2019 Volume 1 Issue 3 Pages 131-136
2019 Volume 1 Issue 3 Pages 131-136
Background: The aim of this study was to determine adequate indication for transcatheter aortic valve replacement (TAVR). We analyzed risk factors of surgical aortic valve replacement (SAVR) not only for mortality, but also for morbidity, including long hospital stay (≥90 days) and patient activity at discharge, in patients who underwent SAVR for aortic stenosis (AS).
Methods and Results: Using the Japan Adult Cardiovascular Surgery Database (JCVSD), 13,961 patients with or without coronary artery bypass grafting who underwent elective SAVR for AS were identified from January 2008 to December 2012. The hospital mortality rate was 3.1%. The percentage of patients who had long hospital stay (≥90 days) and who had moderately or severely decompressed activity at discharge (modified Rankin scale ≥4) was 2.9% and 6.5%, respectively. Eleven and 20 preoperative predictors of hospital mortality and morbidity, respectively, including long hospital stay and compromised status at discharge, were identified. Based on these risk factors, the risk model predicted hospital mortality (area under the curve [AUC], 0.732) and morbidity (AUC, 0.694).
Conclusions: Using JCVSD, a risk model of SAVR was developed for AS. This model can identify patients at high risk not only for mortality, but also for mortality and morbidity, including long hospital stay and status at discharge.
Transcatheter aortic valve replacement (TAVR) has become the alternative treatment modality for aortic stenosis (AS) in patients ineligible for surgical aortic valve replacement (SAVR), who have an extremely high risk for open surgery.1,2 Currently, the indication for TAVR tends to extend to those with a moderate risk or to the younger population. The durability of the TAVR prosthesis has not fully been determined and is thought to be inferior to that of surgical aortic valve replacement (SAVR). In the context of increasing average life expectancy, TAVR is not indicated only according to advanced age. Moreover, minimally invasive cardiac surgery and newly developed devices, such as the sutureless valve,3 might contribute to improve the clinical outcome of SAVR, with promising durability of the prosthetic valve. Therefore, the criteria for SAVR or TAVR for AS continue to be a concern.
Of the various factors affecting the selection of procedure for severe AS, postoperative functional status is one of the most important issues. Longer hospital stay might result in impairment of functional status,4 and compromised general condition at discharge might outweigh the benefit of recovering cardiac function. TAVR is superior to SAVR regarding early recovery of functional status and shorter hospital stay after surgery. But, given the aforementioned reasons, we consider it important to clear early recovery of general condition and duration of hospital stay in addition to factors that affect these variables after SAVR.
In the present study, we performed a large-scale analysis of 13,961 patients who had elective SAVR with or without coronary artery bypass grafting (CABG) for AS using the Japan Adult Cardiovascular Surgery Database (JCVSD). The aim of this study was to determine the contemporary clinical outcome of SAVR and the risk factors of SAVR not only for mortality, but also for morbidity, including long hospital stay (≥90 days) and patient activity at discharge.
The JCVSD was initiated in 2000 to estimate surgical outcomes after cardiovascular procedures in many centers throughout Japan. The JCVSD adult cardiovascular division currently captures clinical information from nearly half of all Japanese hospitals performing cardiovascular surgery. The data collection form has approximately 300 variables (definitions are available online at http://www.jacvsd.umin.jp), and these are almost identical to those in the Society of Thoracic Surgeons (STS) National Database (definitions are available online at http://sts.org). The JCVSD has developed software for a Web-based data collection system through which the data manager of each participating hospital electronically submits the data to the central office. Although participation in the JCVSD is voluntary, data completeness is a high priority. Accuracy of submitted data is maintained by a data audit that is achieved by monthly visits by administrative office members to the participating hospital to check data against clinical records. Validity of data is further confirmed by independent comparison of the volume of cardiac surgery at a particular hospital entered in the JCVSD with that reported to the Japanese Association for Thoracic Surgery annual survey.5 Informed consent to register clinical data in the JCVSD was obtained from each patient. The JCVSD Review Board approved the present study.
We examined 13,961 cases of elective SAVR for AS with or without CABG enrolled in this database between 1 January 2008 and 31 December 2012. Exclusion criteria were as follows: urgent/emergency/salvage surgery, concomitant cardiac surgery other than CABG, aortic regurgitation dominant (grade 3 or 4), rheumatic heart disease, active infective endocarditis, annular abscess, graft infection, Marfan syndrome, aortitis, Behcet’s disease, and previous AVR (mechanical or bioprosthesis). Records with missing data or out-of-range data for age, sex, or 30-day status were also excluded. After data cleaning, the population for this risk model analysis consisted of 13,961 patients.
DefinitionsMortality was defined as death at 30 days or before hospital discharge. Morbidity was defined as long hospital stay ≥90 days and/or daily activity that was moderately or severely compromised (modified Rankin scale 4 or 5) at discharge.
Statistical AnalysisVariables are expressed as mean±SD or percentage. To develop risk models of SAVR with or without CABG, we conducted multivariate stepwise logistic regression analysis for each outcome. Stability of the model was checked every time a variable was eliminated. When all statistically non-significant variables (P<0.10) had been eliminated from the model, the goodness of fit was evaluated. The area under the receiver operating characteristic curve (AUC) was used to assess how well the model could discriminate between patients who lived without morbidity from those who had died or were complicated by morbidity.
Baseline subject characteristics are listed in Table 1. Mean age was 74.2±8.4 years. Age was categorized into 6 groups as follows: age <60 years, 6%; 60–64 years, 8%; 65–69 years, 14%; 70–74 years, 22%; 75–79 years, 27%; and ≥80 years, 23%. A total of 1,542 patients (11.0%) were hemodialysis dependent and 431 (3.1%) had more than moderate chronic lung disease. A total of 1,286 patients (8.9%) also had peripheral artery disease (PAD). A total of 2,307 (16.5%) had congestive heart failure (CHF) in the 2 weeks before the operation, whereas 363 (2.6%) were in New York Heart Association (NYHA) class IV. Concomitant CABG was performed in 3,848 patients (27.6%), and 10,076 (72.2%) underwent isolated SAVR. Bioprosthesis were implanted in 10,076 patients (72.2%).
| SAVR (n=13,961) | |
|---|---|
| Age (years) | 74.2±8.4 |
| ≥80 | 23 |
| 75–79 | 27 |
| 70–74 | 22 |
| 65–69 | 14 |
| 60–64 | 8 |
| <60 | 6 |
| Female gender | 7,642 (54.7) |
| BMI ≥30 kg/m2 | 601 (4.3) |
| DM | 3,954 (28.3) |
| DM insulin use | 925 (6.6) |
| Renal dysfunction (Cr ≥2 mg/dL) | 1,995 (14.3) |
| Preoperative dialysis | 1,542 (11.0) |
| CLD ≥moderate | 431 (3.1) |
| History of stroke/TIA | 1,283 (9.2) |
| History of psychoneurotic disorder | 26 (0.2) |
| Liver cirrhosis (Child-Pugh B/C) | 19 (0.1) |
| Carotid stenosis | 697 (5.0) |
| Extracardiac vascular lesion | 1,536 (11.0) |
| PAD | 1,246 (8.9) |
| Concomitant malignant neoplasm | 292 (2.1) |
| Congestive heart failure | 2,307 (16.5) |
| Atrial fibrillation | 790 (5.7) |
| Previous cardiac surgery | 290 (2.1) |
| History of coronary intervention | 1,371 (9.8) |
| Coronary lesion ≥2 vessels | 2,658 (19.0) |
| LMT lesion | 674 (4.8) |
| LV function | |
| Medium (EF 0.3–0.6) | 3,977 (21.3) |
| Bad (EF <0.3) | 306 (2.2) |
| Concomitant mitral stenosis | 413 (3.0) |
| Concomitant MR ≥2 | 2,930 (21.0) |
| NHYA class 4 | 363 (2.6) |
| Preoperative shock | 43 (0.3) |
| Use of bioprosthesis | 10,076 (72.2) |
| Concomitant CABG | 3,848 (27.6) |
Data given as mean±SD, % or n (%). BMI, body mass index; CABG, coronary artery bypass grafting; CLD, chronic lung disease; Cr, creatinine; DM, diabetes mellitus; EF, ejection fraction; LMT, left main trunk; LV, left ventricular; MR, mitral regurgitation; NYHA, New York Heart Association; PAD, peripheral artery disease; SAVR, surgical aortic valve replacement; TIA, transient ischemic attack.
In-hospital outcomes are shown in Table 2. Thirty-day mortality and 30-day/in-hospital mortality rates were 2.0% and 3.1%, respectively. Long hospital stay (≥90 days) was observed in 407 patients (2.9%) for the following reasons: neurological causes, n=41 (10.1%); rehabilitation, n=165 (40.5%); transfer to a different department, n=88 (21.6%); assisted circulation, n=12 (2.9%); and other, n=174 (42.8%). Patient activity at discharge was as follows: 745 (5.3%) had moderate compromise (modified Rankin scale score 4: unable to walk without assistance and unable to attend to own bodily needs without assistance) and 164 (1.2%) had severe compromise (modified Rankin scale score 5: bedridden, incontinent, and required constant nursing care and attention). A total of 1,521 patients (10.9%) had complications of mortality and morbidity.
| n (%) or n | |
|---|---|
| Mortality | |
| 30-day mortality | 281 (2.0) |
| 30-day or in-hospital mortality | 435 (3.1) |
| Morbidity | |
| Hospital stay ≥90 days | 407 (2.9) |
| Neurological cause | 41 |
| Rehabilitation | 165 |
| Transfer to a different department | 88 |
| Assisted circulation | 12 |
| Others | 174 |
| Status at discharge | |
| Moderately compromised (m-Rankin 4†) | 745 (5.3) |
| Severely compromised (m-Rankin 5‡) | 164 (1.2) |
†Unable to walk without assistance and unable to attend to own bodily needs without assistance; ‡bedridden, incontinent, and requires constant nursing care and attention. m-Rankin, modified Rankin score; SAVR, surgical aortic valve replacement.
Multivariate predictors of operative mortality are listed in Table 3. For hospital mortality, the following 11 factors were identified: age category (odds ratio [OR], 1.26), preoperative dialysis (OR, 3.44), chronic lung disease (OR, 1.83), non-cardiac vascular disease (OR, 2.15), CHF (OR, 1.37), atrial fibrillation (AF; OR, 2.08), NYHA class ≥3 (OR, 1.52), history of stroke/transient ischemic attack (TIA; OR, 1.38), concomitant CABG (OR, 1.38), shock (OR, 3.97), and concomitant mitral regurgitation (OR, 1.52). AUC was 0.732 (P<0.001; Figure A). For mortality/morbidity, the following 20 factors were identified: age category (OR, 1.24), preoperative dialysis (OR, 1.86), chronic lung disease (OR, 1.83), non-cardiac vascular disease (OR, 1.49), CHF (OR, 1.42), AF (OR, 1.69), NYHA class ≥3 (OR, 1.27), ≥4 (OR, 2.10), history of stroke/TIA (OR, 1.32), concomitant CABG (OR, 1.8), left ventricular (LV) function <30% (OR, 1.67), 30–60% (OR, 1.18), insulin-dependent diabetes mellitus (OR, 1.40), history of psychoneurotic disorder (OR, 3.57), renal dysfunction (creatinine ≥2.0 mg/dL; OR, 1.43), concomitant mitral stenosis (OR, 1.45), body mass index ≥30 (OR, 1.4), coronary lesion 2- or 3-vessel disease (OR, 1.24), left main trunk disease (OR, 1.30), and female sex (OR, 1.14). AUC was 0.694 (P<0.001, Figure B).
| Variable | Mortality RR | Mortality/morbidity RR |
|---|---|---|
| Age category | 1.26*** | 1.24*** |
| Preoperative dialysis | 3.44*** | 1.86*** |
| CLD ≥Moderate | 1.83** | 1.83*** |
| Non-cardiac vascular disease | 2.15 (PAD)*** | 1.49*** |
| Congestive heart failure | 1.37* | 1.42*** |
| Atrial fibrillation | 2.08*** | 1.69*** |
| NYHA class | 1.52 (class ≥3)*** | 1.27 (class 3)** |
| 2.10 (class 4)*** | ||
| History of stroke/TIA | 1.38* | 1.32** |
| Concomitant CABG | 1.38** | 1.8* |
| Shock | 3.97*** | – |
| Concomitant mitral regurgitation ≥2 | 1.52** | – |
| LV function | – | 1.67 (bad)** |
| 1.18 (medium)* | ||
| DM | – | 1.15* |
| 1.40 (insulin use)** | ||
| History of psychoneurotic disorder | – | 3.57** |
| Renal dysfunction (Cr ≥2 mg/dL) | – | 1.43** |
| Concomitant mitral stenosis | – | 1.45** |
| BMI ≥30 | – | 1.40* |
| Coronary lesion | – | 1.24 (≥2VD)* |
| 1.30 (LMT)* | ||
| Female gender | – | 1.14* |
| C statistics | 0.732*** | 0.694*** |
*P<0.05; **P<0.01; ***P<0.001. 2VD, 2-vessel disease; RR, relative risk. Other abbreviations as in Table 1.
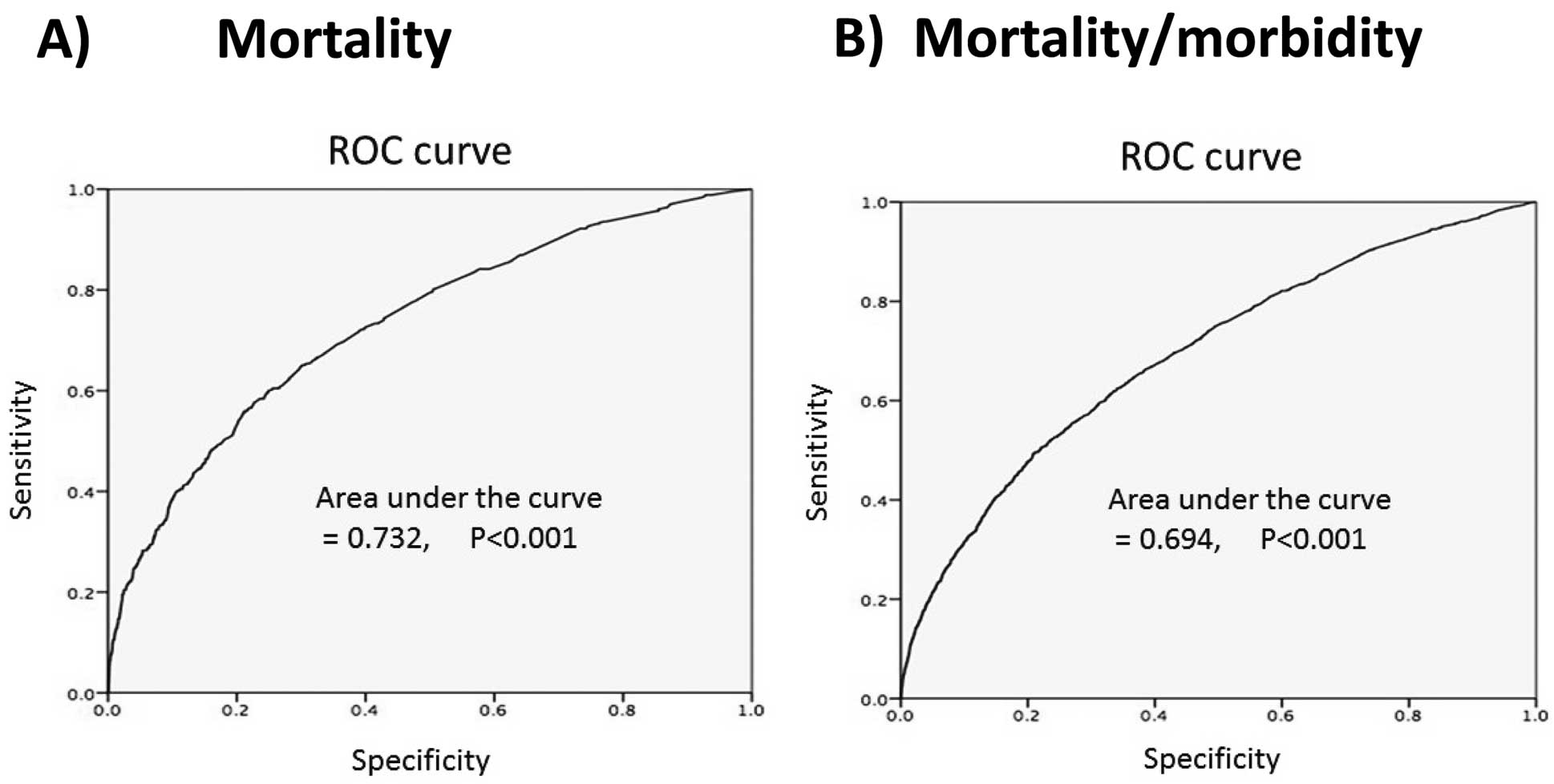
Receiver operating characteristics (ROC) curve for (A) mortality and (B) mortality and morbidity after surgical aortic valve replacement for aortic stenosis.
TAVR has shown excellent results in patients with high risk scores or with contraindications for surgery, such as porcelain aorta.1,2 Because of recent advances in prosthetic valves for TAVR, the indication for TAVR tends to extend to those with a moderate risk or to the younger population. The durability of the TAVR prosthesis, however, has not been fully established. In contrast, the durability of prosthetic valves used for SAVR has been well established and shows excellent long-term results,6,7 which are superior to those of TAVR. In Japan, a life expectancy of approximately 10 years has been reported for men and women aged 80 years. Therefore, TAVR is not indicated only according to advanced age, and its use in younger patients carries a considerable possibility of the need for reoperation.
Appropriate selection of treatment modality for AS requires discussion of various issues, such as operative mortality,8 operative morbidity,9,10 long-term survival and the cardiac event-free rate,11–13 durability of the prosthetic valve,14,15 postoperative quality of life,16 and cost-effectiveness of each treatment.17–20 In the present study, we focused on general functional status at discharge and hospitalization period, and investigated the factors that affected these variables. No previous large-scale studies have focused on general status at discharge and hospitalization period. These 2 factors are thought to be closely related to postoperative quality of life and have become benchmarks for AS treatment selection. With regard to general condition, we focused on inability of ambulatory discharge, which worsens the quality of life of not only the patients, but also their family. Therefore, we used the modified Rankin scale for assessment of general status at discharge. Longer hospitalization is also considered to weaken general status after discharge.4 STS score was used to calculate the risk of long hospital stay. The STS definition of long hospital stay is postoperative hospital stay >14 days, which might have a smaller effect on postoperative quality of life. In the present study, we defined long hospital stay as ≥90 days, which is considered to have a larger effect on the general condition of patients undergoing cardiac surgery. In addition, hospital stay in Japan tends to be longer because most of the medical cost is covered by national health insurance for all patients in Japan. In the USA, most patients are discharged to lower acuity long-term care facilities, mostly driven by insurance and health system demands. Long hospitalization and compromised general status burden not only medical finance, but medical resources. Medical expenses for older patients are currently one of the major concerns in an aging society, because they are becoming a burden on national health-care finance. The medical cost of octogenarians undergoing SAVR using cardiopulmonary bypass with a longer hospitalization was approximately 1.5-fold higher than those without.13 Hospital costs have been reported to be 20% higher in older patients.21 The balance between medical costs and clinical outcome is also important.
Older age,13 preoperative renal function including hemodialysis requirement,22 poor LV dysfunction,23 chronic obstructive pulmonary disease,24 preoperative heart failure,25 PAD,26 and AF27 have been shown to be independent risk factors for postoperative cardiac mortality. These factors are used in the EuroSCORE, STS score, and Japan score, which are representative risk calculators of mortality after cardiac surgery. In the present study, we showed that these factors also affected general condition at discharge and longer hospitalization. Patients with the aforementioned factors have a worse baseline risk profile and require a longer hospitalization period with a compromised general status at discharge. Therefore such risk calculators could predict the postoperative general status to some extent. In a previous study on octogenarians undergoing SAVR, the incidence of Rankin scale ≥4 at discharge increased in proportion to EuroSCORE II (EuroSCORE <5, 8%; 5<EuroSCORE<10, 14.3%; EuroSCORE >10, 18.3%).13 Interestingly, preoperative mental illness was a strong independent risk factor (OR, 3.57) that affected general condition at discharge and the hospitalization period.13 Jakobsen et al reported that severe mental illness is likely to have an adverse effect on prognosis, including adverse cardiac events following ST-elevation.28 Galyfos et al showed that preoperative neurological disease was an independent risk factor for postoperative delirium.29 The reasons why mental illness has an adverse effect after cardiac surgery are thought to be as follows. These patients are considered to have a worse baseline risk factor compared with those without mental illness.28 Furthermore, the incidence of delirium, which is considered to delay postoperative cardiac rehabilitation, is higher in patients with mental illness than in those without mental illness. Rubino noted that perioperative problems should be anticipated by the neurologist, in order to prevent as many complications as possible.30
Study LimitationsFrailty and intraoperative parameters were not evaluated in the present study. Some specific factors that may affect the operative results, such as porcelain aorta or small aortic annulus, were not included in the possible factors. The SAVR in the present study were performed between 1 January 2008 and 31 December 2012, in which TAVR were performed only in clinical trial study. Therefore, very high-risk patients, for whom TAVR would be selected in the current status, may have been included and the clinical outcome of the present study might be a little different from that in the era of widespread of TAVR.
Using the JCVSD, a large database, we have developed a risk model of SAVR for AS that can identify patients at high risk not only for mortality, but also for mortality and morbidity. Although risk factor analysis in patients undergoing TAVR is also important, risk analysis for SAVR may help to determine adequate indications for TAVR.
The authors thank the data managers at each cardiovascular institute in the JCVSD for their great efforts in registering clinical data.
The authors declare no conflicts of interest.