2013 Volume 61 Issue 11 Pages 1105-1113
2013 Volume 61 Issue 11 Pages 1105-1113
A series of isatin derivatives, 1-butyl-5/7-chloro/fluoro-3-((4-methoxybenzyl)imino)indolin-2-ones (3a–d), 6-butyl-chloro/fluoro-6H-indolo[2,3-b]quinoxalines (4a–h), and 5/7-chloro/fluoro-3-((4-methoxybenzyl)imino)indolin-2-ones (5a–h) were synthesized and characterized by using Fourier transform (FT)-IR, 1H- and 13C-NMR spectroscopy, mass spectrometric and elemental analysis. The substances were further subjected to in vitro cytotoxicity evaluation against HeLa, SK-BR-3, and MCF-7 cells. The results showed that quinoxalines 4d, 4e, and 4g; and indolin-2-one 5f display significant in vitro cytotoxic activities against HeLa cells and further the compound 4d has resulted in highest cytotoxicity in the entire series studied. In addition, 5f was shown to display substantial activity against all the three cell lines used in the current study.
Cancer is one of the most serious diseases afflicting mankind and a leading cause of human deaths worldwide. Consequently, a considerable effort has been given to the search for new anticancer drugs that have limited or no harmful side effects.1) During the past few decades, these studies have led to the discovery of a number of small synthetic molecules that display potent anti-carcinogenic activities. Because cancer is characterized by uncontrolled and rapid cell growth and proliferation, effective strategies for its treatment have been aimed at finding novel compounds that inhibit uncontrolled cancer cell proliferation pathways. Numerous synthetic and naturally derived substances have been screened to evaluate their cytotoxic activities.2) Nitrogen heterocycles have retained a high level of interest as privileged structures in medicinal chemistry and have gained great attention owing to their ubiquitous presence in therapeutic agents. Isatin (1H-indole-2,3-dione),3) a naturally occurring member of the vast group of known N-heterocycles, is found in number of plants including those of the genus, Isatis.4) This substance has been reported to possess a wide range of central nervous system activities.5,6) Moreover, -isatin moiety is present in numerous medicinal agents that display broad spectrum of biological properties, such as antiviral,7,8) antifungal,9) antibacterial,10,11) anticancer,12–19) anti-poxvirus,20) and antiproliferation activities.21)
Owing to its chemical reactivity profile and, in particular to the electrophilicity of its C-3 carbonyl group; isatin has been transformed to a wide variety of biologically active 3-substituted indoline-2-ones.22–24) For example, Schiff’s and Mannich base derivatives of isatin have been reported to exhibit anticonvulsant,25) antioxidant,26) and anti-human immunodeficiency virus (HIV) activities.27) In addition, previous studies suggest that isatin derivatives containing halogens and alkyl groups as well as those derived by transformation of the C-3 ketone into imine groups possess enhanced biological properties.28–32) In an investigation aimed to discover new cytotoxic substances, we have utilized the previous observations made out of earlier efforts to design a number of selected halogen substituted isatin compounds containing alkyl and non-alkyl nitrogen substituents. Below, we describe the synthesis and characterization of newly prepared 1-butyl-5/7-chloro/fluoro-2,3-diones (2a–d), 1-butyl-5/7-chloro/fluoro-3-((4-methoxybenzyl)imino)indolin-2-ones (3a–d), 6-butyl-chloro/fluoro-6H-indolo[2,3-b]quinoxalines (4a–h), and 5/7-chloro/fluoro-3-((4-methoxybenzyl)imino)indolin-2-ones (5a–h), and their in vitro cytotoxic activities against HeLa, SK-BR-3, and MCF-7 tumor cells.
The isatin derivatives 2a–d, 3a–d, 4a–h, and 5a–h were synthesized by using the processes as depicted in Chart 1. Commercially available choro/fluoro substituted isatins were treated with n-butyl bromide in dimethyl formamide solution containing cesium carbonate to form the 1-butyl isatins 2a–d. In addition, the 3a–d were generated through the reactions of corresponding halogenated isatins with selected benzylamines in the presence of acetic acid. In a similar manner, reactions of the halogenated isatins with a variety of 1,2-diaminobenzenes in refluxing ethanol, led to the formation of 4a–h. Finally, the 5a–h were produced by the reaction of chloro/fluoro substituted isatins with 4-methoxybenzylamine in refluxing in ethanol containing acetic acid .
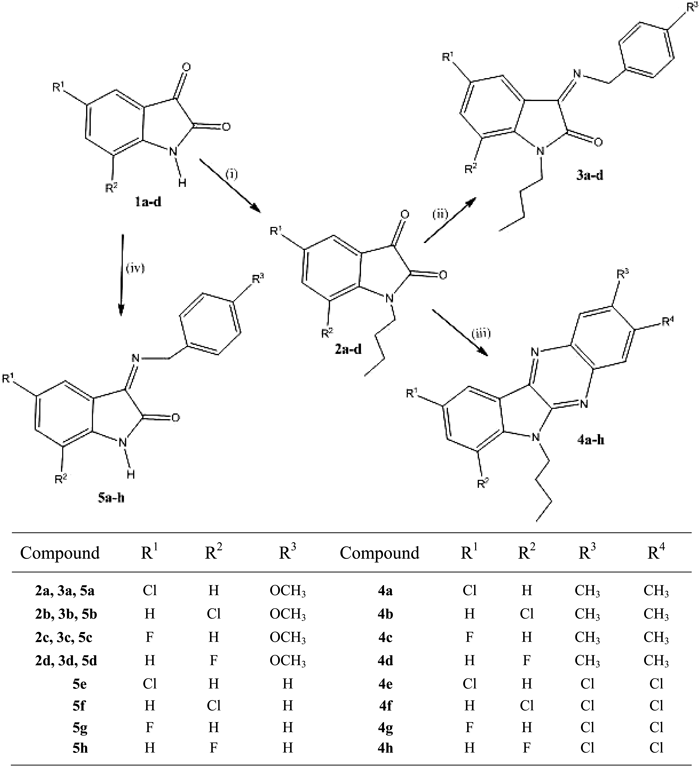
Reagents and conditions: (i) n-BuBr–DMF (ii) benzylamine–ethanol, (iii) 1,2-diaminobenzene–ethanol, and (iv) benzylamine–ethanol.
These structures of the synthetic substances were determined by using Fourier transform (FT)-IR, 1H- and 13C-NMR spectroscopy, GC-mass spectrometric (GC-MS) and elemental analysis. For example, the existence of NH groups and C=N functionality in 5a–h was demonstrated by the presence of respective broad bands at 3408–3421 cm−1 and two sharp bands between 1611–1655 cm−1 in their FT-IR spectra. The NH band is not present in the FT-IR spectra of the 1-butyl-isatin derivatives 2a–d. In addition, an amide C=O stretching band was observed at 1685–1701 cm−1 in the FT-IR spectra of 2a–d, 3a–d, and 5a–d. The GC-MS spectra of all of the synthesized substances display intense molecular ion peaks at expected m/z values. In addition, low intensity peaks corresponding to the loss of CO from the molecular ions are also observed in the GC-MS spectra of 2a–d, 3a–d, and 5a–h. Finally, all substances prepared in this work were observed to contain resonance in their 1H- and 13C-NMR spectra with chemical shift values close to those expected for their structures; and having elemental composition consistent with their proposed structures.
The structure activity study demonstrated that the molecules such as n-butyl isatins 4a–h were fused with diamines that resulted in all the good anticancer activity to Hela, SKBR-3, MCF-7 cell lines. The presence of electron releasing groups, such as methoxy at the para position on a benzene ring, in some compounds 5a–d was associated with less activity. Interestingly, the absence of methoxy group in the compounds 5f, 5g showed the good activity. Particularly, 5f showed the excellent activity against HeLa, SKBR-3, MCF-7 cell lines with IC50 values of 2.33, 6.18, 5.27 µg/mL, respectively. In this fashion, 7-floro indoline derivative 5h was inactive to three cell lines. The introduction of electron releasing group such as n-butyl onto the nitrogen atom (3a–d) resulted in moderate activities.
Cytotoxic ActivityThe cytotoxic activities of the newly synthesized isatin derivatives were determined using HeLa (human cervical cancer), SK-BR-3 (human breast adenocarcinoma), and MCF-7 (human breast adenocarcinoma) cells. The results of the cytotoxicity determinations, made by measuring the number of live cells after 24 h of treatment (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay), are represented by the IC50 values listed in Table 1. The results revealed that all the substances exhibit high anti-proliferative activities against the cancer cells, with IC50 values ranging from 1.78 to >100 µg/mL. Usually, when a compound displays high inhibition rate of more than 50% at 1 µM or more than 85% at 10 µM concentration, it will be considered as strongly cytotoxic. Based on these standards, isatin derivative 5f is of particular interest, because it exhibits marked growth inhibitory activities with IC50 values of 2.33±0.49 µg/mL, 6.18±0.972 µg/mL and 5.27±0.961 µg/mL against HeLa, SK-BR-3 and MCF-7 cells, respectively. However, when considering growth inhibition of only HeLa cells, 4d displays the highest anti-proliferative activity. Moreover 4c, 4f, and 5h were found to have no anti-proliferative activity even when administered at concentrations higher than 100 µg/mL.
| Compound | (IC50b) in µg/mL) | ||
|---|---|---|---|
| HeLa | SK-BR-3 | MCF-7 | |
| 2a | 64.98±1.76 | 71.13±1.21 | 69.20±1.29 |
| 2b | 67.09±1.64 | 85.26±1.16 | 76.73±1.91 |
| 2c | >100 | >100 | >100 |
| 2d | >100 | 89.11±1.12 | 69.47±1.25 |
| 3a | 24.07±1.61 | 29.12±1.09 | 31.79±1.27 |
| 3b | 35.99±1.99 | 42.36±2.31 | 17.34±1.76 |
| 3c | >100 | >100 | >100 |
| 3d | 60.03±2.95 | 68.91±3.21 | 79.67±3.41 |
| 4a | 63.39±3.08 | 58.41±2.95 | 38.41±1.93 |
| 4b | 28.60±1.52 | 26.55±1.20 | 32.30±1.57 |
| 4c | >100 | >100 | >100 |
| 4d | 1.78±0.16 | 43.28±2.33 | 71.98±3.01 |
| 4e | 3.24±0.63 | 29.34±1.46 | 33.97±1.78 |
| 4f | >100 | >100 | >100 |
| 4g | 4.34±0.86 | 59.54±2.89 | 82.92±3.81 |
| 4h | 19.67±1.32 | 31.06±1.28 | 24.61±1.01 |
| 5a | 41.51±2.21 | 37.69±1.91 | 31.94±1.42 |
| 5b | >100 | >100 | 33.09±1.65 |
| 5c | >100 | 62.21±2.33 | 57.91±2.14 |
| 5d | >100 | >100 | 79.67±3.01 |
| 5e | 71.92±3.71 | 87.36±3.02 | >100 |
| 5f | 2.33±0.49 | 6.18±0.972 | 5.27±0.961 |
| 5g | 15.09±1.31 | 46.22±2.67 | 50.01±2.03 |
| 5h | >100 | >100 | >100 |
| Etoposide | 13.73±0.52 | 9.73±0.42 | 15.51±0.66 |
| Camptothecin | 3.1±0.12 | 2.83±0.11 | 3.73±0.24 |
a) Exponentially growing cells were treated with different concentrations of test compounds for 24 h and cell growth inhibition was analyzed through MTT assay. b) IC50 is defined as the concentration, which results in a 50% decrease in cell number as compared with that of the control cultures in the absence of an inhibitor. The values represent the mean±S.D. of five individual observations. Mean percent decrease in cell number of five independent experiments was used to calculate the linear regression equation.
The cytomorphological abnormalities in all of the cell lines caused by isatin derivative 5f were observed by using phase-contrast microscopy. Analysis of a control group of cells, not treated with the isatin derivatives, shows that they contain intact nuclei without any cytological abnormalities (Fig. 1A). In contrast, cells treated with IC50 concentrations of 5f for 24 h display obvious morphological changes, including bulging of the cellular membranes, chromatin condensation, fragmentation and formation of apoptotic bodies (Fig. 1B). Most of the treated cells were found to exhibit the symptoms of apoptosis but, in some cases the damage caused by 5f was so severe that the cell membrane ruptured with subsequent release of cytoplasm (e.g., HeLa cells shown in Fig. 1B). Interestingly, SK-BR-3 cells treated with 5f appear to be circular and nearly all of the treated cells have enlarged membranes (Fig. 1D). The results further indicated that the cell damage promoted by 5f takes place slowly, except in the case of HeLa cells. Also, the cytotoxicity of MCF-7 cells cannot be well delineated owing to clustering of apoptotic bodies within the cellular mass (Fig. 1F).

Left panel corresponds to untreated/normal HeLa, SK-BR-3, and MCF-7 cells (A, C, E, respectively) and the right panel corresponds to HeLa, SK-BR-3, and MCF-7 cells (B, D, F, respectively) treated with IC50 concentrations (2.33, 6.18, 5.27 µg/mL, respectively) of 5f.
The results of the light microscopy studies are consistent with those emanating from investigations carried out using fluorescence microscopy on Hoechst 33342 nuclear stained control and treated cells (Fig. 2). The images of cells treated with 5f contain bright spots corresponding to condensed chromatin, which is a clear indication of early apoptosis leading to deformed nuclear cytoplasmic consistency followed by transformation of chromatin into a horseshoe shaped structure. In addition, karyorrhexis and pyknosis are the characteristic nuclear deformities that are well evident in the treated cells. The current cytotoxic and apoptotic findings are consistent with the previous reports on the concentration and cell line dependent activities of isatin.33,34)

Fluorescent micrographs of normal and treated HeLa, SK-BR-3, and MCF-7 cells. Left panel corresponds to Hoechst 33342 stained nuclei of untreated/normal HeLa, SK-BR-3, and MCF-7 cells (A, C, E, respectively) and the right panel corresponds to HeLa, SK-BR-3, and MCF-7 cells (B, D, F, respectively) treated with IC50 concentrations (2.33, 6.18, 5.27 µg/mL, respectively) of 5f. The cells were detected by using fluorescence light microscopy at 360 nm/470 nm excitation/emission.
An evaluation of apoptosis of HeLa cells promoted by the isatin analogue 4d was carried out by using the dual staining method with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI). The results showed that much greater change takes place in treated as compared to untreated cells (Fig. 3). However, the change is not large when compared to typical Annexin V-FITC/PI dual staining data, a finding which may be a possible consequence of the fact that a single apoptotic cell may produce several individual nuclear fragments. This phenomenon would result incorporation of some of the apoptotic cells in the debris and, as a result, an under estimation of their number. It is worth mentioning that an increase in the amount of greenish brown to red color cell debris takes place in the treated cells in compared to the control (non-fluorescent), a possible result of hyper fragmentation of apoptotic nuclei.

Fluorescence micrographs of control (A) and treated (B) HeLa cells imaged at 200×.
A DNA fragmentation study was performed on HeLa cells treated with 4d. After treatment with various concentrations (5, 10, 20 µg/mL) of 4d, inter-nucleosomal DNA in HeLa cells was observed to undergo fragmentation, as revealed by a DNA ladder evaluation using agarose gel electrophoresis. The genomic DNA extracted from the exposed cells after 24 h was found to display a characteristics ladder pattern of discontinuous DNA fragments, while an untreated control (represented as concentration 0) does not show any signs of fragmentation (Fig. 4). The results, which are consistent with those coming from previous studies,35) suggest that 4d induces apoptosis by internucleosomal DNA cleavage, a biochemical event used as a definitive apoptotic marker that signifies the possibility that 4d is a potential drug lead for cancer chemotherapy.

HeLa cells were treated with different concentrations (0, 5, 10, 20 µg/mL) of 4d for 24 h, DNA from cells was extracted and electrophoresed through a 1.8% agarose gel and visualized by staining with ethidium bromide.
Observations made in the study described above show that 2a–d, 3a–d, 4a–h, and 5a–h display anti-proliferation activities against HeLa, SK-BR-3, and MCF-7 cell lines. Significantly, 4d, 4e, 4g, and 5f were observed to all have remarkable anticancer activities against HeLa cells. Among these substances, 4d exhibits the highest cytotoxicity against HeLa cells and 5f displays substantial activities against all of the cells.
All reagents were used as purchased from commercial suppliers without further purification unless otherwise noted. Melting points were determined by using a Gallenkamp melting-point apparatus and are uncorrected. Thin-layer chromatography (TLC) was performed on Silica Gel F254 plates with visualization by UV or iodine vapor. 1H- and 13C-NMR spectra of DMSO-d6 solutions (tetramethylsilane (TMS) as an internal standard) were recorded on Varian 400 MHz spectrometer. The IR spectra were measured on a Nicolet 380 FT-IR spectrophotometer as KBr pellets or film. The mass spectra were obtained with Bruker 320 GC-MS spectrometer.
General Procedure for the Synthesis of the 1-Butyl-Isatins 2a–dA mixture of 5-chloro isatin (1.81 mg, 10 mmol), 1-bromobutane (2 mL, 18 mmol) and cesium carbonate (13.03 g, 40 mmol) in dimethylformamide (100 mL) was stirred at room temperature overnight. After extraction with ethyl acetate, the organic layer was washed with hydrochloric acid (0.4 N) and water, dried over MgSO4 and concentrated under vacuum, giving a residue that was washed with a mixture of heptane–ethyl acetate to afford 1-butyl-5-chloroindoline-2,3-dione (2a, 2.13 g, 90%) as a red solid. The same procedure was used to prepare 2b–d.
1-Butyl-5-chloroindoline-2,3-dione (2a): Red solid, yield: 90%, mp 113–114°C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.57 (d, J=6.8 Hz, 1H, H-7), 7.47 (s, 1H, H-4), 7.08 (d, J=7.8 Hz, 1H, H-6), 4.13 (t, J=6.8 Hz, 2H, N–CH2), 1.65 (m, 2H, N–CH2–CH2), 1.35 (m, 2H, –CH2–H3), 0.97 (t, J=7.32 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 161.3, 156.3, 146.4, 142.3, 132.4, 126.4, 123.9, 113.4, 49.3, 30.2, 19.8, 13.7; GC-MS: m/z (%): [M+·] 237.11 (100.0), 239.12 (32.91), 211.19 (26.42), 134.16 (38.24), 124.12 (28.76), 209.19 (36.87), 124.15 (36.85), 108.12 (33.39), 91.13 (87.98), 57.12 (42.19), 43.12 (39.12); IR (KBr) cm−1: 1723 C=O, 1678 C=O; Anal. Calcd for C12H12ClNO2: C, 60.64; H, 5.09; N, 5.89. Found C, 60.60; H, 5.11; N, 5.87.
1-Butyl-7-chloroindoline-2,3-dione (2b): Yellow solid, yield: 79%, mp 109–110°C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.45 (d, J=7.4 Hz, 1H, H-6), 7.02 (t, J=7.0 Hz, 1H, H-4), 6.87 (d, J=7.4 Hz, 1H, H-5), 4.11 (t, J=6.6 Hz, 2H, N–CH2), 1.63 (m, 2H, N–CH2–CH2), 1.35 (m, 2H, –CH2–CH3), 0.97 (t, J=7.30 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 164.2, 157.7, 141.2, 136.1, 131.4, 130.7, 124.9, 119.4, 43.3, 31.3, 19.9, 14.2; GC-MS: m/z (%): [M+·] 237.13 (100.0), 239.12 (32.91), 210.17 (29.37), 180.12 (33.17), 114.12 (30.22), 112.13 (28.54), 91.10 (54.21), 57.17 (35.13), 43.14 (30.17); IR (KBr) cm−1: 1740 C=O, 1682 C=O; Anal. Calcd for C12H12ClNO2: C, 60.64; H, 5.09; N, 5.89. Found C, 60.61; H, 5.07; N, 5.86.
1-Butyl-5-fluoroindoline-2,3-dione (2c): Yellow solid, yield: 82%, mp 121–122°C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.57 (d, J=7.2 Hz, 1H, H-7), 7.41(s, 1H, H-4), 7.01 (d, J=7.4 Hz, 1H, H-6), 4.10 (t, J=6.8 Hz, 2H, N–CH2), 1.65 (m, 2H, N–CH2–CH2), 1.37 (m, 2H, –CH2–CH3), 0.99 (t, J=7.26 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 167.5, 164.4, 159.2, 144.3, 130.1, 123.1, 121.0, 112.8, 43.0, 32.1, 20.5, 17.1; GC-MS: m/z (%): [M+·] 221.15 (100.0), 122.12 (28.88), 210.19 (36.87), 193.12 (36.85), 134.19 (38.32), 108.12 (32.43), 91.12 (65.13), 43.17 (44.11); IR (KBr) cm−1: 1728 C=O, 1675 C=O; Anal. Calcd for C12H12FNO2: C, 65.15; H, 5.47; N, 6.33. Found C, 65.18; H, 5.46; N, 6.30.
1-Butyl-5-fluoroindoline-2,3-dione (2d): Pale yellow solid, yield: 79%, mp 124–125°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.89 (d, J=7.2 Hz, 1H, H-4), 7.01 (t, J=7.2 Hz, 1H, H-6), 7.47 (d, J=7.2 Hz, 1H, H-6), 4.11 (t, J=6.6 Hz, 2H, N–CH2), 1.64 (m, 2H, N–CH2–CH2), 1.37 (m, 2H, –CH2–CH3), 0.97 (t, J=7.30 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 167.8, 164.0, 161.3, 135.8, 129.5, 122.2, 120.6, 119.3, 44.0, 33.2, 21.6, 17.9; GC-MS: m/z (%): [M+·] 221.12 (100.0), 163.13 (34.12), 136.17 (30.23), 108.12 (32.43), 91.12 (65.13), 52.12 (38.19), 43.11 (39.54); IR (KBr) cm−1: 1723 C=O, 1678 C=O; Anal. Calcd for C12H12FNO2: C, 65.15; H, 5.47; N, 6.33. Found C, 65.17; H, 5.49; N, 6.31.
General Procedure for the Synthesis of 3a–hA solution of 1-butyl-5-chloroindoline-2,3-dione (2a) (0.71 g, 3 mmol) and 4,5-dimethylbenzene-1,2-diamine (0.40 g, 3 mmol) in acetic acid (10 mL) was stirred at reflux for 30 min. The progress of the reaction was monitored by using TLC (n-hexane–ethyl acetate 5 : 1). The pale yellow colored precipitate obtained upon cooling was isolated by filteration and washed with water. The precipitate was triturated with chloroform to yield a solid that was crystallized from acetic acid to afford yellow solid, 1-butyl-5-chloro-3-((4-methoxybenzyl)imino)indolin-2-one (3a, 0.85 g, 80%). The same experimental procedure was used to prepare 3b–d.
1-Butyl-5-chloro-3-((4-methoxybenzyl)imino)indolin-2-one (3a): Yellow solid, yield: 80%, mp 124–125°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.76–7.84 (m, 7H, ArH), 4.13 (t, J=6.88 Hz, 2H, N–CH2), 4.86 (s, 2H, Ar–CH2), 3.84 (s, 3H, O–CH3), 1.67 (m, 2H, N–CH2–CH2), 1.38 (m, 2H, –CH2–CH3), 0.98 (t, J=7.28 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 163.2, 162.2, 157.8, 145.8, 141.1, 131.7, 131.6, 130.8, 129.8, 125.3, 124.3, 114.5, 57.6, 55.9, 48.2, 29.3, 19.5, 13.6; GC-MS: m/z (%): [M+·] 356.14 (100.0), 358.13 (31.98), 328.12 (60.15), 326.24 (23.56), 291.21 (29.47), 269.15 (31.34), 263.19 (26.29), 122.12 (28.88), 210.19 (36.87), 124.15 (36.85), 108.12 (33.39), 91.13 (87.98), 57.12 (42.19); IR (KBr) cm−1: 1740 C=O, 1621 C=N; Anal. Calcd for C20H21ClN2O2: C, 67.32; H, 5.93; N, 7.85. Found C, 67.28; H, 5.91; N 7.87.
1-Butyl-7-chloro-3-((4-methoxybenzyl)imino)indolin-2-one (3b): Yellow solid, yield: 79%, mp 116–118°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.79–7.85 (m, 7H, ArH), 4.85 (s, 2H, Ar–CH2), 4.15 (t, J=6.84 Hz, 2H, N–CH2), 3.82 (s, 3H, O–CH3), 1.69 (m, 2H, N–CH2–CH2), 1.39 (m, 2H, –CH2–CH3), 0.96 (t, J=7.26 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 163.4, 162.4, 157.9, 145.9, 141.0, 131.7, 131.4, 130.7, 129.7, 125.6, 124.5, 114.6, 57.6, 55.8, 48.5, 29.5, 19.8, 13.9; GC-MS: m/z (%): [M+·] 356.11 (100.0), 358.14 (31.65), 328.14 (42.17), 326.17 (36.67), 291.13 (33.46), 269.12 (34.35), 263.17 (26.29), 124.25 (46.15), 122.13 (38.12), 108.13 (39.47), 91.12 (55.24), 57.14 (37.56); IR (KBr) cm−1: 1743 C=O, 1621 C=N; Anal. Calcd for C20H21ClN2O2: C, 67.32; H, 5.93; N, 7.85. Found C, 67.28; H 5.91; N, 7.87.
1-Butyl-5-fluoro-3-((4-methoxybenzyl)imino)indolin-2-one (3c): Reddish brown solid, yield: 86%, mp 131–135°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.76–7.88 (m, 7H, ArH), 4.81 (s, 2H, Ar–CH2), 4.17 (t, J=6.80 Hz, 2H, N–CH2), 3.85 (s, 3H, O–CH3), 1.69 (m, 2H, N–CH2–CH2), 1.38 (m, 2H, –CH2–CH3), 0.99 (t, J=7.24 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 163.9, 163.0, 158.2, 145.7, 141.3, 131.9, 131.1, 130.1, 129.7, 125.9, 124.6, 114.8, 57.7, 55.8, 48.3, 29.4, 19.8, 13.7; GC-MS: m/z (%): [M+·] 340.14 (100.0), 312.11 (58.25), 283.13 (42.43), 233.18 (27.32), 163.12 (37.54), 145.13 (35.57), 108.12 (54.12), 91.14 (51.41), 57.13 (42.45); IR (KBr) cm−1: 1731 C=O, 1616 C=N; Anal. Calcd for C20H21FN2O2: C, 70.57; H, 6.22; N, 8.23. Found C, 70.60; H, 6.20; N, 8.21.
1-Butyl-7-fluoro-3-((4-methoxybenzyl)imino)indolin-2-one (3d): Yellow solid, yield: 82%, mp 121–123°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.77–7.86 (m, 7H, ArH), 4.83 (s, 2H, Ar–CH2), 4.14 (t, J=6.68 Hz, 2H, N–CH2), 3.85 (s, 3H, O–CH3), 1.71 (m, 2H, N–CH2–CH2), 1.39 (m, 2H, –CH2–CH3), 0.96 (t, J=7.40 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 165.1, 163.3, 161.3, 160.7, 158.3, 134.2, 131.9, 128.8, 127.1, 125.3, 117.2, 59.1, 57.0, 43.5, 31.2, 30.7, 21.4, 14.3; GC-MS: m/z (%): [M+·] 340.11 (100.0), 312.15 (39.33), 283.21 (31.23), 210.13 (30.55), 163.11 (30.25), 145.18 (40.75), 108.11 (32.33), 91.14 (32.25), 57.13 (37.38); IR (KBr) cm−1: 1742 C=O, 1637 C=N; Anal. Calcd for C20H21FN2O2: C, 70.57; H, 6.22; N, 8.23. Found C, 70.59; H, 6.20; N, 8.21.
General Procedure for the Synthesis of 4a–hA solution of 1-butyl-5-chloroindoline-2,3-dione (2a) (0.71 g, 3 mmol) and 4, 5-dimethylbenzene-1,2-diamine (0.40 g, 3 mmol) in 25 mL ethanol containing 5 drops of acetic acid was stirred at reflux for 1 h. The progress of the reaction was monitored by using TLC methanol (chloroform, 3 : 7). After 3 h the reaction mixture was cooled to room temperature and extracted with chloroform. The chloroform extracts were dried over Na2SO4 and concentrated under reduced pressure to give a residue that was crystallized from acetic acid to afford 6-butyl-9-chloro-2,3-dimethyl-6H-indolo-[2,3-b]quinoxaline (4a, 0.84 g, 83%) as a reddish brown solid. The same experimental procedure was employed to prepare 4b–h.
6-Butyl-9-chloro-2,3-dimethyl-6H-indolo-[2,3-b]quinoxaline (4a): Reddish brown solid, yield: 83%, mp 112–114°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.86–7.92 (m, 5H, ArH), 4.21 (t, J=6.80 Hz, 2H, N–CH2), 2.23 (s, 6H, Ar–CH3), 1.71 (m, 2H, N–CH2–CH2), 1.39 (m, 2H, –CH2–CH3), 0.95 (t, J=7.44 Hz, 3H, –CH3); 0.95 (t, J=7.44 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 163.4, 162.4, 145.9, 141.5, 141.0, 136.8, 131.5, 129.8, 129.7, 125.6, 124.5, 48.6, 29.5, 19.5, 18.9, 13.8; GC-MS: m/z (%): [M+·] 337.13 (100.0), 339.13 (31.85), 302.13 (40.13), 280.17 (33.45), 234.13 (40.55), 178.18 (29.19), 105.35 (23.16), 91.13 (38.12), 57.34 (32.26); IR (KBr) cm−1: 1639 C=N, 1611 C=N; Anal. Calcd for C20H20ClN3: C, 71.10; H, 5.97; N, 12.44. Found C, 71.08; H, 5.94; N, 12.41.
6-Butyl-7-chloro-2,3-dimethyl-6H-indolo-[2,3-b]quinoxaline (4b): Faint yellow solid, yield: 81%, mp 119–121°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.88–7.91 (m, 5H, ArH), 4.18 (t, J=6.84 Hz, 2H, N–CH2), 2.19 (s, 6H, Ar–CH3), 1.75 (m, 2H, N–CH2–CH2), 1.37 (m, 2H, –CH2–CH3), 0.98 (t, J=7.62 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 165.7, 163.5, 146.7, 141.9, 141.4, 136.1, 132.2, 129.9, 129.4, 127.8, 124.8, 49.6, 31.3, 21.2, 14.3; GC-MS: m/z (%): [M+·] 337.17 (100.0), 339.14 (33.20), 302.16 (29.85), 273.11 (30.26), 234.18 (37.30), 178.11 (32.28), 105.13 (34.23), 91.13 (38.47), 57.11 (37.15); IR (KBr) cm−1: 1655 C=N, 1630 C=N; Anal. Calcd for C20H20ClN3: C, 71.10; H, 5.97; N, 12.44. Found C, 71.14; H, 5.93; N, 12.41.
6-Butyl-9-fuloro-2,3-dimethyl-6H-indolo-[2,3-b]quinoxaline (4c): Brown solid, yield: 77%, mp 125–127°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.89–7.87 (m, 5H, ArH), 4.15 (t, J=6.78 Hz, 2H, N–CH2), 2.25 (s, 6H, Ar–CH3), 1.78 (m, 2H, N–CH2–CH2), 1.35 (m, 2H, –CH2–CH3), 0.96 (t, J=7.80 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 163.7, 162.6, 145.7, 142.1, 141.3, 137.7, 131.5, 129.5, 129.2, 128.1, 125.7, 124.3, 48.9, 29.1, 19.9, 13.8; GC-MS: m/z (%): [M+·] 321.17 (100.0%), 295.20 (34.45), 256.23 (33.30), 189.17 (39.50), 105.15 (30.50), 91.10 (51.27), 57.31 (36.15); IR (KBr) cm−1: 1639 C=N, 1612 C=N; Anal. Calcd for C20H20FN3: C, 74.74; H, 6.27; N, 13.07. Found C, 74.71; H, 6.25. N, 13.09.
6-Butyl-7-fuloro-2,3-dimethyl-6H-indolo-[2,3-b]quinoxaline (4d): Yellow solid, yield: 80%, mp 125–127°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.86–7.93 (m, 5H, ArH), 4.18 (t, J=6.86 Hz, 2H, N–CH2), 2.17 (s, 6H, Ar–CH3), 1.76 (m, 2H, N–CH2–CH2), 1.38 (m, 2H, –CH2–CH3), 0.99 (t, J=7.24 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 165.1, 163.2, 162.6, 146.0, 141.7, 141.2, 136.9, 131.3, 129.7, 129.5, 125.7, 124.8, 48.5, 29.3, 19.1, 13.0. GC-MS: m/z (%): [M+·] 321.13 (100.0%), 265.13 (30.15), 219.12 (28.27), 162.13 (21.22), 158.16 (23.21), 91.17 (82.42), 77.15 (27.21), IR (KBr) cm−1: 1643 C=N, 1619 C=N; Anal. Calcd for C20H20FN3: C, 74.74; H, 6.27; N, 13.07. Found C, 74.70; H, 6.29; N, 13.05.
6-Butyl-2,3,9-trichloro-6H-indolo[2,3-b]quinoxaline (4e): Brown solid, yield: 88%, mp 107–109°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.78–7.84 (m, 5H, ArH), 4.19 (t, J=6.80 Hz, 2H, N–CH2), 1.75 (m, 2H, N–CH2–CH2), 1.39 (m, 2H, –CH2–CH3), 0.98 (t, J=7.80 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 149.9, 148.2, 144.3, 140.9, 138.3, 137.7, 135.9, 130.8, 126.9, 124.6, 123.1, 113.7, 58.7, 33.3, 22.1, 14.2; GC-MS: m/z (%): [M+·] 377.04 (100.0%), 378.13 (19.50), 379.11 (89.24), 381.09 (27.35), 362.05 (20.10), 293.19 (30.51), 257.18 (33.17), 182.15 (39.23), 105.23 (30.17), 91.15 (42.17), 57.32 (36.13); IR (KBr) cm−1: 1638 C=N, 1612 C=N; Anal. Calcd for C18H14Cl3N3: C, 57.09; H, 3.73; N, 11.10. Found C, 57.12; H, 3.75; N, 11.11.
6-Butyl-2,3,7-trichloro-6H-indolo[2,3-b]quinoxaline (4f): Brown solid, yield: 78%, mp 112–114°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.79–7.88 (m, 5H, ArH), 4.18 (t, J=6.84 Hz, 2H, N–CH2), 1.75 (m, 2H, N–CH2–CH2), 1.37 (m, 2H, –CH2–CH3), 0.98 (t, J=7.62 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 163.5, 162.4, 145.8, 143.1, 141.4, 136.8, 135.9, 131.4, 129.7, 129.5, 126.7, 124.5, 49.2, 29.5, 19.5, 13.9; GC-MS: m/z (%): [M+·] 377.11 (100.0%), 378.10 (20.24), 379.15 (86.35), 381.11 (25.50), 362.12 (32.15), 293.45 (34.10), 257.11 (33.17), 182.15 (39.23), 105.11 (32.24), 91.11 (33.12); IR (KBr) cm−1: 1643 C=N, 1624 C=N; Anal. Calcd for C18H14Cl3N3: C, 57.09; H, 3.73; N, 11.10. Found C, 57.06; H, 3.78; N, 11.14.
6-Butyl-2,3-dichloro-9-fluoro-6H-indolo[2,3-b]quinoxaline (4g): Yellow solid, yield: 79%, mp 109–101°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.78–7.89 (m, 5H, ArH), 4.15 (t, J=6.92 Hz, 2H, N–CH2), 1.79 (m, 2H, N–CH2–CH2), 1.39 (m, 2H, –CH2–CH3), 0.97 (t, J=7.80 Hz, 3H, –CH3): 13C-NMR (100 MHz, DMSO-d6) δ: 163.3, 162.6, 145.7, 142.1, 141.3, 136.7, 135.7, 131.5, 129.7, 129.5, 125.7, 124.4, 48.9, 28.8, 19.9, 14.1, GC-MS: m/z (%): [M+·] 363.13 (67.25), 365.13 (13.50), 362.17 (18.90), 361.12 (100.0), 310.15 (30.14), 282.17 (32.16), 234.18 (34.20), 178.18 (30.23), 105.23 (32.18), 91.15 (39.15), 57.44 (37.19); IR (KBr) cm−1: 1639 C=N, 1611 C=N; Anal. Calcd for C18H14Cl2FN3: C, 59.68; H, 3.90; N, 11.60. Found C, 59.65; H, 3.93; N, 11.57.
6-Butyl-2,3-dichloro-7-fluoro-6H-indolo[2,3-b]quinoxaline (4h): Brown solid, yield: 84%, mp 141–143°C. 1H-NMR (400 MHz, DMSO-d6) δ: 6.79–7.87 (m, 5H, ArH), 4.18 (t, J=6.88 Hz, 2H, N–CH2), 1.76 (m, 2H, N–CH2–CH2), 1.38 (m, 2H, –CH2–CH3), 0.99 (t, J=7.86 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 149.5, 148.9, 147.8, 144.3, 141.0, 138.3, 130.4, 128.9, 124.1, 122.3, 118.9, 110.6, 59.3, 34.4, 21.2, 14.6; GC-MS: m/z (%): [M+·] 363.17 (69.13), 365.11 (12.85), 362.13 (19.92), 361.15 (100.0), 310.12 (32.25), 234.11 (30.75), 189.13 (30.55), 105.12 (22.11), 91.10 (33.30), 57.13 (30.10); IR (KBr) cm−1: 1645 C=N, 1618 C=N; Anal. Calcd for C18H14Cl2FN3: C, 59.68; H, 3.90; N, 11.60. Found C, 59.65; H, 3.93; N, 11.57.
General Procedure for the Synthesis of 5a–hA solution of 5-chloroisatin (1.09 g, 6 mmol) and 4-methoxybenzylamine (0.65 mL, 6 mmol) in 25 mL ethanol containing 5 drops of acetic acid was stirred at reflux for 1 h. The progress of the reaction was monitored by using TLC (n-hexane–ethyl acetate, 2 : 1, v/v). After completion of reaction, the mixture was cooled to room temperature. The formed brown solid was isolated by filtration, washed with ice-cold water and then crystallized from hot ethanol to afford 5-chloro-3-((4-methoxybenzyl)imino)indolin-2-one (5a, 1.41 g, 78%) as a crystalline brown solid. The same experimental procedure was used to prepare 5b–h.
5-Chloro-3-((4-methoxybenzyl)imino)indolin-2-one (5a): Brown solid, yield: 78%, mp 127–129°C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.46 (br s, 1H, NH), 6.87–7.90 (m, 7H, ArH), 4.86 (s, 2H, Ar–CH2), 3.86 (s, 3H, O–CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 160.2, 157.7, 151.2, 147.6, 135.1, 132.6, 132.1, 131.5, 130.2, 129.8, 124.3, 114.7, 114.51, 58.1, 55.9, GC-MS: m/z (%): [M+·] 300.12 (34.15), 302.17 (34.15), 287.14 (30.50), 272.14 (62.15), 235.25 (40.25), 178.12 (33.33), 124.12 (36.85), 153.17 (58.50), 105.19 (53.50), 91.10 (68.25), 57.13 (30.28); IR (KBr) cm−1: 3421 NH, 1690 C=O, 1619 C=N; Anal. Calcd for C16H13ClN2O2: C, 63.90; H, 4.36; N, 9.31. Found C, 63.96; H, 4.39; N, 9.27.
7-Chloro-3-((4-methoxybenzyl)imino)indolin-2-one (5b): Pale yellow solid, yield: 73%, mp 151–153°C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.13 (br s, 1H, NH), 6.84–7.89 (m, 7H, ArH), 4.87 (s, 2H, Ar–CH2), 3.85 (s, 3H, O–CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 160.4, 157.9, 151.5, 147.7, 135.3, 132.9, 132.6, 130.4, 130.1, 129.9, 124.5, 114.9, 58.3, 55.6, GC-MS: m/z (%): [M+·] 300.17 (34.15), 302.13 (34.15), 280.19 (45.45), 272.11 (57.45), 234.35 (30.50), 180.26 (30.25), 153.14 (51.78), 124.15 (41.55), 105.15 (23.60), 91.17 (40.50), 57.10 (30.28); IR (KBr) cm−1: 3419 NH, 1693 C=O, 1612 C=N; Anal. Calcd for C16H13ClN2O2: C, 63.90; H, 4.36; N, 9.31. Found C, 63.95; H, 4.37; N, 9.35.
5-Fluoro-3-((4-methoxybenzyl)imino)indolin-2-one (5c): Brown solid, yield: 77%, mp 124–126°C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.36 (br s, 1H, NH), 6.87–7.90 (m, 7H, ArH), 4.86 (s, 2H, Ar–CH2), 3.88 (s, 3H, O–CH3); 13C-NMR (100 MHz, DMSO-d6) δ: 160.5, 157.9, 151.3, 147.8, 135.3, 132.7, 130.6, 130.1, 128.8, 124.5, 114.8, 114.1, 58.3, 45.7; GC-MS: m/z (%): [M+·] 284.15 (100.0), 256.13 (64.30), 235.11 (55.15), 178.21 (35.50), 137.18 (56.12), 108.13 (40.70), 105.20 (23.80), 91.13 (59.65), 57.18 (33.50); IR (KBr) cm−1: 3408 NH, 1687 C=O, 1615 C=N; Anal. Calcd for C16H13FN2O2: C, 67.60; H, 4.61; N, 9.85. Found C, 67.64; H, 4.65; N, 9.81.
7-Fluoro-3-((4-methoxybenzyl)imino)indolin-2-one (5d): Reddish brown solid, yield: 80%, mp 119–121°C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.38 (br s, 1H, NH), 6.87–7.89 (m, 7H, ArH), 4.87 (s, 2H, Ar–CH2), 3.87 (s, 3H, O–CH3); 13C-NMR (100 MHz, DMSO-d6) δ :160.4, 157.8, 151.4, 147.8, 135.4, 132.7, 132.3, 130.5, 130.0, 129.8, 124.4, 114.6, 58.2, 55.8; GC-MS: m/z (%): [M+·] 284.13 (100.0), 256.16 (55.57), 254.25 (32.15), 178.24 (34.75), 137.13 (62.34), 108.11 (52.65), 105.50 (35.25), 91.15 (41.60), 57.19 (30.25); IR (KBr) cm−1: 3413 NH, 1692 C=O, 1613 C=N; Anal. Calcd for C16H13FN2O2: C, 67.60; H, 4.61; N, 9.85. Found C, 67.55; H, 4.65; N, 9.82.
3-(Benzylimino)-5-chloroindolin-2-one (5e): Brown solid, yield: 72%, mp 127–129°C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.06 (br s, 1H, NH), 6.87–7.92 (m, 7H, ArH), 4.86 (s, 2H, Ar–CH2); 13C-NMR (100 MHz, DMSO-d6) δ: 160.5, 157.6, 151.3, 147.6, 135.4, 132.5, 130.9, 130.2, 129.9, 124.5, 114.7, 114.1, 45.8; GC-MS: m/z (%): [M+·] 270.13 (100.0), 272.12 (33.2), 251.17 (40.25), 242.14 (58.46), 189.23 (29.50), 153.15 (55.57), 124.13 (52.30), 105.24 (32.10), 91.19 (38.80), 57.45 (30.25); IR (KBr) cm−1: 3420 NH, 1685 C=O, 1616 C=N; Anal. Calcd for C15H11ClN2O: C, 66.55; H, 4.10; N, 10.35. Found C, 66.51; H, 4.14; N, 10.32.
3-(Benzylimino)-7-chloroindolin-2-one (5f): Yellow solid, yield: 76%, mp 151–153°C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.14 (br s, 1H, NH), 6.89–7.91 (m, 7H, ArH), 4.85 (s, 2H, Ar–CH2); 13C-NMR (100 MHz, DMSO-d6) δ: 160.3, 157.7, 151.4, 147.6, 135.1, 132.8, 132.1, 130.5, 129.8, 124.3, 114.7, 114.0, 45.9; GC-MS: m/z (%): [M+·] 270.13 (100.0), 272.12 (33.2), 242.11 (65.25), 234.12 (29.50), 178.20 (33.10), 153.11 (59.34), 124.15 (62.50), 105.25 (33.75), 91.11 (39.25), 57.34 (32.60); IR (KBr) cm−1: 3419 NH, 1701 C=O, 1613 C=N; Anal. Calcd for C15H11ClN2O: C, 66.55; H, 4.10; N, 10.35. Found C, 66.59; H, 4.13; N, 10.30.
3-(Benzylimino)-5-fluoroindolin-2-one (5g): Reddish brown solid, yield: 81%, mp 124–126°C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.75 (br s, 1H, NH), 6.86–7.89 (m, 7H, ArH), 4.86 (s, 2H, Ar–CH2); 13C-NMR (100 MHz, DMSO-d6) δ: 168.5, 163.3, 159.2, 140.1, 138.3, 129.3, 128.1, 127.3, 126.7, 119.7, 113.8, 111.7, 58.6; GC-MS: m/z (%): [M+·] 254.19 (100.0), 228.14 (30.18), 226.12 (60.15), 178.23 (41.23), 137.11 (49.54), 108.17 (36.45), 105.12 (41.19), 91.18 (56.50), 57.14 (34.25); IR (KBr) cm−1: 3410 NH, 1697 C=O, 1617 C=N; Anal. Calcd for C15H11FN2O: C, 70.86; H, 4.36; N, 11.02. Found C, 70.91; H, 4.42; N, 11.05.
3-(Benzylimino)-7-fluoroindolin-2-one (5h): Brown solid, yield: 77%, mp 119–121°C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.03 (br s, 1H, NH), 6.87–7.91 (m, 7H, ArH), 4.85 (s, 2H, Ar–CH2); GC-MS: m/z (%): [M+·] 254.17 (100.0), 245.15 (40.11), 226.15 (47.37), 178.17 (39.21), 137.13 (49.55), 108.14 (58.23), 105.18 (33.18), 91.11 (33.11), 57.14 (31.19); 13C-NMR (100 MHz, DMSO-d6) δ: 168.9, 166.1, 163.3, 139.7, 129.3, 128.7, 128.0, 126.8, 125.6, 125.4, 125.0, 119.1, 58.4; IR (KBr) cm−1: 3412 NH, 1689 C=O, 1620 C=N; Anal. Calcd for C15H11FN2O: C, 70.86; H, 4.36; N, 11.02. Found C, 70.93; H, 4.39; N, 10.08.
Cell CultureHeLa (human cervical cancer), SK-BR-3 (human breast adenocarcinoma), and MCF-7 (human breast adenocarcinoma) cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from BioWhittaker®; RPMI-1640 media, fetal bovine serum (FBS) and other cell culture materials were purchased from Sigma® (U.S.A.). Cells were cultured in DMEM media supplemented with 10% (v/v) heat-inactivated FBS, 100 units/mL penicillin and 100 µg/mL streptomycin. Cells were maintained in culture media at 37°C in an atmosphere of 5% CO2. Paraformaldehyde and Bisbenzimide Hoechst 33342 stain were procured from Sigma-Aldrich Corp., St. Louis, MO, U.S.A.; MTT from Biosesang Inc., Korea.
MTT AssayCell proliferation/viability was measured using the MTT assay.36) Both HeLa and MCF-7 cells were cultured in T-75 tissue culture flasks (Nunc, Denmark) at 37°C in a 5% CO2 humidified incubator using appropriate media supplemented with DMEM containing 10% heat-inactivated FBS. Similarly, SK-BR-3 cells were cultured in RPMI-1640 media. Cells were seeded in each well containing 100 µL medium at a final density of 2×104 cells/well, in a 96 well microtiter plates at identical conditions. After overnight incubation, the cells were treated with different concentrations of test compounds (1–100 µg/mL) or dimethyl sulfoxide (DMSO) (carrier solvent) in a final volume of 200 µL with three replicates each. After 24 h, 10 µL of MTT (5 mg/mL) was added to each well and the plate was incubated at 37°C in the dark for 4 h. Then the media along with MTT was removed and the formazan crystals were solubilized by adding DMSO (100 µL/well). Finally, the reduction of MTT was quantified by reading the absorbance at 570 nm by using GENios® microplate reader (Tecan Austria GmbH, Austria). Effects of the test compounds on cell viability were calculated using cells treated with DMSO as control. The data were subjected to linear regression analysis and the regression lines were plotted for the best straight-line fit. The IC50 (the concentration at which 50% of the cells are dead) concentrations were calculated using the respective regression equation.
Microscopic StudiesThe altered morphologies of exposed cells (1×105/well) at different concentrations were evaluated after 24 h using a phase contrast microscope (DMI6000B, Leica Microsystems, Wetzlar, Germany). Subsequently, the cells were Hoechst stained to observe the occurrence of nuclear/chromosomal condensation promoted by treatment of the synthesized compounds. For cell staining, 96 well cell culture plates were used to culture the cells (1×104 cells/well) in three replicates for treatment with 5f. The cells were then incubated at 37°C overnight and the media was removed to wash the cells twice with phosphate buffered saline (PBS). The cells were then fixed with 4% paraformaldehyde in PBS for 1 d at −4°C. The cells were stained with 1 µg/mL of the fluorescent DNA-binding dye, Bisbenzimide Hoechst 33342 stain and incubated for 20 min at room temperature. The Hoechst-stained cells were visualized and photographed by using fluorescence microscope (CTR 6000; Leica, Wetzlar, Germany).
Annexin-PI Dual Staining for Apoptosis AssessmentAnnexin V-FITC/PC dual staining was performed to determine the level of apoptosis and necrotic cell death in HeLa cells. The staining solutions used were the components of FITC Annexin V in an apoptosis Detection Kit (BD Pharmingen™). Initially, HeLa cells (1×105 cells/well) were treated and untreated (control) with 4d at the IC50 concentration (1.78±0.16 µg/mL) and incubated at 37°C in 5% CO2 atmosphere for 24 h. The cells were harvested using 1X trypsin-ethylenediaminetetraacetic acid (EDTA) solution prepared in serum free DMEM and then washed twice with cold PBS and resuspended in 1X binding buffer (provided with the Kit) at a concentration of 1×105 cells/mL. Then 100 µL of the solution (1×105 cells) was transferred to a 5 mL culture tube followed by the addition of 5 µL of FITC Annexin V, gentle vortexing and incubation for 15 min at room temperature (25°C) in the dark. Subsequently, 2 µL of PI staining solution was added to the Annexin V-FITC labeled cells, which were then incubated for 5 min under similar conditions. Finally, 400 µL of 1X binding buffer was added to each tube. Cells were mounted on glass slides and observed using a fluorescence microscope (CTR 6000; Leica, Wetzlar, Germany) to monitor the presence of apoptotic cells.
DNA Fragmentation AssaySince the best activity is observed specifically in HeLa cells by the compound 4d among all the compounds, DNA damage studies were performed using HeLa cells and 4d as per the previously described method.35) Initially, HeLa cells were harvested, washed with phosphate-buffered saline (PBS), and then lysed with digestion buffer containing 50 mM Tris–HCl, 0.5% sodium dodecyl sulfate (SDS), 1 µg/mL proteinase k (pH 8.0), and 10 mM EDTA at 37°C overnight. The cells were then treated with RNase A (0.5 µg/mL) for 1 h at 37°C. DNA was extracted with phenol–chloroform–isoamyl alcohol (25 : 24 : 1) before loading and analyzed by using 1.8% agarose gel electrophoresis. The agarose gels were run at 50 V for 120 min in Tris-borate/EDTA electrophoresis buffer. Approximately 20 µg of DNA was loaded in each well, visualized under UV light, and photographed.
This work was supported by a Grant from the Basic Science Research Program through NRF funded by the Ministry of Education, Science and Technology of S. Korea (Grant Number: 20100029128).