Abstract
Background:
The vasopressin type-2 receptor antagonist tolvaptan is an essential tool in the management of decompensated heart failure (HF) in the inpatient setting for short-term use with careful monitoring. There is conflicting evidence, however, for its long-term use.
Methods and Results:
In this prospective, multi-center, open-labeled, randomized control trial, Assessment of QUAlity of life during long-term treatment of ToLVaptan in refractory HF (AQUA-TLV study), patients with congestive HF refractory to furosemide ≥60 mg/day were randomly assigned to a control group or tolvaptan add-on group and followed for 6 months, after confirmation of baseline urine osmolality ≥350 mOsm/L. Twenty-nine patients (median age, 60 years; 22 male) were enrolled and assigned to a control group (n=16) or a tolvaptan group (n=13). Minnesota Living with Heart Failure Questionnaire score improved significantly in the tolvaptan group (from 58 to 10, P=0.030). In the tolvaptan group, diuretics dose reduced (P=0.001), serum creatinine decreased (P=0.040), and hyponatremia tended to improve (P=0.12). The tolvaptan group had a lower HF readmission rate compared with the control group (0.213 vs. 1.242 events/year, P=0.13).
Conclusions:
Six-month tolvaptan therapy improved quality of life and renal function and reduced HF readmissions, when given to the estimated responders (UMIN Clinical Trial Registry Number: UMIN 000009604).
Although conventional diuretics including loop diuretics have been essential tools to manage patients with decompensated heart failure (HF), they have various limitations including stimulation of the renin-angiotensin-aldosterone system, intravascular hypovolemia, worsening renal function, and sodium and potassium wasting in the urine.1,2
Furthermore, patients with advanced HF often experience refractoriness to conventional diuretics.
Tolvaptan, a vasopressin type-2 antagonist, has been widely used for the past decade as a novel and unique aquaretic agent, because it overcomes various disadvantages of conventional diuretics by ameliorating congestive symptoms and improving hyponatremia while maintaining hemodynamics and renal function. Its use, however, has been proven only in a strictly monitored, short-term inpatient setting.3
Many patients with advanced HF have reduced quality of life (QOL) owing to frequent readmissions from worsening congestion, despite use of a considerable amount of diuretics. In contrast to the favorable evidence for the use of short-term tolvaptan therapy, the implications of long-term tolvaptan therapy remains controversial.4
Several retrospective observational studies with small sample sizes showed favorable outcomes for long-term tolvaptan therapy,5–8
whereas a large-scale randomized control trial (RCT) could not demonstrate an advantage of tolvaptan over conventional diuretics.9
Our team previously demonstrated that elevated baseline urine osmolality in the fasting condition (≥350 mOsm/L) was a predictive marker of tolvaptan responders. These patients had an increase in urine output following tolvaptan treatment, likely due to preserved function of the collecting duct of the kidney.10
We hypothesized that long-term tolvaptan therapy might be effective when tolvaptan is given in the aforementioned defined responders. In this prospective, multi-center, open-labeled, RCT, Assessment of QUAlity of life during long-term treatment of ToLVaptan in refractory HF (AQUA-TLV study), we investigated QOL during 6 months of therapy in patients with advanced HF and baseline urine osmolality ≥350 mOsm/L, randomized into a control arm with conventional diuretic therapy, or a tolvaptan add-on arm (UMIN Clinical Trial Registry Number: UMIN 000009604).11
Methods
Patient Selection
In this prospective RCT, patients were considered to be enrolled from 8 institutions when they had HF refractory to conventional medical therapy at the time of admission between March 2013 and December 2014. Patients were randomized into the control group (conventional medical therapy without tolvaptan) and the tolvaptan group (tolvaptan add-on) and followed for 6 months to observe QOL. Inclusion and exclusion criteria are detailed previously,11
but are briefly stated in the following section. The study protocol was approved by the Ethics Committee of the Graduate School of Medicine, University of Tokyo and other institutes (UMIN Clinical Trial Registry Number: UMIN 000009604). Informed consent was obtained from all patients before enrollment.
Inclusion Criteria
Patients who were hospitalized due to decompensated HF, with New York Heart Association (NYHA) functional class III or IV, refractory to the conventional diuretics equivalent to furosemide ≥60 mg/day, and with a history of at least 1 HF hospitalization in the past 6 months, were included in this study.
Exclusion Criteria
Patients with general contraindications to tolvaptan therapy were excluded, that is, those with end-stage renal function on hemodialysis, hypernatremia with serum sodium >145 mEq/L, on mechanical circulatory support, or with impaired consciousness.
Urine Osmolality
We enrolled patients with baseline urine osmolality ≥350 mOsm/L, considered to be a predictor of response to tolvaptan therapy, as demonstrated in a previous study (response was defined as any increase in urine output following tolvaptan treatment).10
Study Design
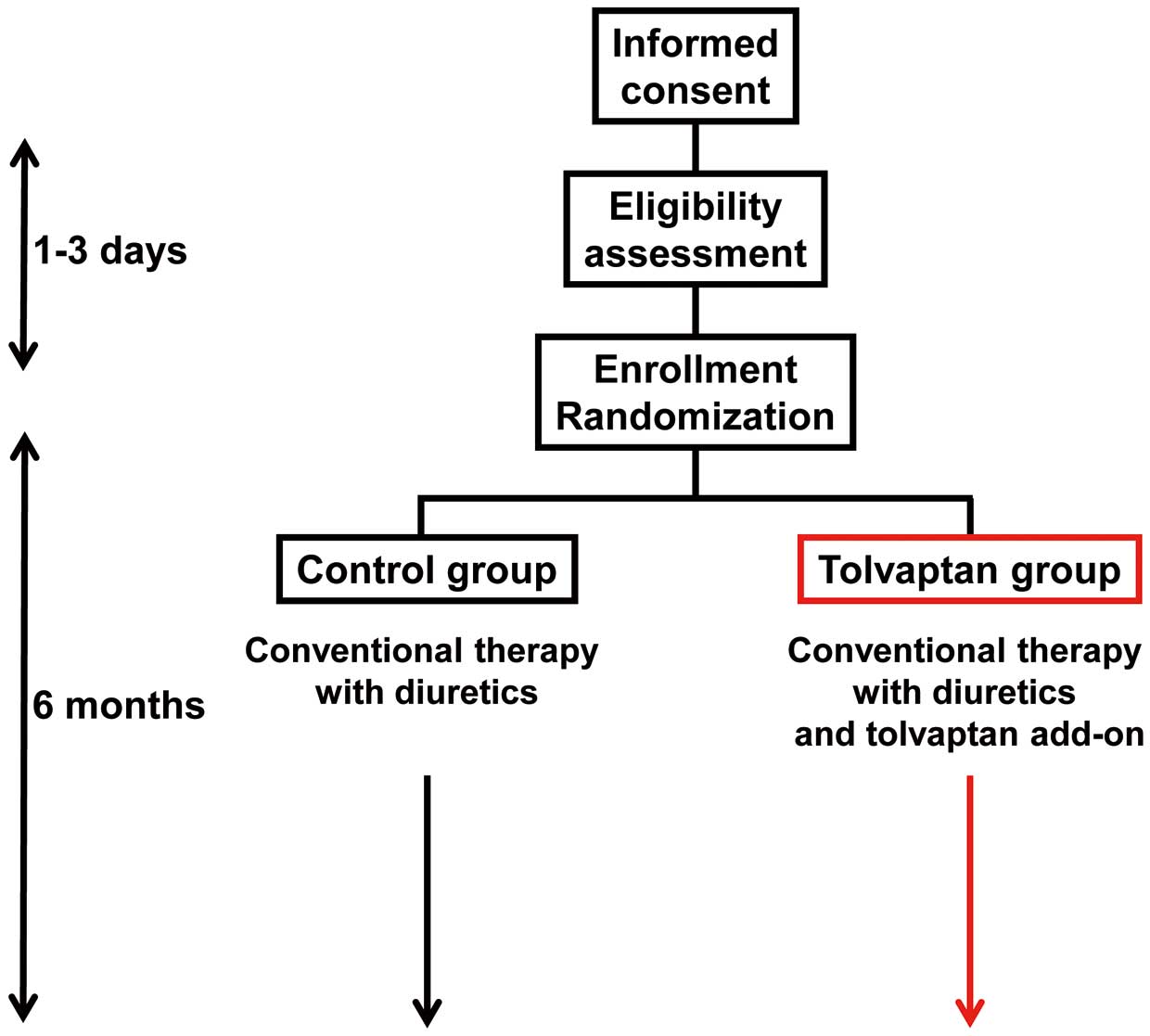
Following informed consent, patients received clinical examinations to confirm their eligibility during a 1–3-day observational period (Figure 1), when baseline characteristics including Minnesota Living with Heart Failure Questionnaire (MLHFQ) and transthoracic echocardiography were obtained. After confirmation of eligibility, patients were randomly assigned into the 2 arms: the tolvaptan arm or the control arm, and followed for 6 months. Target sample size was calculated as 100 patients (50 vs. 50) for the condition of α error probability 0.05, power 0.80, and effect size 0.5.
Endpoints
The primary endpoint was improvement in QOL assessed on a reduction in MLHFQ score. The secondary endpoints were improvements in other clinical outcomes including serum sodium and creatinine, body weight, left ventricular ejection fraction (LVEF), plasma B-type natriuretic peptide (BNP), and HF readmissions.
Statistical Analysis
Statistical analysis was performed with SPSS Statistics 22 (SPSS, Chicago, IL, USA). Two-sided P<0.05 was considered statistically significant. Continuous variables are expressed as median (IQR) and were compared between groups using the Mann-Whitney U-test, considering the relatively small sample size. Trends of continuous variables were compared using the Friedman test.
Freedom from HF readmission was compared between 2 groups using the log-rank test. Event rates are expressed as per patient-year and were compared between 2 groups on negative binominal regression analysis.
Results
Baseline Characteristics
In total, 29 patients were enrolled (Table). Median age was 60 years (IQR, 46–74 years) and 22 were male. All patients had at least 1 history of HF admission in the past 6 months. All patients had serum sodium ≤145 mEq/L, urine osmolality ≥350 mOsm/L, and were on furosemide ≥60 mg/day. Eleven patients (38%) had serum sodium <135 mEq/L. Median LVEF was 21% (IQR, 15–30%).
Table.
Baseline Characteristics
| |
Total
(n=29) |
Tolvaptan
(n=13) |
Control
(n=16) |
P-value |
| Demographics |
| Age (years) |
60 (46–74) |
60 (48–79) |
62 (44–73) |
0.40 |
| Male |
22 (76) |
9 (69) |
13 (81) |
0.45 |
| Body weight (kg) |
63.6 (51.2–69.5) |
60.9 (52.1–68.8) |
65.1 (50.8–69.7) |
0.69 |
| Body mass index (kg/m2) |
22.4 (21.0–25.3) |
24.0 (20.8–26.9) |
22.4 (20.8–24.9) |
0.61 |
| Ischemic etiology for HF |
6 (21) |
2 (15) |
4 (25) |
0.53 |
| HF readmission before 6 months |
2 (1–3) |
2 (2–3) |
2 (1–2) |
0.12 |
| Comorbidities |
| History of stroke |
5 (17) |
2 (15) |
3 (19) |
0.81 |
| Hypertension |
13 (45) |
7 (54) |
6 (38) |
0.38 |
| Diabetes mellitus |
13 (45) |
6 (46) |
7 (44) |
0.60 |
| Dyslipidemia |
13 (45) |
6 (46) |
7 (44) |
0.60 |
| Hyperuricemia |
16 (55) |
7 (54) |
9 (56) |
0.60 |
| Chronic kidney disease |
17 (59) |
8 (62) |
9 (56) |
0.54 |
| Laboratory data |
| Urine osmolality (mOsm/L) |
481 (413–579) |
423 (391–586) |
482 (425–581) |
0.32 |
| Urine osmolality ≥350 mOsm/L |
29 (100) |
13 (100) |
16 (100) |
– |
| Serum sodium (mEq/L) |
137 (134–139) |
136 (134–140) |
137 (134–139) |
0.97 |
| Serum sodium <135 mEq/L |
10 (34) |
5 (38) |
5 (31) |
0.68 |
| Serum potassium (mEq/L) |
4.1 (3.8–4.4) |
4.1 (3.9–4.4) |
4.0 (3.7–4.4) |
0.54 |
| Serum creatinine (mg/dL) |
1.3 (1.1–1.6) |
1.2 (1.0–1.8) |
1.3 (1.2–1.5) |
0.81 |
| Serum creatinine >1.5 mg/dL |
10 (34) |
6 (46) |
4 (25) |
0.23 |
| Plasma BNP (log pg/mL) |
2.8 (2.4–3.0) |
2.8 (2.4–3.1) |
2.7 (2.4–2.9) |
0.41 |
| Hemodynamics |
| SBP (mmHg) |
96 (84–101) |
96 (84–123) |
96 (86–101) |
0.52 |
| DBP (mmHg) |
59 (50–65) |
59 (50–68) |
58 (50–61) |
0.52 |
| Heart rate (beats/min) |
71 (65–81) |
70 (59–85) |
71 (70–79) |
0.63 |
| LVEF (%) |
21 (15–30) |
20 (14–62) |
21 (16–29) |
0.98 |
| MLHFQ score |
58 (35–69) |
58 (36–76) |
55 (31–68) |
0.63 |
| Medication |
| Tolvaptan (mg/day) |
– |
3.75 (3.75–7.5) |
0 |
– |
| Furosemide (mg/day) |
60 (60–80) |
60 (60–90) |
70 (60–80) |
0.65 |
| β-blocker |
25 (86) |
10 (77) |
15 (94) |
0.19 |
| ACEI/ARB |
23 (79) |
10 (77) |
13 (81) |
0.78 |
| Aldosterone antagonist |
22 (76) |
9 (69) |
13 (81) |
0.45 |
| I.v. inotrope infusion |
7 (24) |
3 (23) |
4 (25) |
0.90 |
Data given as median (IQR) or n (%). *P<0.05 (Mann-Whitney U-test or Fisher’s exact test. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; DBP, diastolic blood pressure; HF, heart failure; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; SBP, systolic blood pressure.
Patients were randomly assigned to the tolvaptan group (n=13) or to the control group (n=16). There were no significant differences in baseline patient characteristics (P>0.05 for all). The tolvaptan group received a median tolvaptan dose 3.75 mg/day (IQR, 3.75–7.5 mg/day). During the 6-month treatment period, no patients had adverse events associated with tolvaptan.
Primary Outcome
QOL was assessed on MLHFQ score, which decreased (i.e., improved) significantly in the tolvaptan group (P=0.030), whereas it remained unchanged in the control group (P=0.23;
Figure 2). As a result, the score was lower in the tolvaptan group compared with the control group at 6 months (P=0.040).
Secondary Outcomes (Clinical Variables)
Loop diuretic dose was reduced significantly following tolvaptan treatment (P=0.001), but remained unchanged in the control group (P=0.68;
Figure 3A). The loop diuretics dose was expressed as an equivalent dose of furosemide, when other diuretics were used. Serum sodium level tended to increase in the tolvaptan group (P=0.12), whereas it remained unchanged in the control group (P=0.78;
Figure 3B). As a result, the tolvaptan group had lower furosemide dose (40 vs. 80 mg/day) and higher serum sodium (140 vs. 137 mEq/L) compared with the control group at 6 months (P=0.003 and P=0.024, respectively).
Body weight remained unchanged in both groups during the 6-month treatment period (P>0.05 for both;
Figure 3C). Serum creatinine decreased significantly in the tolvaptan group (P=0.040), whereas it remained unchanged in the control group (P=0.53;
Figure 3D). As a result, serum creatinine tended to be lower in the tolvaptan group compared with the control group (1.0 vs. 1.3 mg/dL, P=0.083).
Plasma BNP reduced significantly in the tolvaptan group (P=0.026) whereas it remained unchanged in the control group (P=0.51;
Figure 3E). Statistically, however, the levels were similar between the 2 groups at 6 months (2.46 vs. 2.34 log pg/mL, P=0.66). LVEF remained unchanged in both group (P>0.05 for both;
Figure 3F).
Secondary Outcomes (HF Readmission)
Five patients were not discharged due to 3 transfers, 1 death, and 1 LV assist device (LVAD) implantation. The remaining 24 patients were discharged from the index hospitalization. Of those, 1 patient was transferred and another received LVAD implantation. The other 22 patients completed 6 months of follow-up.
Six-month freedom from HF readmission was numerically higher in the tolvaptan group (90% vs. 54%, P=0.092;
Figure 4A). HF readmission rate was numerically lower in the tolvaptan group (0.213 vs. 1.242 events/patient-year; incidence rate ratio, 5.71; 95% CI: 0.61–53.2; P=0.13;
Figure 4B).
Discussion
In this prospective, multi-center, open-labeled, RCT, AQUA-TLV study, we investigated the impact of 6-month tolvaptan therapy on QOL and other clinical outcomes in NYHA functional class III or IV patients with symptomatic decompensated HF with a history of HF admission, and who were estimated to be responders to tolvaptan, defined as baseline urine osmolality ≥350 mOsm/L.
The main findings are as follows: (1) all 13 patients in the tolvaptan group had no adverse events associated with tolvaptan during the 6-month treatment period; (2) in the tolvaptan group, QOL assessed on MLHFQ score improved during the 6-month treatment period and was more improved at 6 months compared with the control group; (3) in the tolvaptan group, the dose of furosemide reduced, serum creatinine recovered, and hyponatremia tended to improve, whereas these remained unchanged in the control group; and (4) the tolvaptan group had fewer HF readmissions compared with the control group.
Inclusion/Exclusion Criteria
In the EVEREST study, long-term tolvaptan therapy had a negative impact,9
but often, in real-world daily practice, a favorable clinical outcome is seen. We hypothesized that the different outcomes might come from patient selection, and that optimal patient selection might be the key to successful tolvaptan therapy.12
To demonstrate the advantage of tolvaptan vs. conventional diuretics, which is also an essential tool to treat mild-moderate congestion, we believe that patients should be “adequately sick” in their HF in order to receive benefit from tolvaptan. Less sick patients would recover solely on conventional diuretics.
Given the previous findings that patients receiving >80 mg/day furosemide had a significantly worse prognosis,13,14
we enrolled those with congestive HF refractory to ≥60 mg/day furosemide. Approximately 40% had serum sodium <135 mEq/L in this study (although we enrolled patients irrespective of the existence of hyponatremia), whereas the EVEREST trial had only 11% with serum sodium <135 mEq/L.9
In contrast, we believe that preserved kidney function is essential for an optimal response to tolvaptan, given the biological mechanism in which tolvaptan affects vasopressin type-2 receptor located on the surface of the collecting duct and inhibits the active re-absorption of free water. We previously showed that elevated urine osmolality and urine aquaporin-2, which indicate the preserved function of the collecting duct to concentrate urine in a fasting condition, was associated with better prognosis during tolvaptan therapy.5
Recent post-marketing surveillance (SMILE study) also showed that the clinical responders whose urine output increased following tolvaptan had lower all-cause mortality compared with the clinical non-responders whose urine output remained unchanged despite tolvaptan.15
Therefore, in order to select appropriate responders, we set the inclusion criterion of baseline urine osmolality ≥350 mOsm/L in this study. In summary, we did our best to enroll suitable patients who would derive optimal clinical benefit from tolvaptan.
Improvement of Hyponatremia
As already shown in many studies,16
hyponatremia improved during 6-month tolvaptan therapy in this study, probably due to significant reduction in the dose of loop diuretics, excretion of free water by vasopressin type-2 receptor blocking, and amelioration of congestion. Hyponatremia is a marker of HF severity as well as a driver of poor outcomes.17
Improvement of hyponatremia is associated with better clinical outcomes in HF patients in general.18
In the EVEREST trial subanalysis, an improvement of hyponatremia by tolvaptan in the subgroup with serum sodium <130 mEq/L was associated with reduced cardiovascular morbidity and mortality.19
The recently conducted SMILE study also showed that the correction of hyponatremia was associated with a higher survival rate.15
Therefore, improvement of hyponatremia in the tolvaptan arm would be one of the major contributors to reduced HF readmissions and improved QOL.
Improvement of Renal Function
Renal dysfunction is another well-known risk factor of worse prognosis in general and in HF patients in particular.20
We recently performed a meta-analysis in which the reduction in diuretic dose given concomitantly with tolvaptan had a linear correlation with the reduction of serum creatinine.4
Particularly, the dose of furosemide remained high (>70 mg/day) during the tolvaptan therapy in the EVEREST trial, and serum creatinine was higher in the tolvaptan arm compared with the control arm.9
In the EVEREST trial, subanalysis patients with higher serum osmolality (≥300 mOsm/L) at discharge, probably due to too much dehydration by tolvaptan and furosemide, had a higher serum creatinine after discharge and worse outcomes compared with the normal serum osmolarity group.21
Improvement in renal function by the dose reduction of loop diuretics following tolvaptan treatment may be another major contributor to favorable clinical outcomes in the tolvaptan arm. Tolvaptan may also have the potential to preserve renal function by inhibiting the proliferation of epithelial cells, as seen in patients with polycystic kidney disease,22
although it is uncertain whether it is applicable to the low-dose tolvaptan given in this study (3.75–7.5 mg).
In the clinical responders, it is well known that urine output increases following tolvaptan treatment, and congestion is ameliorated. As a result, we can prevent the recurrence of decompensated congestive HF. Both improvement of hyponatremia and preservation of renal function would also have a favorable effect on the prevention of worsening HF during long-term follow-up. Both relief of daily HF symptom and prevention of worsening HF would improve QOL.
Limitations and Future Perspective
There are several limitations in this study. We could not complete the target enrollment of 100 patients11
because clinicians tended to hesitate to enroll patients into the RCT given their favorable experience with long-term tolvaptan. Despite a moderate sample size, we still observed significant advantages of tolvaptan therapy in the primary endpoint, although several secondary outcomes did not reach statistical significance. This study was open-labeled, similar to other tolvaptan studies,4
because blinded trials are not realistic considering the strong aquaretic effect of tolvaptan. Body weight remained unchanged in both groups. We believe that body weight may not be a good indicator of fluid retention for long-term follow-up. Loss of body weight may instead represent cardiac cachexia. The present trial involved 6 months of follow-up, considering the natural course of this sick population, therefore the longer term effects of tolvaptan remain uncertain. Considering the severity of the enrolled cohort, we did not fix the doses of other medications. We cannot completely exclude the effect of other medications, although all enrolled patients were already well-treated at baseline.
Although the short-term efficacy of tolvaptan is well known, we could not observe significant differences at 4 weeks between the 2 arms. Given the relatively small sample size, the very sick population, and the low dose of tolvaptan, it might have taken time (over months) to observe a significant effect of tolvaptan.
Because we do not have data on the patients who were excluded due to lower baseline urine osmolality, we do not know the prevalence of responders to tolvaptan in this study. In our previous study that enrolled a similar advanced HF cohort, a total of 61% were clinical responders.10
More responders might be found in less sick populations.
In contrast, we enrolled only 4 patients aged >80 years, whereas tolvaptan is used in a much older population with relatively less severe HF in real-world practice.15
Given that renal function depends on age, some non-responders may be included in such an aged population. Given the lower cost-effectiveness and higher readmission rate in the non-responders receiving tolvaptan, tolvaptan therapy is not recommended in such populations.
Conclusions
On comparison with a randomly assigned control group receiving conventional diuretic therapy, 6-month tolvaptan therapy improved QOL, recovered renal function, and reduced heart failure readmissions in decompensated HF patients, who were estimated to be responders to tolvaptan on urine osmolality.
Funding
This study was supported by a Grant-in-Aid from Japan Heart Foundation.
Conflict of Interest
T.I. receives financial support from Teraura-Sayoko Memorial Scholarship Foundation. K.K. receives a lecture fee from Otsuka Pharmaceutical. I.K. receives scholarship donation from Otsuka Pharmaceutical. H.T. receives honoraria from Otsuka Pharmaceutical.
N.H., I.K., and K.K. are members of
Circulation Reports
’ Editorial Team. The other authors declare no conflicts of interest.
References
- 1.
Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 2002; 105: 1348–1353.
- 2.
Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure: Activation of the neurohumoral axis. Ann Intern Med 1985; 103: 1–6.
- 3.
Kinugawa K, Sato N, Inomata T. Effects of tolvaptan on volume overload in patients with heart failure. Int Heart J 2018; 59: 1368–1377.
- 4.
Imamura T, Kinugawa K. Update of acute and long-term tolvaptan therapy. J Cardiol 2019; 73: 102–107.
- 5.
Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, Hatano M, et al. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J 2014; 78: 2240–2249.
- 6.
Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Ishikawa S, et al. Clinical benefit of tolvaptan in patients with acute decompensated heart failure and chronic kidney disease. Heart Vessels 2016; 31: 1643–1649.
- 7.
Nakano Y, Mizuno T, Niwa T, Mukai K, Wakabayashi H, Watanabe A, et al. Impact of continuous administration of tolvaptan on preventing medium-term worsening renal function and long-term adverse events in heart failure patients with chronic kidney disease. Int Heart J 2018; 59: 105–111.
- 8.
Nakamura M, Sunagawa O, Kinugawa K. Tolvaptan improves prognosis in responders with acute decompensated heart failure by reducing the dose of loop diuretics. Int Heart J 2018; 59: 87–93.
- 9.
Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007; 297: 1319–1331.
- 10.
Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients: Association between non-responders and chronic kidney disease. Circ J 2013; 77: 397–404.
- 11.
Imamura T, Kinugawa K, Ohtani T, Sakata Y, Higo T, Kinugawa S, et al. Assessment of quality of life during long-term treatment of tolvaptan in refractory heart failure: Design and rationale of the AQUA-TLV study. Int Heart J 2014; 55: 264–267.
- 12.
Imamura T. Aquaporin-2-guided tolvaptan therapy in patients with congestive heart failure accompanied by chronic kidney disease. Int Heart J 2014; 55: 482–483.
- 13.
Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006; 97: 1759–1764.
- 14.
Imamura T, Kinugawa K. Prognostic impacts of hyponatremia, renal dysfunction, and high-dose diuretics during a 10-year study period in 4,087 Japanese heart failure patients. Int Heart J 2016; 57: 657–658.
- 15.
Kinugawa K, Sato N, Inomata T, Yasuda M, Shimakawa T, Fukuta Y. Real-world effectiveness and tolerability of tolvaptan in patients with heart failure: Final results of the Samsca Post-Marketing Surveillance in Heart Failure (SMILE) Study. Circ J 2019; 83: 1520–1527.
- 16.
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006; 355: 2099–2112.
- 17.
Leier CV, Dei Cas L, Metra M. Clinical relevance and management of the major electrolyte abnormalities in congestive heart failure: Hyponatremia, hypokalemia, and hypomagnesemia. Am Heart J 1994; 128: 564–574.
- 18.
Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009; 122: 857–865.
- 19.
Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 2013; 19: 390–397.
- 20.
Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005; 46: e1–e82.
- 21.
Cook TD, Greene SJ, Kalogeropoulos AP, Fonarow GC, Zea R, Swedberg K, et al. Temporal changes in postdischarge mortality risk after hospitalization for heart failure (from the EVEREST Trial). Am J Cardiol 2016; 117: 611–616.
- 22.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367: 2407–2418.