2024 年 64 巻 2 号 p. 105-108
2024 年 64 巻 2 号 p. 105-108
A 75-year-old man developed sudden-onset tetraparesis preceded by chest pain. MRI of the cervical spine on the day of onset showed no abnormalities. Although his motor symptoms improved gradually, the weakness of the muscles innervated by the C5 nerve root persisted. Sensory and autonomic deficits were detected on an additional neurological examination, and follow-up MRI eight days after onset revealed spinal cord infarction at the right anterior horn at C3–C4. This case suggests that motor symptoms mimicking a radiculopathy could be present during the course of spinal cord infarction.
Spinal cord infarction is a relatively rare disorder, accounting for approximately 1% of all admissions related to vascular pathology of the nervous system1). Many etiologies are associated with the disease, including atherosclerosis, embolism, aortic diseases, vascular surgeries, trauma, and degenerative spinal diseases2)3). Symptoms of the disease include motor paralysis, sensory deficits, and autonomic dysfunctions, corresponding to the neurological functions of the affected segments of the spine. More than half of the cases develop due to occlusion of the anterior spinal artery that supplies the anterior two-thirds of the spinal cord4). This occlusion typically shows acute-onset paraplegia with loss of pain and temperature sensation; however, the severity of these symptoms varies among cases. Other cases of spinal cord infarction result from occlusions of other arteries, such as the posterior spinal arteries. Cases developing unilateral symptoms are not necessarily rare4). As the clinical phenotype of spinal cord infarction is quite variable, rapid and correct diagnosis is sometimes challenging.
The case of a patient with spinal cord infarction presenting with unilateral C5 palsy in the subacute phase is described.
The patient was a 75-year-old, right-handed man who visited a hospital with transient chest pain lasting 40 min. He had no recent episodes of trauma and excessive rotation of the neck. He had hypertension and regularly took amlodipine 5 mg/day. His chest X-ray, electrocardiogram, transthoracic echocardiography, and coronary CT angiography showed no serious findings indicating acute coronary syndrome or aortic dissection. However, 2 h after the onset of chest pain, he suddenly developed tetraparesis with difficulty walking. This motor weakness was right-side dominant, and the upper extremities were preferentially involved. Head MRI showed no acute lesions, and MR angiography (MRA) showed that bilateral vertebral arteries had no severe stenosis (Fig. 1A, B). Cervical MRI 3 h after the onset of tetraparesis showed cervical spondylosis, whereas no abnormal lesions were detected in the spinal cord (Fig. 1C, D). He was admitted to the hospital. Although the motor weakness of his left arm and bilateral legs gradually improved, the weakness of the right proximal arm persisted. Seven days after onset, he was transferred to our hospital for further investigations and treatment.

(A) Head MRI shows no acute ischemic infarctions on diffusion-weighted images. (B) MR angiography shows no severe stenoses in intracranial arteries. (C) (D) Cervical MRI shows cervical spondylosis, whereas no abnormal lesions are observed in the spinal cord. (C) is a sagittal image, and (D) is an axial image at the C4 level. In each figure, “R” indicates the right side, and “A” indicates the anterior side.
On examination at our hospital, his consciousness was intact, and eye movements, pupillary light reflexes, and facial sensation were all normal. Bulbar signs and symptoms were not evident, and there was no weakness of bilateral trapezius and sternocleidomastoid muscles. No muscle atrophy was observed in the limbs or trunk, and there were no fasciculations or other involuntary movements. The arm Barre sign and Mingazzini’s sign were absent bilaterally. The results of the manual muscle test (MMT) were as follows: trapezius 5/5, sternocleidomastoid 5/5, deltoid 2/5, supraspinatus 2/5, infraspinatus 3/5, rhomboids 3/5, biceps brachii 3/5, triceps brachii 5/5, brachioradialis 3/5, supinator 5/5, pronator teres 5/5, abductor pollicis brevis 5/5, abductor digiti minimi 5/5, iliopsoas 5/5, quadriceps 5/5, hamstrings 5/5, tibialis anterior 5/5, and triceps surae 5/5. Notably, the MMT showed weakness of muscles innervated by the right C5 nerve root. His grip strength was right 12 kg and left 17 kg. Deep tendon reflexes were hypoactive in his arms bilaterally, and Babinski’s sign was absent on both sides. On sensory examination, pain and thermal sensation were decreased below the left C8 dermatome, whereas deep sensation was intact. Limb and truncal ataxia was not evident. He could walk alone without assistance. As autonomic symptoms, he had constipation and urinary retention. Anal tone was preserved.
The results of blood analyses were mostly unremarkable, except for an elevated D-dimer level (3.4 μg/ml). Anti-nuclear antibody, anti-nuclear cytoplasmic antibodies, and anti-aquaporin 4 antibody were negative. Anticardiolipin IgG and IgM antibodies and anti-beta 2 glycoprotein 1 antibody were negative. Soluble interleukin-2 receptor was not elevated. Cerebrovascular fluid analysis showed mildly elevated protein (42 mg/dl) without pleocytosis (1/μl). No embolic sources were detected on 12-lead and Holter electrocardiography examinations and transthoracic echocardiography. Ultrasound of the neck showed no apparent vascular dissections, and blood flows were normal at bilateral vertebral arteries. Chest CT did not show aortic dissection or an aneurysm. On the other hand, follow-up cervical MRI eight days after onset showed a high-intensity lesion at the right anterior horn at C3–C4 on diffusion-weighted imaging (DWI) and T2-weighted imaging (Fig. 2A–C). On the apparent diffusion coefficient (ADC) map, the lesion showed iso-intensity (Fig. 2D). The lesion showed partial gadolinium enhancement (Fig. 2E, F). Spinal MRI of the thoracic and lumbar spines did not show other lesions.
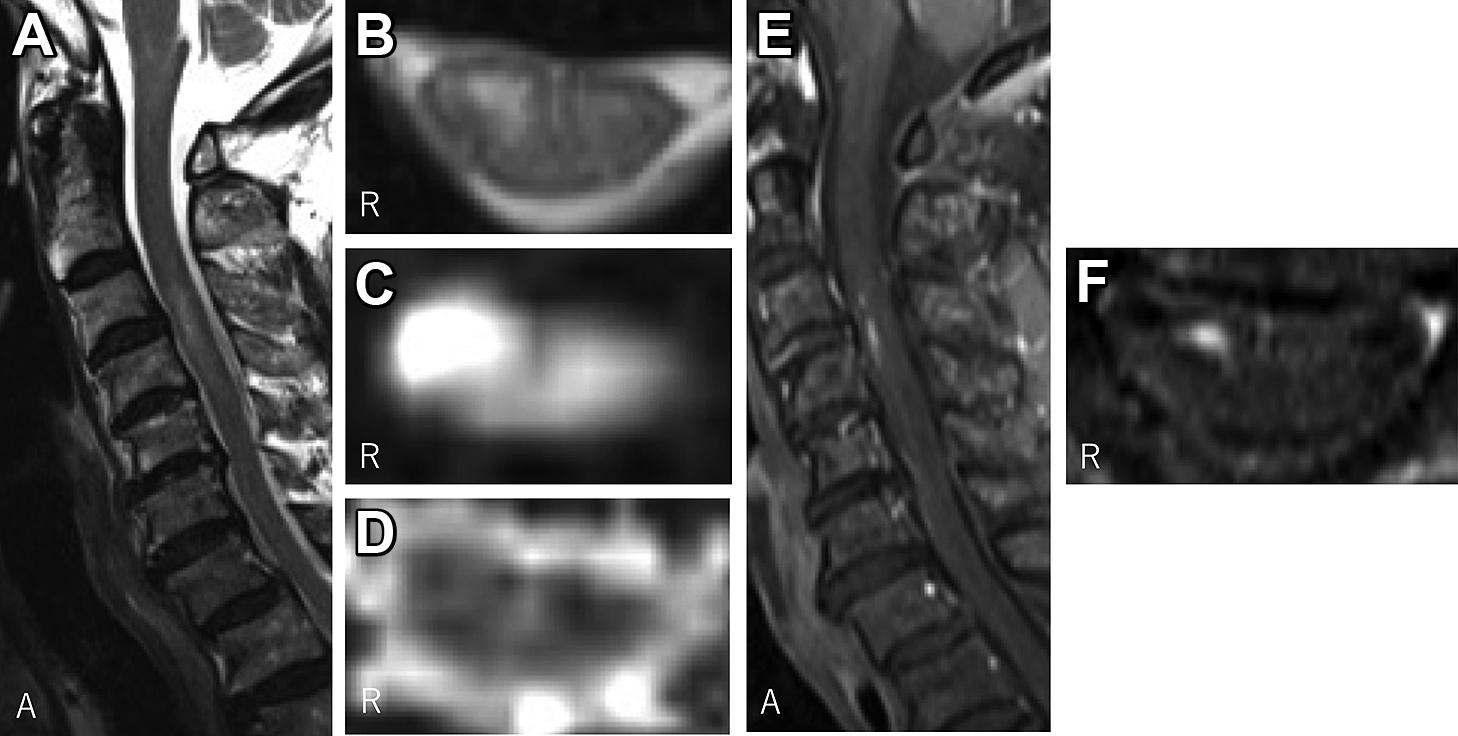
(A) A sagittal T2-weighted image shows a high-intensity lesion at the C3–C4 level. (B) (C) Axial images at the C4 level show a high-intensity lesion at the right anterior horn. (B) is a T2-weighted image, (C) is a diffusion-weighted image, and (D) is an apparent diffusion coefficient map. (E) and (F) are gadolinium-enhanced T1-weighted images. Partial enhancement is observed in the lesion. In each figure, “R” indicates the right side, and “A” indicates the anterior side.
He was diagnosed with a cervical cord infarction. He was treated with cilostazol and underwent physical and occupational rehabilitation. During the course, his bladder disturbance and constipation improved gradually, although his C5 palsy and sensory symptoms persisted. He was returned to the previous hospital 24 days after onset.
A case of cervical cord infarction was described. The present patient initially developed tetraparesis preceded by chest pain. As a cause of acute tetraparesis, cervical cord infarction should be considered along with spinal epidural hematoma, brainstem infarction, transverse myelitis, and Guillain-Barre syndrome5). Acute onset pain often develops with spinal cord infarction4). In the present case, cervical MRI 3 h after onset of tetraparesis did not show ischemic lesions, making diagnosis difficult. However, a previous study reported that only 12.8% of spinal cord infarction cases showed lesions on the first MRI, which means that negative MRI results in the acute phase cannot exclude the disease6). Above all, his clinical features at onset were consistent with spinal cord infarction in many ways.
On the other hand, a prominent complaint of the present patient on admission to our hospital was difficulty in lifting his right upper limb, and neurological examination showed weakness of the muscles innervated by the right C5 root. As for proximal arm weakness, a previous study reported that 3 of 55 patients with spinal cord infarction showed weakness of the proximal upper limbs, so called ‘man-in-the-barrel syndrome’, although all three patients showed the weakness bilaterally7). Generally, monoparesis due to spinal cord infarction is rare, and there are only a few case reports describing such patients8)–10). However, most of these reports did not fully describe the detailed distribution of muscle weakness. Therefore, it remains unknown how often patients with spinal cord infarction show a single nerve root palsy mimicking a radiculopathy. The present case demonstrates the need to consider spinal cord infarction even in patients with monoparesis or a single nerve root palsy.
The vascular etiology and pathophysiology of the present case are not fully understood. Because the bloodstream in the upper cervical cord is usually supplied by vertebral arteries, atherosclerosis or dissection of the arteries could be a cause of cervical cord infarction11). However, the present patient did not show abnormal findings on head MRA and neck ultrasonography. Alternatively, he had cervical spondylosis, which might suggest the possibility of fibrocartilaginous embolism due to dislodged material from the fibrocartilaginous pulposus nucleus12). The tetraparesis at onset indicated broad ischemia in a territory of the anterior spinal artery. The dissociative sensory loss on neurological examination also suggested damage in the vascular area. However, this ischemia would be transient, and damage seemed reversible in most of the lesion. On the other hand, a limited area including the right anterior horn developed infarction, probably due to its lower tolerance for ischemia7). Although why the infarction was limited to the right side is unknown, hypoplasia of the right central sulcal artery or laterality of collateral circulation might have contributed.
Finally, the neurological-radiological correlation in the present patient needs to be considered based on the neuroanatomy of the spinal cord. The motor neurons that supply the axons comprising the C5 nerve root are mainly located on the anterior horn at the C3–4 level, suggesting that the infarction detected by MRI was a cause of his C5 palsy. On the other hand, the present patient showed decreased thermal and pain sensation below the C8 dermatome. Although the reason for this discrepancy was unclear, the arrangement of sensory fibers in the lateral spinothalamic tract might be responsible. The bundle of afferent fibers from the cervical nerve roots is localized at the dorsomedial zone in the tract, whereas fibers from the thoracic and lumbosacral roots lie in the ventrolateral zone13). Considering this anatomy, the infarction in the present patient might have preferentially involved the ventrolateral zone in the tract, sparing the fibers from the upper and middle cervical nerve roots. As for bladder and rectal dysfunction, central spinal damage could be a cause. The infarction in the present case partially involved this area, and several previous cases with unilateral spinal cord infarctions also developed urinary retention, suggesting that a hemicord lesion could be a cause of bladder and rectal dysfunction8)10). In general, with spinal cord lesions, there are often discrepancies between the neurological symptoms and the radiological findings. Understanding the neuroanatomy of the spinal cord is essential for correct diagnosis.
In conclusion, the present case suggested a diversity of clinical symptoms and courses of spinal cord infarction. It could present as monoparesis or a single nerve root palsy, which should be properly differentiated from other diseases, including cerebral infarction and spondylosis. A detailed medical interview and neurological and radiological evaluations will lead to the correct diagnosis.
Abstract of this work was presented at the 125th Kinki Regional Meeting of the Japanese Society of Neurology and recommended by the conference chairperson for the publication to Rinsho Shinkeigaku.