Abstract
Background:
N-terminal pro-B-type natriuretic peptide (NT-proBNP) is known to increase in heart failure patients. Given that no reports have described the association between NT-proBNP and chronic kidney disease (CKD) incidence in Asian populations, we investigated this association in the Japanese population.
Methods and Results:
We followed up 867 participants without CKD from the general population of Ohasama, Japan. We defined CKD as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2
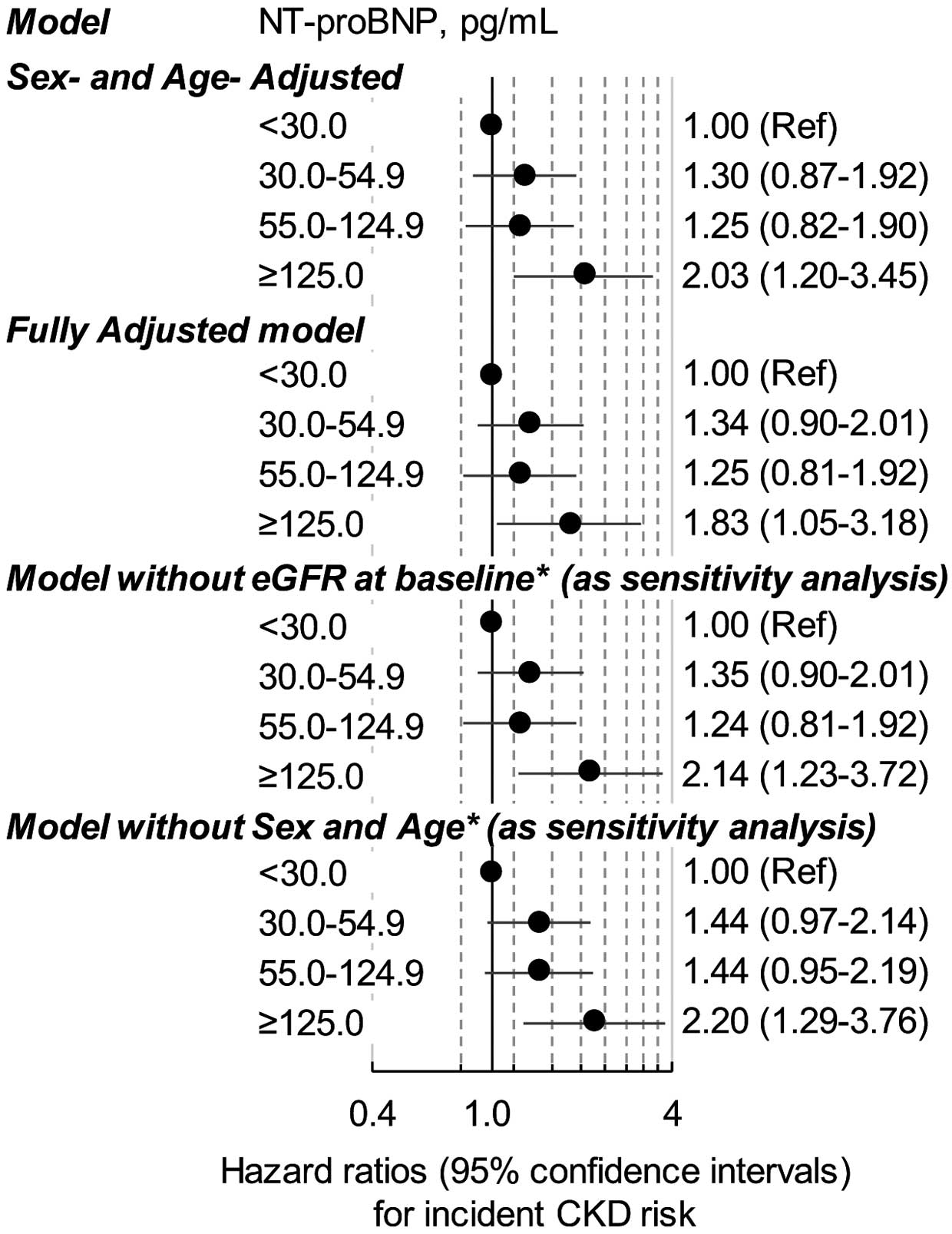
and/or proteinuria. In accordance with previous studies, the participants were classified into 4 groups according to NT-proBNP level (<30.0, 30.0–54.9, 55.0–124.9, and ≥125.0 pg/mL). The Cox model was applied to assess adjusted hazard ratios (HR) for CKD incidence after full adjustment including baseline eGFR. Participant mean age was 59.1 years, and 587 (67.7%) were women. During the mean follow-up period of 9.7 years, 177 participants developed CKD. When the group with NT-proBNP <30.0 pg/mL was used as the reference, adjusted HR for CKD incidence in the 30.0–54.9, 55.0–124.9, and ≥125.0 pg/mL groups were 1.34 (95% CI: 0.90–2.01), 1.25 (95% CI: 0.81–1.92), and 1.83 (95% CI: 1.05–3.18), respectively.
Conclusions:
NT-proBNP can be significantly predictive for CKD incidence in Asian populations.
Chronic kidney disease (CKD) is a major global health burden, and the global incidence and associated mortality have increased over the last several decades.1
In addition, decreased kidney function is an independent risk factor for cardiovascular disease.2,3
Therefore, the prevention of CKD is beneficial to public health.
Brain natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are secreted from the cardiac ventricles in response to ventricular wall stretch and tension.4,5
NT-proBNP is increased in heart failure patients and is associated with an increased risk of future cardiovascular disease and all-cause mortality.4–7
In addition, several studies have demonstrated an association between progression of CKD and NT-proBNP or BNP.8–11
Most of those studies, however, evaluated patients with CKD or renal insufficiency8–10
and used BNP.8,9
NT-proBNP is considered a more discerning marker than BNP to evaluate cardiac dysfunction.12
NT-proBNP was reported to be related to CKD incidence in the US general population, but that study’s participants were limited to those aged ≥65 years.11
Differences in the association between NT-proBNP and CKD incidence according to ethnicity have been reported,13–15
and African-American and Asian people have an increased risk of CKD and of end-stage kidney disease, respectively.14,15
Therefore, association between NT-proBNP and CKD incidence in the US population aged ≥65 years cannot be generalized to other populations such as that of Asia.11
Furthermore, it is completely unknown whether NT-proBNP is a predictor of CKD incidence in individuals aged <65 years in a general population. Therefore, we investigated the association of NT-proBNP with the risk of CKD in a general Asian population.
Methods
Design
This study was based on the Ohasama study, which is an ongoing cohort that began in 1986 in Ohasama, Iwate Prefecture, Japan. We previously reported the details of the Ohasama study along with the socioeconomic and demographic characteristics of this region.16–19
The Institutional Review Boards of Teikyo University, Tohoku Medical and Pharmaceutical University, and Tohoku University approved the study protocol.
Participants
The flow chart of participant selection is shown in
Figure 1. Farmers, self-employed individuals, pensioners, and dependants aged ≥35 years are eligible for annual health check-ups in Japan. The total population of Ohasama in 1997 was 7,318, and 2,719 individuals aged ≥35 years were eligible for annual health check-ups. Of these, 1,831 individuals participated in check-ups that year, and 1,622 individuals provided written informed consent to take part in the Ohasama study. We excluded the participants who had no medical data for NT-proBNP (n=270) or proteinuria (n=19). In addition, 134 individuals diagnosed with CKD at baseline, which was based on either proteinuria or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, were excluded. Moreover, we excluded participants who had a history of ischemic heart disease (n=71) or atrial fibrillation (n=1), given that these conditions considerably affect NT-proBNP level. Finally, 260 individuals who lacked medical data for eGFR from 2002 to 2014 were excluded. As a result, a final total of 867 individuals were analyzed.

Blood samples were collected in chilled ethylenediaminetetraacetic acid (EDTA) tubes, and NT-proBNP was measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Tokyo, Japan). The inter- and intra-assay coefficients of variation for NT-proBNP were 3.1% at 46 pg/mL and 1.9% at 64 pg/mL, respectively. We gathered information on smoking status, alcohol consumption, antihypertensive medication, and histories of diabetes mellitus, hypercholesterolemia, ischemic heart disease and cerebrovascular disease by reviewing medical records or a questionnaire survey. We classified smoking status and alcohol consumption into current or former/never. Diabetes mellitus was defined as random blood glucose ≥11.1 mmol/L (≥200 mg/dL), hemoglobin A1c ≥6.5% according to the Japan Diabetes Society, treatment with insulin or oral hypoglycemic agents, or history of diabetes mellitus. Hypercholesterolemia was defined as total cholesterol ≥5.7 mmol/L (≥220 mg/dL), the use of medication to treat hypercholesterolemia, and/or a history of hypercholesterolemia. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). After participants were seated and rested for ≥2 min, blood pressure was measured twice by trained nurses at local medical centers using a semi-automatic blood pressure-measuring device. Serum creatinine (SCr) was measured using the Jaffe method before 2002 and the enzymatic method thereafter.
We determined renal function from eGFR, which was estimated from SCr using the modified Japanese equation: eGFR (mL/min/1.73 m2)=194×(SCr in enzymatic method)−1.094×Age−0.287(×0.739, if female).20
We subtracted 0.2 from any SCr value that was measured using the Jaffe method (mg/dL) to convert the value to an equivalent enzymatically determined value.21
Proteinuria was determined with a dipstick test for spot-urine; the test was considered positive at ≥1+, corresponding to urinary protein >30 mg/dL. CKD was defined as eGFR <60 mL/min/1.73 m2
and/or proteinuria. According to a previous study (2012 Kidney Disease: Improving Global Outcomes [KDIGO] Clinical Practice Guideline for the Evaluation and Management of CKD), CKD grade (G) was classified as follows: G1A2–3, eGFR ≥90 mL/min/1.73 m2
and moderately to severely increased proteinuria; G2A2–3, 60≤eGFR<90 mL/min/1.73 m2
and moderately to severely increased proteinuria; G3aA1, 45≤eGFR<60 mL/min/1.73 m2
and normal to mildly increased proteinuria; G3aA2–3, 45≤eGFR<60 mL/min/1.73 m2
and moderately to severely increased proteinuria; and G3bA1, 30≤eGFR<45 mL/min/1.73 m2
and normal to mildly increased proteinuria.22
Follow-up and Outcomes
The primary outcome was CKD diagnosed during the annual check-ups from 2002 to 2014. The date of CKD diagnosis was defined as the midpoint between the most recent date when the individual did not have CKD and the date of the first diagnosis of CKD. If a participant had more than 1 CKD event during follow-up, only the first event contributed to the analysis. The monitoring period was from the baseline to the date of CKD diagnosis or final check-up.
Statistical Analysis
We divided participants into 4 groups: NT-proBNP <30.0 pg/mL, 30.0–54.9 pg/mL, 55.0–124.9 pg/mL, and ≥125.0 pg/mL.7,23
Such groupings were done because NT-proBNP 125 pg/mL has been approved as a marker of heart failure in the European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure (2012).24
The cut-off 55.0 pg/mL was also used in previous studies.7,23
Given that approximately 60% of the participants had NT-proBNP <55.0 pg/mL (Supplementary Figure), we used the approximate median NT-proBNP to stratify these participants into 2 groups (NT-proBNP <30.0 pg/mL and ≥30.0 pg/mL). The means and proportions were compared using Student’s t-test, chi-squared test, analysis of variance (ANOVA), analysis of covariance (ANCOVA), and multiple logistic regression analysis. When we calculated CKD incidence rates in the 4 NT-proBNP groups, sex- and age-standardization (<65/≥65 years) was done using the direct method. The Cox proportional hazards model was applied to assess adjusted hazard ratio (HR) for CKD incidence in the NT-proBNP groups using the NT-proBNP <30.0 pg/mL group as a reference. Covariates in the fully adjusted model were sex, age, BMI, current or ex-smoker, current or ex-drinker, diabetes mellitus, hypercholesterolemia, history of cerebrovascular disease, systolic blood pressure (SBP), use of antihypertensive drugs, and baseline eGFR, given that these have been identified as risk factors for CKD in previous studies.25,26
The range of the variance inflation factor for these covariates was 1.03–2.51, indicating no multicollinearity. We interpolated missing values of BMI (n=10) from the regression slope on age. Given that sex and age were used in the calculation of eGFR, we performed the sensitivity analyses after excluding eGFR, or sex and age, from the fully adjusted model. In addition, we added the median number of follow-up visits to the fully adjusted model and performed sensitivity analyses. When NT-proBNP was treated as a continuous variable, natural log-transformed (ln) NT-proBNP was used because of its positively skewed distribution (Supplementary Figure). In the stratification analyses, the age cut-off was determined based on the World Health Organization definition of elderly. Hypertension was defined as SBP/diastolic blood pressure ≥140/90 mmHg according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2019) and 2018 ESC/European Society of Hypertension (ESH) Guidelines for the management of arterial hypertension.27,28
Median baseline eGFR was used as the cut-off. All data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA). P<0.05 was considered statistically significant.
Results
Characteristics
Mean participant age was 59.1 years and 67.7% were women. Median NT-proBNP was 41.3 pg/mL (IQR, 23.5–70.0 pg/mL). The number of participants with NT-proBNP <30.0 pg/mL, 30.0–54.9 pg/mL, 55.0–124.9 pg/mL, and ≥125.0 pg/mL was 299, 257, 237, and 74, respectively. Higher NT-proBNP was associated with female sex, advanced age, and lower eGFR. After adjustments for sex and age, higher NT-proBNP was positively associated with antihypertensive medication use and higher SBP but inversely associated with BMI (Table 1). After adjustments for sex and age, the association between higher NT-proBNP and hypercholesterolemia was significant but not linear.
Table 1.
Baseline Participant Characteristics vs. NT-proBNP Category
| |
NT-proBNP (pg/mL) |
P-value |
Sex and
age-adjusted
P-value |
<30.0
(n=299) |
30.0–54.9
(n=257) |
55.0–124.9
(n=237) |
≥125.0
(n=74) |
| Women |
54.8 |
77.4 |
71.7 |
73.0 |
<0.0001 |
<0.0001† |
| Age (years) |
55.0±9.7 |
58.3±9.7 |
62.6±9.1 |
67.1±8.2 |
<0.0001 |
<0.0001† |
| BMI (kg/m2) |
24.2±3.0 |
23.5±2.9 |
23.4±3.0 |
22.8±3.1 |
0.0008 |
<0.0001 |
| Current or ex-smoker |
33.4 |
15.6 |
21.1 |
16.2 |
<0.0001 |
0.68 |
| Current or ex-drinker |
49.5 |
33.9 |
38.8 |
23.0 |
<0.0001 |
0.12 |
| Hypercholesterolemia |
30.4 |
25.7 |
20.7 |
24.3 |
0.083 |
0.0002 |
| Diabetes mellitus |
8.4 |
5.8 |
7.2 |
4.1 |
0.49 |
0.087 |
| History of CVD |
1.0 |
0.40 |
3.0 |
8.1 |
0.0001 |
0.053 |
| Antihypertensive medication |
10.4 |
17.9 |
30.4 |
41.9 |
<0.0001 |
0.0001 |
| SBP (mmHg) |
127.9±12.7 |
129±12.2 |
131.2±14.2 |
135.3±15.2 |
<0.0001 |
0.032 |
| DBP (mmHg) |
72.4±8.8 |
72.7±8.3 |
73.4±9.1 |
74±9.2 |
0.40 |
0.56 |
| Hematuria |
3.3 |
1.6 |
3.0 |
0.0 |
0.26 |
0.79 |
| eGFR (mL/min/1.73 m2) |
87.2±15.6 |
87.1±17.5 |
85.9±19.2 |
78.3±13.3 |
0.0006 |
–‡ |
| NT-proBNP (pg/mL) |
18.4 (11.2–24.4) |
40.7 (34.6–47.1) |
73.7 (64.1–92.5) |
161.4 (143.2–206.0) |
<0.0001 |
<0.0001 |
| CKD at follow-up |
16.1 |
21.4 |
21.1 |
32.4 |
0.016 |
0.25 |
Data given as %, mean±SD or median (IQR). †The association of sex with NT-proBNP category was adjusted for age. The association of age with NT-proBNP category was adjusted for sex. ‡Sex- and age-adjusted P-value was not calculated because sex and age were used in the calculation of eGFR. BMI, body mass index; CKD, chronic kidney disease; CVD, cerebrovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; SCr, serum creatinine.
The 260 subjects who did not participate in follow-up examinations were older and had lower eGFR and higher NT-proBNP than the 867 included in the present analysis (Table 2). After adjustments for sex and age, the excluded participants had higher blood pressure and higher prevalence of antihypertensive medication use than those included in the analysis (Table 2).
Table 2.
Baseline Participant Characteristics vs. Follow-up Data Status
| Characteristics |
Total
(n=1,127) |
Followed up
(n=867) |
Not followed up
(n=260) |
P-value |
Sex- and
age-adjusted
P-value |
| Women |
66.6 |
67.7 |
63.1 |
0.17 |
0.27† |
| Age (years) |
60.2±10.9 |
59.1±10.2 |
63.7±12.3 |
<0.0001 |
<0.0001† |
| BMI (kg/m2)‡ |
23.7±3.2 |
23.6±3.0 |
23.8±3.7 |
0.51 |
0.45 |
| Current or ex-smoker |
24.5 |
23.3 |
28.5 |
0.090 |
0.17 |
| Current or ex-drinker |
39.1 |
39.7 |
37.3 |
0.49 |
0.72 |
| Hypercholesterolemia |
25.6 |
25.8 |
25.0 |
0.79 |
0.56 |
| Diabetes mellitus |
7.3 |
6.9 |
8.5 |
0.40 |
0.68 |
| History of CVD |
2.8 |
2.0 |
5.4 |
0.0031 |
0.18 |
| Antihypertensive medication |
23.7 |
20.8 |
33.5 |
<0.0001 |
0.036 |
| SBP (mmHg) |
130.7±13.9 |
129.7±13.4 |
134.1±14.9 |
<0.0001 |
0.0024 |
| DBP (mmHg) |
73.3±9.1 |
72.9±8.8 |
74.6±9.8 |
0.0080 |
0.043 |
| Hematuria |
2.7 |
2.4 |
3.5 |
0.36 |
0.49 |
| eGFR (mL/min/1.73 m2)‡ |
85.5±17.1 |
86.0±17.2 |
83.6±16.5 |
0.046 |
–§ |
| NT-proBNP (pg/mL)‡ |
43.0 (24.9–76.3) |
41.3 (23.5–70.0) |
54.6 (29.2–104.0) |
<0.0001 |
0.016 |
Data given as %, mean±SD or median (IQR). Followed up individuals (n=867) constituted the present participants. †The association of sex with NT-proBNP category was adjusted for age. The association of age with NT-proBNP category was adjusted for sex. ‡Excluded participants had medical data for BMI (n=246). §Sex- and age-adjusted P-value was not calculated because sex and age were used in the calculation of eGFR. Abbreviations as in Table 1.
The number of follow-up visits was 1, 2, 3, and ≥4 in 175, 154, 260, and 278 participants, respectively. During a mean follow-up period of 9.74±4.10 years (median, 9.66 years; IQR, 6.59–11.9 years), 177 participants developed CKD. Of these, 156 participants had eGFR <60 mL/min/1.73 m2
and 31 had proteinuria. In addition, the number of participants with CKD stage G1A2–3, G2A2–3, G3aA1, G3aA2–3, and G3bA1 was 3, 18, 144, 10, and 2, respectively. No participants developed advanced CKD stage G4–5. The participants with CKD at follow-up were older and had lower eGFR (Table 3). After adjustments for sex and age, the participants with CKD at follow-up had significantly higher NT-proBNP than those without CKD (Table 3).
Table 3.
Baseline Participant Characteristics vs. CKD Incidence
| Characteristics |
CKD incidence at follow-up |
P-value |
Sex- and age-adjusted
P-value |
| Absent (n=690) |
Present (n=177) |
| Women |
67.2 |
69.5 |
0.57 |
0.43† |
| Age (years) |
58.5±10.2 |
61.5±9.7 |
0.0005 |
0.0005† |
| BMI (kg/m2) |
23.6±3.0 |
23.8±3.0 |
0.37 |
0.40 |
| Current or ex-smoker |
23.0 |
24.3 |
0.73 |
0.18 |
| Current or ex-drinker |
40.3 |
37.3 |
0.47 |
0.68 |
| Hypercholesterolemia |
25.8 |
26.0 |
0.96 |
0.71 |
| Diabetes mellitus |
6.5 |
8.5 |
0.36 |
0.57 |
| History of CVD |
1.9 |
2.3 |
0.75 |
0.98 |
| Antihypertensive medication |
19.6 |
25.4 |
0.087 |
0.45 |
| SBP (mmHg) |
129.4±13.3 |
131±13.6 |
0.16 |
0.48 |
| DBP (mmHg) |
72.8±8.7 |
73.5±9.1 |
0.34 |
0.45 |
| Hematuria |
2.9 |
0.60 |
0.072 |
0.10 |
| eGFR (mL/min/1.73 m2) |
88.7±17.4 |
75.5±11.3 |
<0.0001 |
–‡ |
| NT-proBNP (pg/mL) |
39.6 (22.7–67.2) |
48.6 (27.6–77.8) |
0.043 |
0.0013 |
Data given as %, mean±SD or median (IQR). †The association of sex with NT-proBNP category was adjusted for age. The association of age with NT-proBNP category was adjusted for sex. ‡Sex- and age-adjusted P-value was not calculated because sex and age were used in the calculation of eGFR. Abbreviations as in Table 1.
The age- and sex-standardized incidence rates for CKD were higher in the NT-proBNP ≥125.0 pg/mL group (42.9/1,000 person-years) than in those with NT-proBNP <30.0 pg/mL (18.1/1,000 person-years;
Figure 2). The risk of CKD in the NT-proBNP ≥125.0 pg/mL group was approximately 2-fold higher than in the NT-proBNP <30.0 pg/mL group on sex- and age-adjusted Cox modeling (P=0.0088,
Figure 3). Even after adjustments for covariates, the participants with NT-proBNP ≥125.0 pg/mL had a significantly higher risk of CKD than those with NT-proBNP <30.0 pg/mL, although the corresponding HR decreased (P=0.033,
Figure 3). Similar results were observed when we removed eGFR, or both sex and age from the fully adjusted model (Figure 3). We further added the median number of follow-up visits (≥3) to the fully adjusted model, but similar results were observed (HR between the groups with NT-proBNP ≥125.0 pg/mL and <30.0 pg/mL was 1.84; 95% CI: 1.06–3.20).

Table 4
lists the HR and chi-squared values for each covariate. Baseline eGFR was the strongest predictor (χ2=81.1), while NT-proBNP (χ2=5.97) was a significant predictor of CKD incidence, as compared with smoking habit (χ2=4.53, P=0.033). We performed stratified analyses according to age (<65/≥65 years), sex, eGFR (<median/≥median 83.8 mL/min/1.73 m2), use of hypertensive medication, or hypertension (≥140/≥90 mmHg). Although no significant interactions between these variables and lnNT-proBNP on the risk of CKD were observed (P for interaction ≥0.33), the lowest HR per 1-SD increase in lnNT-proBNP was noted in the participants aged <65 years (Table 5).
Table 4.
HR for CKD Development per 1-SD Increase
| Variables |
HR (95% CI) |
χ2 |
P-value |
| Sex (men=1, women=0) |
0.80 (0.60–1.06) |
2.37 |
0.12 |
| Age (1-SD [10.2 years] increase) |
1.22 (0.97–1.52) |
2.95 |
0.086 |
| BMI (1-SD [3.02 kg/m2] increase) |
1.03 (0.88–1.21) |
0.17 |
0.68 |
| Current or ex-smoker (=1, non-smoker=0) |
1.32 (1.02–1.70) |
4.53 |
0.033 |
| Current or ex-drinker (=1, non-drinker=0) |
1.07 (0.89–1.30) |
0.55 |
0.46 |
| Hypercholesterolemia (=1, non-hypercholesterolemia=0) |
0.92 (0.79–1.07) |
1.10 |
0.29 |
| Diabetes mellitus (=1, non-diabetes mellitus=0) |
1.13 (0.98–1.30) |
2.98 |
0.085 |
| History of CVD (=1, no history of CVD=0) |
0.99 (0.86–1.14) |
0.017 |
0.90 |
| Antihypertensive medication (=1, no antihypertensive medication=0) |
1.01 (0.87–1.17) |
0.0097 |
0.92 |
| SBP (1-SD [=13.4-mmHg] increase) |
1.06 (0.91–1.24) |
0.57 |
0.45 |
| eGFR (1-SD [=17.2-mL/min/1.73 m2] decrease) |
3.18 (2.47–4.09) |
81.1 |
<0.0001 |
| lnNT-proBNP (1-SD [=0.87] increase) |
1.26 (1.05–1.51) |
5.97 |
0.015 |
Abbreviations as in Table 1.
Table 5.
HR for CKD Development per 1-SD Increase in lnNT-proBNP
| Strata |
Event/total no. |
HR
(95% CI) |
P-value |
P for
interaction |
| All |
177/867 |
1.26 (1.05–1.51) |
0.015 |
|
| Sex |
|
|
|
0.82 |
| Men |
54/280 |
1.40 (1.02–1.92) |
0.036 |
|
| Women |
123/587 |
1.19 (0.95–1.49) |
0.13 |
|
| Age |
|
|
|
0.89 |
| <65 years |
67/266 |
1.05 (0.78–1.41) |
0.75 |
|
| ≥65 years |
110/601 |
1.29 (1.02–1.62) |
0.031 |
|
| eGFR |
|
|
|
0.60 |
| <83.8 mL/min/1.73 m2 † |
141/433 |
1.21 (0.98–1.48) |
0.074 |
|
| ≥83.8 mL/min/1.73 m2 † |
36/434 |
1.64 (1.07–2.51) |
0.024 |
|
| Antihypertensive medication |
|
|
|
0.88 |
| Present |
45/180 |
1.17 (0.81–1.69) |
0.42 |
|
| Absent |
132/687 |
1.30 (1.05–1.61) |
0.016 |
|
| BP ≥140/≥90 mmHg |
|
|
|
0.33 |
| Present |
47/194 |
1.34 (0.94–1.90) |
0.11 |
|
| Absent |
130/673 |
1.23 (0.99–1.53) |
0.060 |
|
1-SD lnNT-proBNP increase was 0.87. †Median. HR (95% CI) was adjusted for sex, age, BMI, current or ex-smoker, current or ex-drinker, diabetes mellitus, hypercholesterolemia, history of CVD, SBP, use of antihypertensive drugs, and baseline eGFR. BP, blood pressure. Other abbreviations as in Table 1.
Discussion
This is the first study to assess the association between NT-proBNP and increased risk of CKD in a general Asian population. Median NT-proBNP in participants who had CKD at follow-up was 48.6 pg/mL, which was higher than the 39.6 pg/mL in those without CKD. Even after full adjustment, the individuals with NT-proBNP ≥125.0 pg/mL had a 1.83-fold higher risk of CKD compared with those with NT-proBNP <30.0 pg/mL. In addition, NT-proBNP was associated with a higher risk of CKD when NT-proBNP was treated as a continuous variable. This suggests that the level of NT-proBNP, compared with other factors (e.g., smoking habit and diabetes mellitus), can robustly predict CKD incidence (Table 4).
Elevation of BNP was reported to be an indicator of increased risk of further kidney function decline in Japanese patients with CKD.8,9
NT-proBNP was also reported to be a more suitable estimate of the risk of CKD progression than BNP, although it was confirmed in non-diabetic patients with mild-to-moderate renal insufficiency.10
The Cardiovascular Health Study observed an association between NT-proBNP and CKD incidence. In that study, Bansal et al reported that participants with NT-proBNP >237 pg/mL (whose mean eGFR at baseline was 62±19 mL/min/1.73 m2) had a 1.38-fold increased risk of CKD compared with those with NT-proBNP ≤58 pg/mL (whose mean eGFR at baseline was 79±19 mL/min/1.73 m2) in 3,752 community-dwelling participants without heart failure (mean follow-up, 6.4 years; mean age, 72 years); furthermore, 18% of the participants were African-American, and median NT-proBNP was 115 pg/mL (IQR, 59–237 pg/mL).11
In the present study, median NT-proBNP was 41.3 pg/mL, and the individuals with NT-proBNP ≥125.0 pg/mL accounted for only 8.5% of all participants. Consequently, there is a potential difference in NT-proBNP between the previous results and the Asian population.
We found that the individuals with NT-proBNP ≥125.0 pg/mL had a higher risk of CKD than those with NT-proBNP <30.0 pg/mL. This association remained significant after adjustment for baseline eGFR. In the stratification analyses, elevated NT-proBNP was significantly associated with CKD incidence even in the eGFR ≥83.8 mL/min/1.73 m2
group. Therefore, the present study shows that NT-proBNP may be predictive for future CKD in the Asian population independent of baseline kidney function. The NT-proBNP cut-off ≥125.0 pg/mL has been used for the detection of suspected heart failure, and this cut-off may be useful for screening individuals at a higher risk of CKD in Asian populations.
In the stratification analyses according to age, sex, baseline eGFR, use of hypertensive medication, or hypertension and the risk of CKD, the association between lnNT-proBNP and risk of CKD was weaker in the participants aged <65 years (Table 5). NT-proBNP concentration increases with advanced age.29
Older adults have higher NT-proBNP than the younger population. There is therefore a possibility that the association between NT-proBNP and CKD incidence can apply only to individuals aged ≥65 years who have NT-proBNP in a relatively high range. Also, Bansal et al reported a significant association between NT-proBNP and CKD incidence based on a general population aged ≥65 years.11
No significant interaction, however, between lnNT-proBNP and age on the risk of CKD was observed, which could have been due to the limited number of participants in the present study. As such, further studies based on a larger sample size may be necessary to verify this issue.
Several mechanisms are proposed to explain the relationship between elevated NT-proBNP and CKD incidence. NT-proBNP is enhanced by ventricular wall stretch, volume overload, and venous congestion.30,31
Volume overload or venous congestion triggers increased central venous pressure and decreased cardiac output, renal artery pressure, and endothelial dysfunction, resulting in renal dysfunction. Increased central venous pressure decreases GFR.32
Decreased cardiac output and renal artery pressure activate the sympathetic nervous system and the renin-angiotensin system. Furthermore, decreased renal artery pressure leads to increased central venous pressure by renin release.33–37
Endothelial dysfunction combined with the production of reactive oxygen species brings about impaired renal function.38,39
Moreover, elevated NT-proBNP suggests a reduced clearance rate caused by subclinical renal dysfunction, because renal excretion is the main route of NT-proBNP clearance.40
Study Limitations
There were several limitations to the present study. First, this study was based on the participants of an annual health check-up. The participants included in the present analysis seemed to be younger and healthier than those who did not participate in the follow-up examination. The present population is more health conscious, and therefore there is a possibility of selection bias. These imbalances, or selection biases, might limit the external validity of the present findings. Second, the diagnosis of CKD in the present study was dependent on the value of creatinine or positive proteinuria on only 1 occasion. The current guideline defines CKD as the presence of kidney damage or GFR <60 mL/min/1.73 m2
for ≥3 months.22
Therefore, the participants who met these criteria of CKD can include those who were temporarily affected by general conditions such as dehydration. In addition, the date of CKD diagnosis was defined as the midpoint between the most recent date when the individual did not have CKD and the date of the first diagnosis of CKD. It is difficult, however, to determine the onset of CKD because the symptoms are not noticeable until the disease has progressed to an advanced stage. We used the same criteria to diagnose CKD as in previous epidemiological studies.17,18,25,41
Third, any treatment that was started during follow-up could have weakened the association between NT-proBNP and CKD incidence. Fourth, given that most participants who developed CKD were classified as CKD stage G3aA1, it was difficult to examine how NT-proBNP level affected the progression of CKD stage in the present study. Fifth, the information on ischemic heart disease such as myocardial infarction and angina pectoris was collected by reviewing medical records or a questionnaire survey in the present study. Given that we did not use uniform diagnostic criteria, there is a possibility of bias in diagnosing heart disease. Furthermore, the participants could also have subclinical heart disease.
Conclusions
The present study is the first to demonstrate the association between NT-proBNP and elevated risk of CKD in an Asian population. The participants with NT-proBNP ≥125.0 pg/mL had a higher risk of CKD than those with NT-proBNP <30.0 pg/mL. This association suggests that NT-proBNP ≥125.0 pg/mL is a useful threshold for CKD incidence. NT-proBNP level is not only a screening marker of heart failure but also can be a significant predictor of CKD incidence in Asian populations. Kidney function should be followed carefully in individuals with NT-proBNP ≥125.0 pg/mL for early detection of CKD development.
Acknowledgments
We are grateful to the residents and staff members in Ohasama and staff members of the Hanamaki City Government, Iwate Prefectural Central Hospital attachment Ohasama Regional Clinical Center, Teikyo University, Tohoku Medical and Pharmaceutical University, and Tohoku University for their valuable support on the Ohasama study project.
Author Contributions
All authors have contributed to this scientific work and approved the final version of the manuscript. S.N. designed this study, performed the data analyses, and wrote the manuscript. M.S. was involved in the design of the study, supervised the data analyses, and co-wrote the manuscript. H.M., T. Murakami, K.A., T.H., M.K., T. Mori, A. Hozawa, Y.I., and T.O. assisted with the data analyses and supervised the drafting of the manuscript. M.S., H.M., K.A., A. Hara, T.H., R.I., M.T.-U., M.K., A. Hozawa, K.N., Y.I., and T.O. were involved in data collection. M.S., M.K., and T.O. were responsible for gathering and cleaning the data. T.O. is the principal investigator of the Ohasama study. All authors had full access to the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This study was supported by Grants for Scientific Research (16H05243, 16H05263, 16K09472, 16K11850, 16K15359, 17H04126, 17H06533, 17K15853, 17K19930, 18K09674, 18K09904, 18K17396, 19K19466, 19H03908, and 19K10662) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan; Grant-in-Aid for Young Scientists of Showa Pharmaceutical University H28-4; the Japan Arteriosclerosis Prevention Fund; Comprehensive Research on Cardiovascular and Life-Style Related Diseases (H26-Junkankitou [Seisaku]-Ippan-001 and H29-Junkankitou-Ippan-003) from the Ministry of Health, Labor, and Welfare; A Scheme to Revitalize Agriculture and Fisheries in Disaster Area through Deploying Highly Advanced Technology (NouEi 2-02) from the Ministry of Agriculture, Forestry and Fisheries, Japan; The Academic Contributions from Pfizer Japan Inc.; Scholarship donations from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd.; Research Support from Astellas Pharma Inc. and Takeda Pharmaceutical Co., Ltd.; The Health Care Science Institute Research Grant.
Disclosures
The authors declare no conflicts of interest. K.N. is a member of
Circulation Reports
’ Editorial Team.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-19-0044
References
- 1.
Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581.
- 2.
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108: 2154–2169.
- 3.
Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: The Hisayama Study. Kidney Int 2005; 68: 228–236.
- 4.
Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998; 339: 321–328.
- 5.
Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL Jr. Biology of the natriuretic peptides. Am J Cardiol 2008; 101: 3–8.
- 6.
Geng Z, Huang L, Song M, Song Y. N-terminal pro-brain natriuretic peptide and cardiovascular or all-cause mortality in the general population: A meta-analysis. Sci Rep 2017; 7: 41504.
- 7.
Satoh M, Murakami T, Asayama K, Hirose T, Kikuya M, Inoue R, et al. N-terminal pro-B-type natriuretic peptide is not a significant predictor of stroke incidence after 5 years: The Ohasama Study. Circ J 2018; 82: 2055–2062.
- 8.
Yasuda K, Kimura T, Sasaki K, Obi Y, Iio K, Yamato M, et al. Plasma B-type natriuretic peptide level predicts kidney prognosis in patients with predialysis chronic kidney disease. Nephrol Dial Transplant 2012; 27: 3885–3891.
- 9.
Yoshitomi R, Nakayama M, Sakoh T, Fukui A, Shikuwa Y, Tominaga M, et al. Plasma B-type natriuretic peptide concentration is independently associated with kidney function decline in Japanese patients with chronic kidney disease. J Hypertens 2016; 34: 753–761.
- 10.
Spanaus KS, Kronenberg F, Ritz E, Schlapbach R, Fliser D, Hersberger M, et al. B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: The Mild-to-Moderate Kidney Disease Study. Clin Chem 2007; 53: 1264–1272.
- 11.
Bansal N, Katz R, Dalrymple L, de Boer I, DeFilippi C, Kestenbaum B, et al. NT-proBNP and troponin T and risk of rapid kidney function decline and incident CKD in elderly adults. Clin J Am Soc Nephrol 2015; 10: 205–214.
- 12.
Seino Y, Ogawa A, Yamashita T, Fukushima M, Ogata K, Fukumoto H, et al. Application of NT-proBNP and BNP measurements in cardiac care: A more discerning marker for the detection and evaluation of heart failure. Eur J Heart Fail 2004; 6: 295–300.
- 13.
Kruger R, Schutte R, Huisman HW, Hindersson P, Olsen MH, Schutte AE. N-terminal prohormone B-type natriuretic peptide and cardiovascular function in Africans and Caucasians: The SAfrEIC study. Heart Lung Circ 2012; 21: 88–95.
- 14.
Muntner P, Newsome B, Kramer H, Peralta CA, Kim Y, Jacobs DR Jr, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol 2012; 7: 101–107.
- 15.
Hall YN, Hsu CY, Iribarren C, Darbinian J, McCulloch CE, Go AS. The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int 2005; 68: 2310–2316.
- 16.
Imai Y, Nagai K, Sakuma M, Sakuma H, Nakatsuka H, Satoh H, et al. Ambulatory blood pressure of adults in Ohasama, Japan. Hypertension 1993; 22: 900–912.
- 17.
Kanno A, Kikuya M, Asayama K, Satoh M, Inoue R, Hosaka M, et al. Night-time blood pressure is associated with the development of chronic kidney disease in a general population: The Ohasama Study. J Hypertens 2013; 31: 2410–2417.
- 18.
Kanno A, Kikuya M, Ohkubo T, Hashimoto T, Satoh M, Hirose T, et al. Pre-hypertension as a significant predictor of chronic kidney disease in a general population: The Ohasama Study [Research Support, Non-U.S. Gov’t]. Nephrol Dial Transplant 2012; 27: 3218–3223.
- 19.
Nakayama M, Metoki H, Terawaki H, Ohkubo T, Kikuya M, Sato T, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population: The Ohasama study [Research Support, Non-U.S. Gov’t]. Nephrol Dial Transplant 2007; 22: 1910–1915.
- 20.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan [Comparative Study Multicenter Study Research Support, Non-U.S. Gov’t]. Am J Kidney Dis 2009; 53: 982–992.
- 21.
Ando M, Minami H, Ando Y, Saka H, Sakai S, Yamamoto M, et al. Multi-institutional validation study of carboplatin dosing formula using adjusted serum creatinine level. Clin Cancer Res 2000; 6: 4733–4738.
- 22.
Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–735.
- 23.
Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Ikeda F, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community: The Hisayama study. Arterioscler Thromb Vasc Biol 2011; 31: 2997–3003.
- 24.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869.
- 25.
Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, et al. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int 2007; 71: 159–166.
- 26.
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: Global dimension and perspectives. Lancet 2013; 382: 260–272.
- 27.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 2019; 42: 1235–1481.
- 28.
Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G Ital Cardiol (Rome) 2018; 19: 3–73 (in Italian).
- 29.
Raymond I, Groenning BA, Hildebrandt PR, Nilsson JC, Baumann M, Trawinski J, et al. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart 2003; 89: 745–751.
- 30.
Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J 1998; 135: 825–832.
- 31.
Koratala A, Kazory A. Natriuretic peptides as biomarkers for congestive states: The cardiorenal divergence. Dis Markers 2017; 2017: 1454986.
- 32.
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53: 582–588.
- 33.
Cannon PJ. The kidney in heart failure. N Engl J Med 1977; 296: 26–32.
- 34.
Stanton RC, Brenner BM. Role of the kidney in congestive heart failure. Acta Med Scand Suppl 1986; 707: 21–25.
- 35.
Kirchheim H, Ehmke H, Persson P. Sympathetic modulation of renal hemodynamics, renin release and sodium excretion. Klin Wochenschr 1989; 67: 858–864.
- 36.
Kishimoto T, Maekawa M, Abe Y, Yamamoto K. Intrarenal distribution of blood flow and renin release during renal venous pressure elevation. Kidney Int 1973; 4: 259–266.
- 37.
Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol 1984; 246: F67–F77.
- 38.
Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, et al. Endothelial cell activation in patients with decompensated heart failure. Circulation 2005; 111: 58–62.
- 39.
Ganda A, Onat D, Demmer RT, Wan E, Vittorio TJ, Sabbah HN, et al. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr Heart Fail Rep 2010; 7: 66–74.
- 40.
Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail 2004; 6: 257–260.
- 41.
Chonchol M, Gnahn H, Sander D. Impact of subclinical carotid atherosclerosis on incident chronic kidney disease in the elderly. Nephrol Dial Transplant 2008; 23: 2593–2598.