Article ID: CJ-20-0201
Article ID: CJ-20-0201
Background: We investigated the long-term clinical and hemodynamic outcomes after aortic valve replacement (AVR) with a 17-mm mechanical valve.
Methods and Results: Between January 2005 and December 2011, 80 patients with aortic stenosis underwent AVR with the 17-mm St. Jude Medical Regent prosthetic valve. Echocardiography was performed preoperatively, at discharge, and at follow-up, which was performed at least 2 years postoperatively (median interval, 7.3 years). Prosthesis-patient mismatch (PPM) was defined as an indexed effective orifice area <0.85 cm2/m2 at discharge and occurred in 25 patients (31%). The median follow-up period was 8.7 years (100% complete). Overall in-hospital mortality was 2.5% (2 patients) with 27 late deaths (34%). The 5- and 10-year survival rates were 78.7% and 63.0%, respectively. Peripheral arterial disease and concomitant mitral valve repair were independent predictors of late mortality. The 5- and 10-year freedom from major adverse valve-related events (MAVRE) rates were 91.6% and 83.5%, respectively. PPM at discharge did not affect long-term survival, freedom from MAVRE, or freedom from heart failure. Echocardiographic data at follow-up revealed a significant reduction in the mean left ventricular mass index (LVMI). LVMI reduction observed at follow-up was similar between patients with and without PPM.
Conclusions: AVR with the 17-mm mechanical prosthesis had acceptable long-term clinical and hemodynamic outcomes. Significant reduction in LVMI was observed regardless of PPM.
Surgical aortic valve replacement (AVR) is the standard therapy for severe aortic stenosis, although transcatheter AVR (TAVR) has rapidly evolved and is a widely accepted option in such patients.1 AVR (surgical or transcatheter approach) is aimed at reducing the transvalvular pressure gradient and left ventricular workload.
In patients undergoing AVR with a small-sized valve, prosthesis-patient mismatch (PPM) results in a persistent abnormally high transprosthetic pressure gradient across the aortic valve. Several studies have reported that PPM negatively affects postoperative clinical status and survival rates.2,3 However, studies describing the effect of PPM on clinical outcomes have reported conflicting results, and the effect of PPM on late outcomes remains unclear.4
Although a bioprosthesis is usually selected in elderly patients, biological valves measuring <19 mm in size are not commercially available. Several surgical options are recommended for patients with a small aortic annulus in whom implantation of a 19-mm bioprosthesis is challenging. Among these strategies, implantation of a mechanical valve measuring <19 mm is technically the most uncomplicated. However, the high incidence and negative effect of PPM after AVR using a 17-mm prosthesis remain notable concerns in clinical practice. A few authors have reported satisfactory mid-term outcomes after AVR using a 17-mm mechanical valve with a follow-up period ranging between 33 and 53 months.5–8 However, the incidence of PPM after AVR with the 17-mm valve is reportedly high (range 10–43%).5–8 It is unknown whether a persistent abnormally high transprosthetic pressure gradient associated with implantation of a 17-mm valve negatively affects long-term outcomes. We have previously reported the early outcomes of patients receiving a 17-mm mechanical valve,5 so the purpose of this study was to report on the long-term clinical and hemodynamic outcomes after AVR with a 17-mm mechanical valve.
This retrospective study included 80 consecutive patients (74 women, mean age, 73.0±7.6 years) who underwent AVR for aortic stenosis using the 17-mm St. Jude Medical Regent prosthetic valve (Abbott, Inc., St. Paul, MN, USA) between January 2005 and December 2011. Of these 80 patients, 60 (75%) were aged ≥70 years. Mean body surface area (BSA) was 1.45±0.12 m2 (range, 1.21–1.77 m2). Patients with concomitant mitral valve replacement were excluded. Preoperative patient characteristics are shown in Table 1. The most common valve pathology was senile degeneration (74%). The study was approved by the Institutional Review Board of Jichi Medical University, and the requirement for individual informed consent was waived.
| Variable | Total (n=80) |
|---|---|
| Age (years) | 73.0±7.7 |
| Male sex | 6 (7.5%) |
| Body surface area (m2) | 1.45±0.12 (1.21–1.77) |
| New York Heart Association class III/IV | 36 (45.0%) |
| Valve pathology | |
| Senile degeneration | 59 (73.8%) |
| Rheumatic | 18 (22.5%) |
| Bicuspid | 3 (3.8%) |
| Hypertension | 51 (63.8%) |
| Diabetes | 13 (16.3%) |
| Ischemic heart disease | 19 (23.8%) |
| Atrial fibrillation | 6 (7.5%) |
| Cerebrovascular disease | 2 (2.5%) |
| Chronic kidney disease* | 10 (12.5%) |
| Hemodialysis | 7 (8.8%) |
| Peripheral arterial disease | 3 (3.8%) |
| Previous cardiac surgery | 2 (2.5%) |
| Left ventricular ejection fraction (%) | 61±13 |
| <50% | 16 (20.0%) |
Values for continuous variables are expressed as mean±standard deviation, and values for categorical variables are expressed as numbers (%). *Serum creatinine >1.5 mg/dL.
All procedures were performed through a standard median sternotomy. Cardiopulmonary bypass was established with cannulation of the ascending aorta and the superior and inferior venae cava. Moderate hypothermia was applied with cold-blood cardioplegic arrest. Prosthesis size was determined by measuring the diameter of the aortic annulus with valve sizers supplied by the manufacturer. The 17-mm Regent prosthetic valve was implanted in the intra-annular position using everting mattress sutures in 67 patients (84%) and interrupted sutures in 12 patients (15%), and was placed in the supra-annular position in 1 patient (1%). In our case series, no patient underwent aortic annulus enlargement. Warfarin sodium therapy was initiated on the day of surgery, and patients were instructed to continue lifelong anticoagulation based on the American College of Cardiology/American Heart Association guidelines.9 Table 2 shows the intraoperative data of the patients included in this study. Concomitant procedures were performed in 29 patients (36%). Coronary artery bypass grafting (CABG) was performed in 14 (18%) and mitral valve repair in 9 patients (11%).
| Variable | Total (n=80) |
|---|---|
| Cardiopulmonary bypass time (min) | 167±55 |
| Cross-clamp time (min) | 137±41 |
| Concomitant procedures | 29 (36.3%) |
| Coronary artery bypass grafting | 14 (17.5%) |
| Tricuspid annuloplasty | 13 (16.3%) |
| Mitral valve repair | 9 (11.3%) |
Values for continuous variables are expressed as mean±standard deviation, and values for categorical variables are expressed as numbers (%).
Echocardiographic data were recorded preoperatively, at discharge, and at follow-up (performed at least 2 years postoperatively). The following variables were measured: left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension, end-diastolic septal thickness, left ventricular end-diastolic posterior wall thickness, effective orifice area (EOA), and the mean transvalvular pressure gradient. The complete Bernoulli equation was used to calculate transvalvular pressure gradients. The EOA was calculated using the continuity equation and was indexed to the BSA. Left ventricular mass was calculated using the Devereux formula.10 PPM was defined based on discharge echocardiography as an EOA index (EOAI) (i.e., EOA divided by BSA) of ≤0.85 cm2/m2. The projected EOAI was used to determine PPM at discharge in 20 patients (25%) in whom EOAI data were missing at the time of discharge.
Follow-up EvaluationLate clinical status including survival and complication rates was obtained by reviewing patients’ charts and postal and telephone questionnaires. Clinical follow-up was updated to October 2018. The follow-up rate was 100% complete, which means that the patients’ status was confirmed at least 4 years postoperatively in all patients except those who died within 4 years postoperatively. The median follow-up period was 8.7 years (range, 0–13.3 years). Echocardiographic data were obtained in 39 patients (76% of the 51 patients who were alive) at follow-up with a median interval of 7.3 years postoperatively. Patients’ perceived quality of life was assessed using the New York Heart Association (NYHA) classification.
Statistical AnalysisContinuous data are expressed as mean±standard deviation and categorical data as frequencies and percentages. Categorical variables were compared using the Fisher exact test. Student’s t-test or the Mann-Whitney U test was used for intergroup comparisons of continuous variables. Mixed-effects models with the Kenward-Roger degrees-of-freedom method and an unstructured covariance model were used for analysis of repeated measures. Long-term survival and freedom from major adverse valve-related events (MAVRE) were estimated using the Kaplan-Meier method, and intergroup comparisons were performed using the log-rank test. MAVRE was defined based on the guidelines for reporting after cardiac valve interventions.11
Forward stepwise Cox proportional hazards regression analysis was performed to identify predictors of late mortality, MAVRE, and heart failure. Variables analyzed included age, sex, BSA, NYHA class III/IV, hypertension, diabetes, ischemic heart disease, atrial fibrillation, cerebrovascular disease, chronic kidney disease (serum creatinine >1.5 mg/dL), hemodialysis, peripheral arterial disease, history of cardiac surgery, LVEF <50%, PPM at discharge, cardiopulmonary bypass time, aortic cross-clamp time, concomitant CABG and mitral valve repair. Variables with a P value <0.25 on univariate analysis were subjected to multivariable Cox proportional hazards regression analysis. Data were analyzed using the SPSS software, version 25 (SPSS Inc. Chicago, ILL, USA), SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), and Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered statistically significant.
The overall in-hospital mortality rate was 2.5% (n=2). The causes of death were respiratory failure and intestinal necrosis. Hemodynamic instability necessitated perioperative intra-aortic balloon pumping and percutaneous cardiopulmonary support in 1 patient (1.3%). Postoperative cerebral infarction occurred in 1 patient (1.3%), and no patient required pacemaker implantation.
Clinical Follow-up and Valve-Related EventsThe Kaplan-Meier curve for overall survival is shown in Figure 1A. Late deaths occurred in 27 patients (34%) during the median follow-up of 8.7 years (range, 0–13.3 years). Causes of late deaths were: infections such as pneumonia (n=5), malignancy (n=4), heart failure (n=4), renal failure (n=3), stroke (n=2), gastrointestinal bleeding (n=1), senile deterioration (n=1), and unknown causes (n=7). The overall 1-, 5-, and 10-year survival rates were 95.0%, 78.7% and 63.0%, respectively. Multivariable analysis showed that age (P=0.006), peripheral arterial disease (P=0.002), and concomitant mitral valve repair (P=0.002) were independent predictors of late mortality (Table 3).
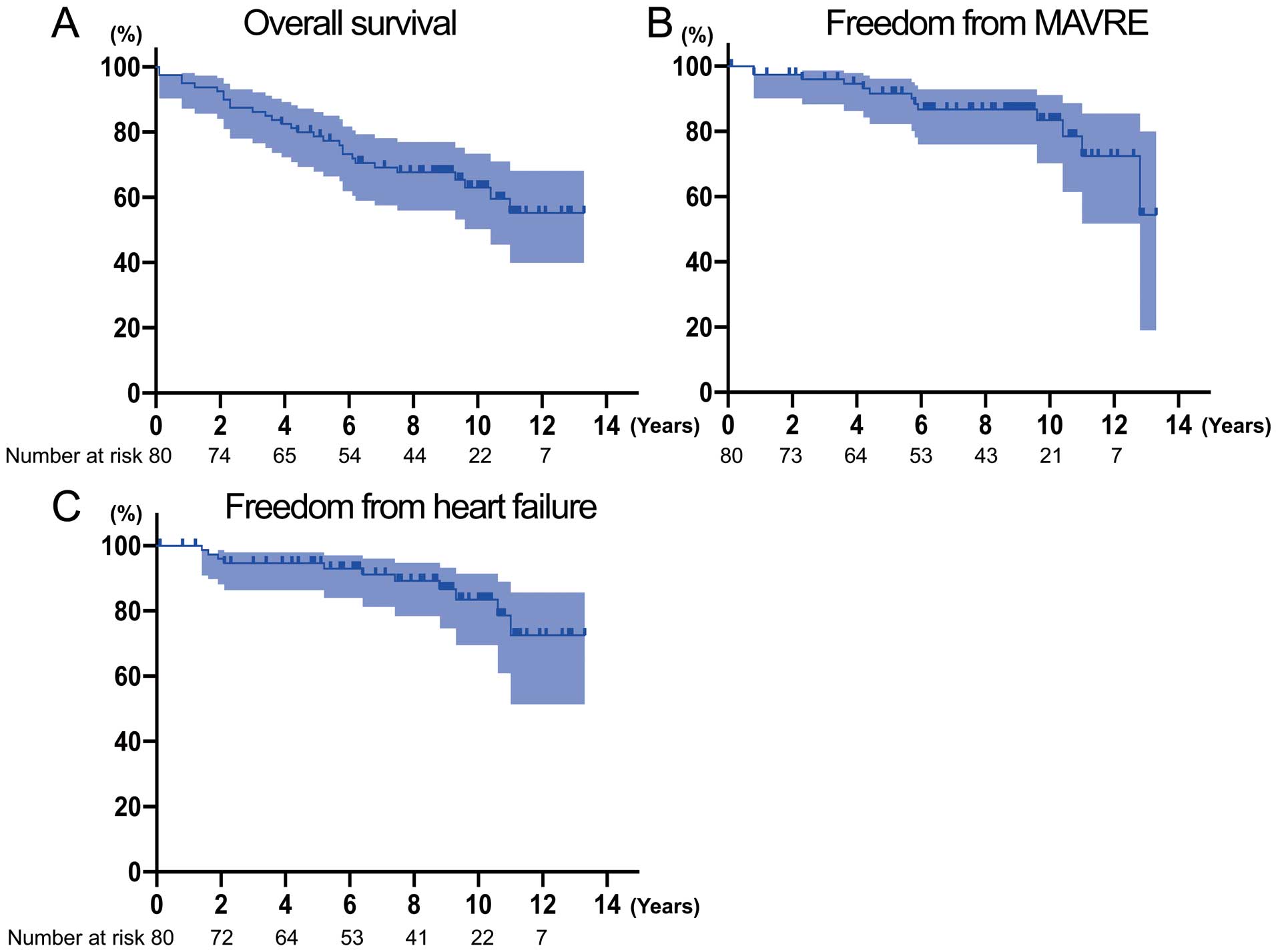
Kaplan-Meier curves showing (A) overall survival, (B) freedom from MAVRE and (C) freedom from heart failure, with 95% confidence intervals. MAVRE, major adverse valve-related events.
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Late mortality | ||||
| Age (years) | 1.10 (1.03–1.17) | 0.002 | 1.12 (1.03–1.22) | 0.006 |
| Male sex | 2.32 (0.80–6.70) | 0.119 | 2.55 (0.60–10.88) | 0.206 |
| Body surface area (m2) | 0.01 (0.00–0.41) | 0.014 | 0.01 (0.00–1.14) | 0.057 |
| NYHA class III/IV | 1.50 (0.72–3.11) | 0.280 | – | |
| Hypertension | 1.84 (0.81–4.16) | 0.146 | – | |
| Diabetes | 2.70 (1.18–6.19) | 0.019 | 2.10 (0.77–5.71) | 0.145 |
| Ischemic heart disease | 1.06 (0.45–2.48) | 0.895 | – | |
| Atrial fibrillation | 1.02 (0.24–4.31) | 0.976 | – | |
| Cerebrovascular disease | 0.05 (0.00–839.13) | 0.541 | – | |
| Chronic kidney disease* | 3.71 (1.47–9.37) | 0.006 | 2.12 (0.67–6.65) | 0.199 |
| Hemodialysis | 2.17 (0.65–7.27) | 0.210 | – | |
| Peripheral arterial disease | 25.76 (5.98–110.98) | <0.001 | 16.33 (2.90–91.83) | 0.002 |
| Previous cardiac surgery | 2.03 (0.27–15.01) | 0.490 | – | |
| LVEF <50% | 1.27 (0.54–3.00) | 0.579 | – | |
| CPB time (min) | 1.00 (0.99–1.01) | 0.911 | – | |
| Cross-clamp time (min) | 1.00 (0.99–1.01) | 0.987 | – | |
| Concomitant CABG | 1.04 (0.40–2.73) | 0.935 | – | |
| Concomitant MVP | 2.44 (0.74–8.12) | 0.145 | 12.00 (2.42–59.54) | 0.002 |
| Postoperative PPM | 0.43 (0.16–1.11) | 0.081 | 0.26 (0.07–1.01) | 0.051 |
| MAVRE | ||||
| Age (years) | 1.04 (0.96–1.12) | 0.369 | – | |
| Male sex | 2.31 (0.49–10.88) | 0.289 | – | |
| Body surface area (m2) | 0.27 (0.00–35.70) | 0.600 | – | |
| NYHA class III/IV | 3.23 (0.99–10.52) | 0.052 | 3.54 (0.99–12.66) | 0.051 |
| Hypertension | 5.14 (1.10–23.98) | 0.037 | 11.17 (1.20–104.41) | 0.034 |
| Diabetes | 3.89 (1.12–13.48) | 0.032 | 3.12 (0.83–11.77) | 0.092 |
| Ischemic heart disease | 0.66 (0.14–3.00) | 0.587 | – | |
| Atrial fibrillation | 1.25 (0.16–9.70) | 0.832 | – | |
| Cerebrovascular disease | 0.05 (0.00–3,136.99) | 0.704 | – | |
| Chronic kidney disease* | 0.04 (0.00–1,578.11) | 0.559 | – | |
| Hemodialysis | 0.05 (0.00–7,254.53) | 0.612 | – | |
| Peripheral arterial disease | 0.05 (0.00–4,559.00) | 0.879 | – | |
| Previous cardiac surgery | 6.16 (0.77–49.51) | 0.088 | 51.13 (2.51–1,040.39) | 0.001 |
| LVEF <50% | 1.57 (0.47–5.25) | 0.466 | – | |
| CPB time (min) | 1.01 (1.00–1.01) | 0.027 | – | |
| Cross-clamp time (min) | 1.01 (1.00–1.02) | 0.080 | – | |
| Concomitant CABG | 1.57 (0.42–5.81) | 0.503 | – | |
| Concomitant MVP | 5.93 (1.25–28.23) | 0.025 | 3.43 (0.66–17.77) | 0.142 |
| Postoperative PPM | 0.58 (0.16–2.12) | 0.409 | – | |
*Serum creatinine >1.5 mg/dL. CABG, coronary artery bypass grafting; CI, confidence interval; CPB, cardiopulmonary bypass; HR, hazard ratio; LVEF, left ventricular ejection fraction; MAVRE, major adverse valve-related events; MVP, mitral valve repair; NYHA, New York Heart Association; PPM, prosthesis-patient mismatch.
A total of 13 MAVRE (16%) were recorded during follow-up. The 1-, 5-, and 10-year freedom from MAVRE rates were 97.4%, 91.6% and 83.5%, respectively (Figure 1B). Multivariable analysis showed that hypertension (P=0.034) and history of cardiac surgery (P=0.010) were independent predictors of MAVRE. A total of 11 patients (14%) were readmitted secondary to heart failure. The 1-, 5-, and 10-year freedom from heart failure rates were 100%, 94.7%, and 83.5%, respectively (Figure 1C). No risk factor for heart failure was identified. Bleeding events occurred in 3 patients (3.8%) (2 patients with cerebral hemorrhage, 1 with gastrointestinal bleeding), and ischemic stroke occurred in 3 patients (3.8%). The linearized rates of MAVRE, heart failure, bleeding, and thromboembolism were 2.2, 1.9, 0.5, and 0.5 per 100 patient-years, respectively, as shown in Table 4. Prosthetic endocarditis and structural failure of the prosthesis did not occur, and reoperation was not required in any patient during the follow-up period.
| No. of events | Linearized rate* | Freedom from events | |||
|---|---|---|---|---|---|
| At 1 year | At 5 years | At 10 years | |||
| MAVRE | 13 | 2.2 | 97.4% (90.1–100%) | 91.6% (82.3–96.2%) | 83.5% (70.3–91.1%) |
| Heart failure | 11 | 1.9 | 100% (100–100%) | 94.7% (86.4–98.0%) | 83.5% (69.5–91.4%) |
| Bleeding | 3 | 0.5 | 98.7% (91.0–99.8%) | 98.7% (91.0–99.8%) | 95.1% (85.5–98.4%) |
| Thromboembolism | 3 | 0.5 | 100% (100–100%) | 98.5% (89.6–99.8%) | 98.5% (89.6–99.8%) |
Linearized rates or percentages (%) (95% confidence interval) are shown. *Per 100 patient-years. MAVRE, major adverse valve-related events.
Symptoms were evaluated using the NYHA classification. Excluding 2 cases of in-hospital death, 87% and 13% of all patients were categorized as NYHA class I and II, respectively.
Cardioprotective drugs prescribed at discharge included β-blockers in 34 patients (42.5%), angiotensin-converting enzyme inhibitors in 10 patients (12.5%), angiotensin-receptor blockers in 18 patients (22.5%), and statins in 37 patients (46.3%).
Long-Term Echocardiographic DataEchocardiographic data recorded over specific time-points are shown in Figure 2. Preoperative mean transvalvular/transprosthetic pressure gradients were significantly higher than those recorded at follow-up (59.0±21.7 vs. 20.6±9.9 mmHg, P<0.001) (Figure 2A,B). The mean EOAI recorded at follow-up was significantly higher than that recorded preoperatively (0.82±0.15 vs. 0.41±0.13 cm2/m2, P<0.001) (Figure 2C,D). The mean left ventricular mass index (LVMI) was significantly reduced at discharge, and the trend of LVMI reduction continued throughout follow-up (pre: 176±52 vs. follow-up: 114±31 g/m2, P<0.001) (Figure 2E,F). The mean reduction in preoperative LVMI and that obtained at follow-up was 33±24%. The LVEF significantly increased from the preoperative value to that of follow-up (pre: 60.9±13.3% vs. follow-up 65.5±8.9%, P=0.013) (Figure 2G,H).

Echocardiographic data recorded at 3 time-points: preoperatively, at discharge, and at follow-up. (A,B) Mean transvalvular/transprosthetic pressure gradients. (C,D) Effective orifice area index. (E,F) Left ventricular mass index. (G,H) Left ventricular ejection fraction. The data of each patient are colored green (moderate PPM and non-PPM) or red (severe PPM). *P<0.05. PPM, prosthesis-patient mismatch.
PPM was documented in 25 patients (31%) and of these 25, severe PPM with an EOAI <0.65 cm2/m2 occurred in 4 patients (5%) at discharge. Of the 4 patients with severe PPM, late death occurred in 1 patient with unknown cause 10 years postoperatively. The other 3 patients with severe PPM were alive at the 13-, 12-, and 11-year follow-up, respectively. Of the 3 surviving patients with severe PPM, intestinal bleeding occurred in 1 patient 6 years postoperatively. Multivariable analysis showed that PPM at discharge was not an independent risk factor for late death, MAVRE, or heart failure. The significant reduction in LVMI values (compared with preoperative values) observed at follow-up was similar between patients with and without PPM (30±26% vs. 31±19%, P=0.857). The rates of cardioprotective drug usage at discharge did not differ between the PPM and non-PPM groups.
Regarding the effect of the severity of PPM on echocardiographic variables, there was no difference in the reduction of LVMI between the moderate and severe PPM groups (24±28% vs. 45±9%, P=0.227) with an equal interval between surgery and follow-up echocardiography (100±37 vs. 122±44 months, P=0.392). The LVEF and mean transprosthetic pressure gradients at follow-up were similar between the moderate and severe PPM groups (61.7±13.2% vs. 67.0±3.0%, P=0.265, 21.3±10.7 mmHg vs. 27.7±8.3 mmHg, P=0.379).
The principal finding in this study was that AVR using the 17-mm Regent valve had acceptable long-term clinical and hemodynamic outcomes. Although 31% of patients showed PPM at discharge, significant LVMI reduction was observed during follow-up, regardless of postoperative PPM.
The surgical options for AVR in patients with a small aortic annulus (<19 mm) include implantation of a 16- or 17-mm mechanical valve, use of a stentless valve, or an annulus enlargement procedure using a larger prosthesis. Although experienced surgeons at high-volume centers advocate aortic annulus enlargement to avoid PPM, we do not routinely perform this procedure because it is time-consuming and technically more challenging in elderly patients. Kulik et al reported that aortic annulus enlargement scored over AVR alone in reducing postoperative transprosthetic gradients; however, this strategy did not improve long-term clinical outcomes.12
The primary drawback associated with implantation of a small-sized valve is persistently increased left ventricular workload. Pibarot et al reported that the ideal EOAI (which correlates with the postoperative transvalvular gradient) should exceed 0.85 cm2/m2 to avoid a residual pressure gradient.2 Based on information available in the Society of Thoracic Surgeons Adult Cardiac Surgery Database, the rate of moderate or severe PPM in patients undergoing isolated surgical AVR is as high as 65%.13 Several authors have investigated this issue and reported that the effect of PPM on clinical outcomes after AVR depends on the severity of PPM, age, sex, body mass index (BMI), and left ventricular function.3,14,15 Bilkhu et al reported that the data regarding the association between PPM and late death are applicable only to patients aged <70 years, those with BMI <30 kg/m2, and those with LVEF <50%.16 Price et al reported that the negative effect of PPM on late death depends on a patient’s age at the time of the operation, suggesting that PPM did not affect late survival in patients aged >70 years.17 Similarly, Mohty et al investigated 2,576 patients and reported that an increase in late deaths in patients with severe PPM was only observed in patients aged <70 years.18
The long-term efficacy and safety of the 17-mm bileaflet mechanical valve remain controversial. Previous studies investigating the utility of the 17-mm mechanical valve have had a mean follow-up period of 33–53 months.5–8 Ogawa et al investigated elderly patients aged >80 years with a small aortic annulus who underwent AVR with the 17-mm St. Jude Medical Regent prosthetic valve and reported satisfactory outcomes in these patients over a mean follow-up of 40 months.7 Hu et al investigated the effect of moderate PPM on clinical outcomes in patients undergoing implantation with the 17-mm Regent valve and observed that moderate PPM was associated with higher late mortality in patients with left ventricular dysfunction.8 Prifti et al reported that clinical and hemodynamic outcomes in patients undergoing AVR with the 17-mm valve were comparable to those observed in patients undergoing AVR with aortic annulus enlargement and implantation of a 19-mm valve.19 However, these were relatively small-sized studies, and the follow-up was <5 years. Our study investigated the long-term clinical and hemodynamic outcomes of AVR with the 17-mm mechanical valve in 80 patients. Low rates of linearized long-term morbidity were observed over a median follow-up of 8.7 years.
LVMI reduction is commonly used as an indicator of relief of left ventricular outflow tract obstruction after AVR. Interestingly, in our study, LVMI reduction observed at follow-up was similar between patients with and without PPM. Price et al reported that elderly patients (aged >70 years) showed lesser LVMI reduction after AVR than younger patients.17 In our series, 75% of patients were aged ≥70 years, which might have affected LVMI reduction and consequently led to similar LVMI reduction rates between patients with and without PPM. Severe PPM occurred in only 5% of the patients in our study, which is not expected to result in a significant difference in survival or late mortality rates between patients with and without PPM.
Comparison of hemodynamic performance between TAVR and surgical AVR has recently been gaining much attention. Pibarot et al reported that PPM is more common and more severe after surgical AVR than after TAVR in patients showing a high surgical risk.20 Furthermore, even in patients with a small annulus, the incidence of severe PPM is lower in patients undergoing TAVR than in those undergoing surgical AVR.21,22 Considering the superior hemodynamic performance of TAVR compared with surgical AVR, TAVR may be preferred as the potential first-line therapy in patients with a small aortic annulus.21 However, to date, limited data are available regarding post-TAVR hemodynamics in patients with an annulus size <19 mm, and whether TAVR is the optimal approach in such patients remains unclear.23
In the multivariable analysis, larger BSA and postoperative PPM had the tendency to lower the late mortality rate as shown in Table 3, although this effect was not statistically significant. One possible explanation is that frailty status, rather than PPM, affected the late outcomes. Frailty is reportedly associated with worse late outcomes after valve surgery.24 This study mainly comprised elderly and lean women as shown in Table 1, so we believe that frail patients with low activity were included to some degree. One of the phenotypes of frailty is shrinking, including weight loss and sarcopenia.25 Presence of frailty associated with BSA, weight, nutritional status, and muscular volume might influence late outcomes more than PPM in a limited patient population study such as ours. In addition, the severity of postoperative PPM was mostly moderate, which might have affected the outcomes to a lesser extent.
A mechanical valve typically necessitates lifelong anticoagulation, which is associated with a risk of bleeding and thromboembolism. In our case series, major bleeding events and thromboembolism occurred in 3 patients each during follow-up, with a linearized rate of 0.5% per patient-year. These results are comparable with a bleeding complication rate of 0.4% per patient-year and thromboembolism rate of 0.6% per patient-year among 1,133 patients undergoing AVR with a bioprosthesis in a study reported by Aupart et al.26 McClure et al reported similar mortality rates at late follow-up between mechanical and biological valve groups despite an increase in the rate of major bleeding events with mechanical valves.27 Although current guidelines recommend the use of a bioprosthesis in elderly patients, a certain percentage of elderly patients continue to receive mechanical valves.7 Vicchio et al reported that despite the need for lifelong anticoagulation associated with mechanical valves, mechanical valves scored over biological valves in octogenarians, with regard to survival rates.28 Hammermeister et al reported that 15 years after AVR, survival rates were better with a mechanical valve than with a bioprosthetic valve, secondary to primary valve failure associated with a bioprosthetic valve.29 Thus, in our view, implantation of the 17-mm mechanical valve is feasible in elderly patients with a small aortic annulus to avoid aortic annulus enlargement and to simplify surgery and minimize its invasiveness.
Study LimitationsThe following are the limitations of this study: (1) retrospective study design; (2) follow-up echocardiography not performed in all patients, which may have resulted in inaccurate interpretation of hemodynamic data; (3) relatively low incidence of PPM, particularly of severe PPM, making it is difficult to conclusively establish the effect of PPM on long-term outcomes; (4) study group primarily comprising Japanese women with a relatively small stature, so applicability of our findings to Western populations remains unclear; and (5) most of the patients in our study were aged >70 years with reduced daily activity owing to aging, which could have affected assessment of symptoms at follow-up.
Our study showed that surgical AVR with the 17-mm Regent prosthetic valve achieved acceptable clinical outcomes with low rates of valve-related complications and significant long-term LVMI reduction. Although the indications for TAVR might be expanded to patients with a small annulus, the 17-mm prosthesis is an acceptable alternative in selected patients with a small aortic annulus (<19 mm).
All authors report no conflicts of interest, grant or sources of funding to disclose.
Institutional Review Board of Jichi Medical University, S17-029.