + | – | + | + |
| Comorbidity | – | – | Stroke | ESRD | – | – | – | – | – |
| Medication at angiography | |||||||||
| CCB | – | – | – | – | – | – | Amlodipine 2.5 mg |
Diltiazem 200 mg |
– |
| β-blocker | – | – | – | – | – | – | – | – | Carvedilol 10 mg |
| ARB/ACEI | – | – | – | – | – | – | – | – | Candesartan 2 mg |
| Statin | – | – | – | – | Rosuvastatin 2.5 mg |
– | Pitavastatin 3 mg |
Atorvastatin 10 mg |
– |
| ERT | – | – | – | – | – | – | + | + | + |
| Coronary angiography | |||||||||
| Organic stenosis |
#4AV 25% | #7 25% | None | #1 25%, #7 25% |
#4AV 25% | #1 25% | #2 25%, #11 25% |
None, #3 aneurysm |
None |
| ACh provocation | |||||||||
| LCA: ACh (μg) |
100 | 20 | 100 | 100 | 50 | 50 | 100 | 50 | 100 |
| LAD | #7 99%, diffuse |
#7 99%, diffuse |
#7 99%, diffuse |
Negative | #7 99%, diffuse |
#8 99%, diffuse |
#7 99%, diffuse |
Negative | Negative |
| LCx | Negative | #13 99%, diffuse |
Negative | Negative | #13 100%, diffuse |
Negative | Negative | #13 99%, focal |
Negative |
| RCA: ACh (μg) |
NA | 50 | NA | 50 | 50 | 50 | 50 | 50 | 50 |
| RCA | NA | #4PD 99%, focal |
NA | #3 99%, diffuse |
Negative | #4PD 99%, diffuse |
Negative | #4PD 100%, focal |
Negative |
| Chest pain | + | + | + | + | + | + | + | + | – |
| ECG change | + | + | + | + | + | + | + | + | – |
| Effect of medical treatment# |
+ | + | ± | + | + | + | ± | + | NA |
*Detailed clinical profiles of cases 7, 8, and 9 described elsewhere.5 #Effect of calcium-channel blockers, nitrate, nicorandil, and ERT: +, completely abolished angina; ±, partially improved angina. α-Gal A, α-galactosidase A; ACh, acetylcholine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BNP, B-type natriuretic peptide; BP, blood pressure; CCB, Calcium-channel blocker; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ERT, enzyme replacement therapy; ESRD, endstage renal disease; GLA, α-Gal A gene; IVS, interventricular septum; LAD, left anterior descending artery; LCA, left coronary artery; LCx, left circumflex artery; LDL-C, low-density lipoprotein cholesterol; LGE, late gadolinium enhancement; NA, not applicable; PW, posterior wall; RCA, right coronary artery.
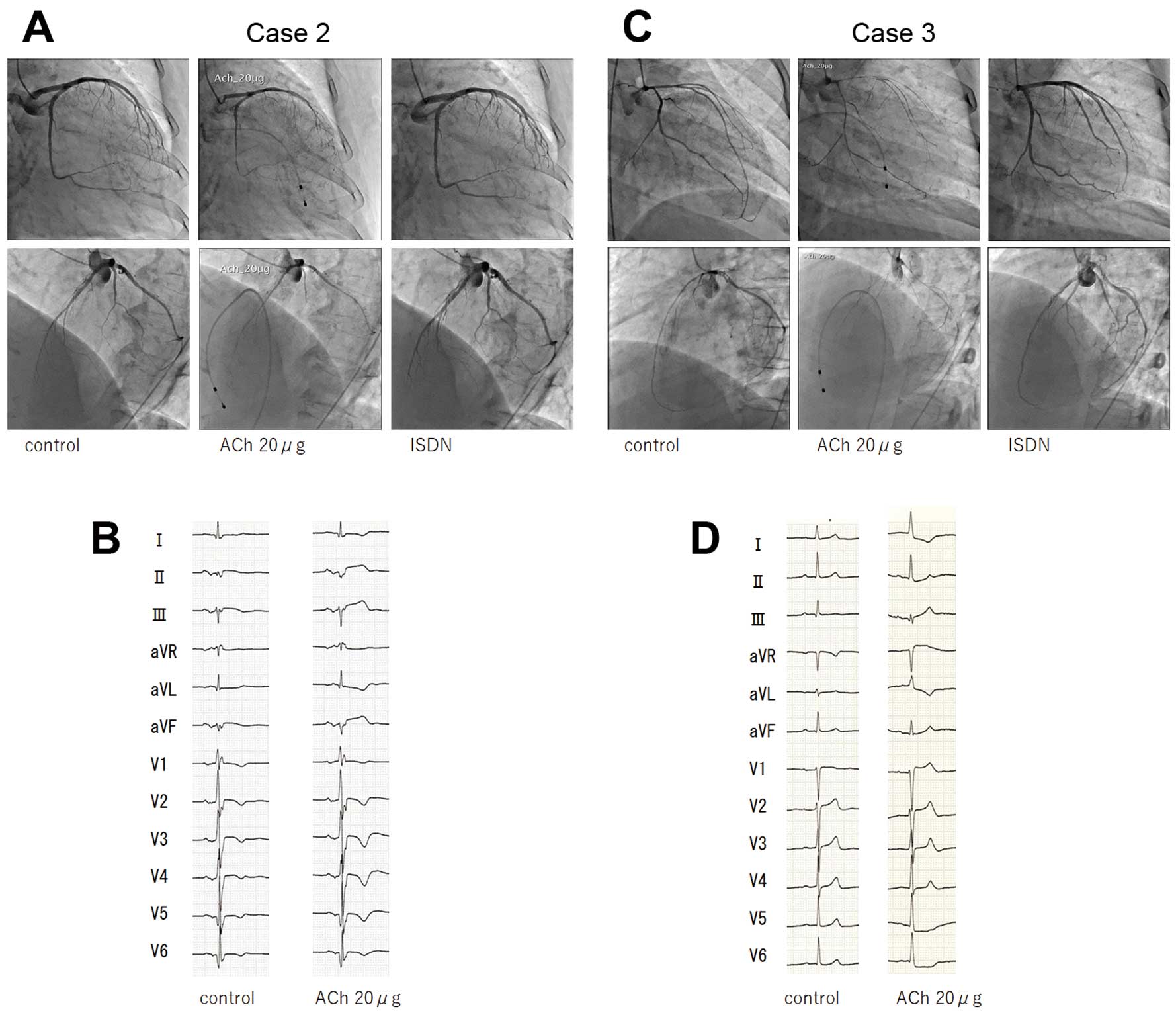
Representative coronary angiograms and electrocardiograms following intracoronary acetylcholine (Ach) infusion. (A,B) Diffuse, severe vasoconstriction of the left coronary artery (LCA) and ST elevation on ECG show ACh dose-dependency in a 67-year-old female patient with a nonsense mutation in exon 5 of the α-galactosidase A gene (case 2 in Table). The spasm and ST elevation disappear after isosorbide dinitrate (ISDN) administration. (C,D) Diffuse, severe vasoconstriction of the LCA and ST elevation on ECG show ACh dose-dependency in a 37-year-old female patient with a deletion in exon 7 of α-galactosidase A (case 3 in Table). The spasm and ST elevation disappear after ISDN administration.
Of the 8 AFD patients with associated CSA, 2 (patients 3 and 7 in Table) did not show either LV hypertrophy or late gadolinium enhancement on their cardiac magnetic resonance studies, suggesting that CSA might be related to early comorbidity preceding the development of LV hypertrophy, regardless of sex, α-Gal A activity, or phenotype of AFD.
We found an unexpectedly high prevalence of CSA in patients with AFD, suggesting that clinicians should consider AFD as a potential cause of CSA.
In patients with AFD, glycosphingolipid accumulation is reported to occur in the coronary arteries, endothelial cells, and smooth muscle cells of cardiac arterioles,3 suggesting that endothelial dysfunction and hyperreactivity of vascular smooth muscle cells play a major role in symptom development. Furthermore, vasospastic neurohormonal stimuli may be also involved. The exact underlying pathology predisposing patients to coronary spasm is not clearly understood, but the most established and accepted mechanisms include endothelial dysfunction, nitric oxide (NO) release, and enhanced vascular smooth muscle cell contractility, all of which determine vascular tone.6 Recently, several reports described NO pathway dysregulation in AFD. Indeed, Aerts et al identified a new lyso-derivative of globotriaosylceramide (Gb3), lyso Gb3 or lyso-CTH, which induces smooth muscle cell proliferation in vitro.7 Secondary to this, endothelial dysfunction may also plausibly occur, as evidenced by the abnormal flow-mediated dilatation observed in patients with AFD.8 Although it has been reported that ERT cleared microvascular endothelial Gb3 deposits,9 further studies investigating the pathophysiology of AFD are clearly needed to help us understand the effects of specific treatments, including ERT,10 and to help develop even more targeted treatments.
Coronary spasm and multivessel coronary spasm have been reported to be more prevalent in Japanese people than in Westerners,11 suggesting that AFD might be an underlying cause of CSA in Japanese patients. Although the most common life-threatening cardiac problem in patients with AFD is arrhythmia,1,2 coronary spasm may also cause infarctions, LV impairment, life-threatening arrhythmias, and ultimately sudden cardiac death. Therefore, coronary spasm should be considered to result from extensive vasomotor and endothelial dysfunction of the coronary circulation in patients with AFD. Although coronary spasm provocation tests may be performed with an acceptable safety profile, using intracoronary administration of ACh and ergonovine, and the tests provide useful diagnostic and prognostic information,12 those provocation tests are not routinely performed outside of Japan because of the possibility of adverse events. Thus, clinicians outside of Japan may consider performing coronary spasm provocation tests in patients with AFD.
Although this study was limited by its small sample size and potential selection bias, we conclude that clinicians should be aware of AFD as a potential cause of CSA, because early initiation of ERT is required for patients with AFD. Therefore, screening of α-Gal A activity might be recommended for patients with CSA, especially when the patient’s family history includes relevant clinical features.
We thank Professor Yoshio Makita for the genetic counseling and Kaori Kanno for her excellent technical assistance.
None.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-0734