2013 Volume 61 Issue 12 Pages 1248-1263
2013 Volume 61 Issue 12 Pages 1248-1263
We describe herein the design, syntheses and structure–activity relationships (SAR) of novel zwitterionic compounds as non-thiazolidinedion (TZD) based peroxisome proliferator activated receptor (PPAR) α/γ dual agonists. In the previous report, we obtained compound 1 showing potent PPARα/γ dual agonistic activities, together with a great glucose lowering effect in the db/db mice. However, this compound possessed fatal issues such as potent cytochrome P450 (CYP)3A4 direct inhibitory activity. Thus, we carried out the medicinal optimization to improve these while maintaining the potent PPAR agonistic activity. As a result, the issues were addressed by changing the furan ring to a low lipophilic 1,3,4-oxadiazole ring. Additionally, these oxadiazole derivatives exhibited a significant decrease in plasma glucose and plasma triglyceride levels without marked weight gain.
The number of people living with diabetes is growing rapidly worldwide. While the number of individuals with diabetes was 151 million in 2002, this number is 366 million at present and is estimated to be 552 million by 2030.1) Of these cases, type 2 diabetes is the majority in both developing and developed countries, whose populations currently account for >90% of all diabetes worldwide.2) In the type 2 diabetic people who show it, insulin resistence poses a cardiovascular risk factor including dyslipidemia, coagulopathy, hypertension and obesity.3) Many drug targets have been investigated to treat type 2 diabetes such as peroxisome proliferator-activated receptor (PPAR), dipeptidyl peptidase IV (DPP-4), glucagon-like peptides-1 (GLP-1), etc. Among these, PPAR is noted as a worthy target to improve metabolic syndrome. PPARs consist of three isoforms, i.e. PPARα, PPARγ and PPARδ, which are members of the superfamily of nuclear transcription factors that include the receptors for steroid, retinoid and thyroid hormones. Activation of PPARγ leads to mitigated insulin resistance by promoting hypertrophied adipocyte differentiation and improving the balance of physiological active substances.4,5) However their activation has been plagued by adverse effects including weight gain, fluid retention and edema.6) On the other hand, PPARα activation has been identified to mediate the lipid-lowering activity in the study with the fibrate class of hypolipidemic drugs. Furthermore, some PPARα agonists have been reported to reduce weight gain in rodents without an effect on food intake.7,8) Therefore, PPARα/γ dual agonists are expected to improve blood glucose levels and lipid parameters, together with lowering side effects, i.e. weight gain, caused by PPARγ activation.
In the previous report,9) we obtained zwitterionic compounds showing the PPARα/γ dual agonistic activities. Especially, compound 1 provided highly potent dual activities (α: EC50=1.7 nM, γ: EC50=4.7 nM, shown in Fig. 1), accompanied by a great glucose lowering effect in the db/db mice. Moreover, it remedied the lipid profile, e.g. lowering triglyceride, without body weight gain. However, profiling of compound 1 revealed that this compound inhibits cytochrome P450 (CYP)3A4 potently. CYPs play an important role in metabolism, and direct or mechanism based CYPs inhibition usually lead to drug–drug interaction (DDI).10) DDI is one of the reasons of attrition in the clinical phase of drug development or the withdrawal of drugs from the market. Consequently, evaluation and avoidance of the potential risks of DDI are of great importance in the preclinical study.
We report herein the optimization of the lipophilic tail and the side chain on nitrogen of our lead with an aim to improve the Absorption-Distribution-Metabolism-Excretion (ADME) profiles. Especially, we focused on reducing the CYP3A4 inhibitory activity by altering physicochemical properties.

a) Value at pH 7.4. b) % remaining value using human microsomes. c) % Inhibition value at 10 µM concentration of compound 1.
First, in order to explore substituent effects on the lipophilic tail, compounds 20a–m were designed and synthesized as depicted in Chart 1. Butanedione monooxime 3 was reacted with corresponding aldehyde 2a–c, and the resulting N-oxide intermediate was converted to chloride 5a–c by treatment with phosphorous oxychloride. The reduction of compound 611) by using a borane-tetrahydrofuran complex and following bromination gave 8. The radical bromination of 912) under the standard N-bromosuccinimide (NBS)/azobisisobutyronitrile (AIBN) condition afforded 10. The chlorides 17a–c were produced by the corresponding oxazole esters 15a–c, which were synthesized utilizing a modified Soukup procedure as follows.13) First, starting with β-keto esters 11a–c, oxime was formed by nitrosation, followed by hydrogenolysis under an acidic condition gave primary amine 13a–c. Acylation of 13a–c with corresponding acid chlorides, followed by cyclization using phosphorus oxychloride as a dehydrating reagent, furnished the oxazole ester 15a–c. Reduction of the ester moieties of 15a–c using lithium borohydride and the chlorination of resulting alcohols with the use of thionyl chloride led to 17a–c. The coupling of secondary amine 18 with the corresponding tailpieces using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) and N-hydroxybenzotriazole (HOBt) in N,N-dimethylformamide (DMF) or using K2CO3 in DMF, followed by hydrolysis provided 20a–m, respectively.

Reagents and conditions: (a) 4 M HCl in EtOAc, 4a: 81%, 4b: 85%, 4c: 47%; (b) POCl3, CHCl3, 5a: 79%, 5b: 14%, 5c: 42%; (c) 1 M BH3–THF solution, THF, 87%; (d) NBS, PPh3, DCM, 92%; (e) NBS, AIBN, CCl4, 45%; (f) NaNO2, AcOH; (g) 10% Pd/C, 1 N HCl–EtOH solution; (h) Corresponding acid chloride reagent, TEA, DCM, 14a: 48% (3 steps), 14b: 78% (3 steps), 14c: 67% (3 steps); (i) POCl3, 15a: 63%, 15b: 88%, 15c: 90%; (j) LiBH4, THF; (k) SOCl2, CHCl3, 17a: 18% (2 steps), 17b: 86% (2 steps), 17c: 89% (2 steps); (l) Corresponding lipophilic tail moieties, EDCI, HOBt, DMF or K2CO3, DMF, 22–91%; (m) 1 M BH3–THF solution, THF, 50%; (n) 1 N NaOH aq. THF; (o) (1) Iso-butyl chloroformate, NMM, THF; (2) NaBH4, 65% (2 steps); (p) Dimethylamine, EDCI, HOBt, DMF, 94%; (q) NaOH, MeOH or TFA, DCM, 37–89%.
The syntheses of the compounds bearing other aryl groups besides the furanyl group, are depicted in Chart 2. The alkylation of secondary amine 219) with corresponding aldehyde, followed by hydrolysis gave 22a–c.

Reagents and conditions: (a) (1) Corresponding commercially available aldehyde reagents, NaBH(OAc)3, DCM; (2) NaOH, MeOH, 22a: 56%, 22b: 32%, 22c: 44%.
The syntheses of the other hetero-ring variants via a key intermediate 26 are depicted in Chart 3. The treatment of the aldehyde 237) with glycine tert-butyl ester under reflux conditions to form the imine, followed by the reduction with NaBH4 gave secondary amine 24. Alkylation of 24 with 4-(chloromethyl)-5-methyl-2-phenyl-1,3-oxazole as the lipophilic tail chloride in the presence of K2CO3 in DMF, followed by acidic hydrolysis provided a common key intermediate monoester 26. The amidation reaction of 26 with acetohydrazide gave 28. Also, the amidation of 26 with Boc-protected hydrazine, followed by acidic hydrolysis gave hydrazide 27. The intramolecular cyclization of 28 using triphenyl phosphine and hexachloroethane, followed by hydrolysis provided 1,3,4-oxadiazole derivatives 29. The cyclization reaction of 27 using triphosgene, followed by hydrolysis afforded oxadiazolone 30. Thiadiazole 31 was synthesized by the alternative cyclization reaction of 28 using Lawesson’s reagent and following hydrolysis. The amidation of 26 with ammonium chloride under the conventional EDCI/HOBt condition afforded the carboxamide 32. The reaction of 32 with N,N-dimethylacetamide dimethyl acetal gave an acylamidine intermediate that was then cyclized by using hydroxylamine to give the oxadiazole variant. The following hydrolysis provided 1,2,4-oxadiazole 33 in good yields for the three steps of 62%.

Reagents and conditions: (a) (1) tert-Butylglycinate, MgSO4, THF, reflux; (2) NaBH4, MeOH, 95%; (b) 4-(Chloromethyl)-5-methyl-2-phenyl-1,3-oxazole, K2CO3, MeCN, reflux, 81%; (c) 4 M HCl–dioxane solution, DCM, 0°C, 82%; (d) Acetohydrazide, EDCI, HOBt, DMF; (e) (1) Hydrazinecarboxylic acid tert-butyl ester, EDCI, HOBt, DMF; (2) TFA, DCM; (f) PPh3, C2Cl6, TEA, DCM; (g) NaOH, MeOH, 29: 70% (3 steps), 30: 84%, 31: 56%, 33: 71%; (h) Triphosgene, 1,4-dioxane, 60°C, 84% (2 steps); (i) Lawesson’s reagent, THF, reflux, 88% (2 steps); (j) NH4Cl, EDCI, HOBt, DMF; (k) (1) N,N-Dimethylacetamide dimethyl acetal, 120°C; (2) Hydroxylamine (50% aqueous solution), acetic acid (70% aqueous solution), 88% (2 steps).
The synthesis of compounds 40a–f bearing various substituents on the phenyl ring at the lipophilic tail group are shown in Chart 4. Chloride 39a–f was synthesized in a similar manner to chloride 5 in Chart 1. Fmoc protection of the secondary amine on 24 and following treatment in an acidic condition afforded mono carboxylic acid 35. 1,3,4-Oxadiazole 37 was cyclized from 35 in a similar manner to compound 29. Following de-protection of the Fmoc group using diethylamine, alkylation with 39a–f and subsequent hydrolysis provided 40a–f.

Reagents and conditions: (a) 9-Fluorenylmethylsuccinimidyl carbonate, MeCN, 94%; (b) TFA, DCM; (c) Acetohydrazide, EDCI, HOBt, MeCN; 67% (2 steps); (d) PPh3, C2Cl6, TEA, DCM, 94%; (e) DBU, THF, 82%; (f) (1) Cs2CO3, DMF, KI, 60°C; (2) NaOH, MeOH, 24–61%.
Novel compounds were evaluated in cell-based transcription assay using GAL4-PPAR chimeric receptors and plasmids for functional analysis-secreted alkaline phosphatase (pFA-SEAP) as reporter vector, and the activities are reported as the EC50 value. Log D values were determined from the partition coefficient for 1-octanol/phosphate buffer saline (PBS) at pH 7.4. CYP3A4 direct inhibitory activity values were shown in % inhibition at 10 µM concentration of the compounds for 60 min incubation.14) Mechanism-based inhibitory activity (MBI) values were shown in % remaining at 100 µM concentration of the compounds reacted with CYP3A4 probe substrates after 30 min preincubation in human liver microsomes.15,16)
 | ||||||
|---|---|---|---|---|---|---|
| Compd. | R | PPARα EC50 (nM) | PPARγ EC50 (nM) | Log Da) | CYP3A4 | |
| Direct inhibitionb) (%) | MBI remainingc) (%) | |||||
| 1 |  | 1.7 | 4.7 | 2.9 | 75 | 91 |
| 20a |  | 2.6 | 1.2 | 2.7 | 53 | 63 |
| 20b |  | 1.5 | 0.39 | 3.1 | 53 | 60 |
| 20c |  | 0.81 | 0.51 | 3.2 | 65 | 65 |
| 20e |  | 8.1 | 18 | 2.5 | 37 | 78 |
| 20f |  | 0.85 | 0.38 | 3.4 | 81 | NTd) |
| 20g |  | 4.6 | 8.8 | 4.1 | 77 | NT |
| 20h |  | 2.8 | 1.6 | 4.4 | 71 | NT |
| 20k |  | 5.5 | 11 | 1.7 | 24 | NT |
| 20l |  | 14 | 210 | 1.7 | 43 | 77 |
| 20m |  | 8.5 | 1.5 | 1.0 | <10 | NT |
a) Log D values were determined from the partition coefficient for 1-octanol/phosphate buffer saline (PBS) at pH 7.4. b) CYP3A4 direct inhibition values were shown in % inhibition at 10 µM concentration of the compounds for 60 min incubation. c) MBI values were shown in % remaining at 100 µM concentration of the compounds reacted with CYP3A4 probe substrates after 30 min preincubation in human liver microsomes. d) Not tested.

Many of the furan derivatives depicted in Table 1 showed the MBI of CYP3A4.15) We then embarked on the conversion of the furan ring, which should be a causative structure of MBI.18) The results on the 2-phenyloxazole scaffold are summarized in Table 2. All variants were devoid of the MBI of CYP3A4.
 | ||||||
|---|---|---|---|---|---|---|
| Compd. | R | PPARα EC50 (nM) | PPARγ EC50 (nM) | Log Da) | CYP3A4 | |
| Direct inhibitionb) (%) | MBI remainingc) (%) | |||||
| 22a |  | 1.6 | 5.5 | 1.8 | 22 | 88 |
| 29 | 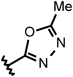 | 2.9 | 33 | 1.1 | 28 | 110 |
| 33 |  | 2.7 | 6.9 | 1.8 | 25 | 83 |
| 30 |  | 17 | 93 | 0.9 | 15 | 124 |
| 22b |  | 2.4 | 8.8 | 2.6 | 42 | 93 |
| 31 |  | 1.9 | 4.2 | 1.6 | NTd) | 84 |
| 22c |  | 110 | 110 | NT | NT | NT |
a) Log D values were determined from the partition coefficient for 1-octanol/phosphate buffer saline (PBS) at pH 7.4. b) CYP3A4 direct inhibition values were shown in % inhibition at 10 µM concentration of the compounds for 60 min incubation. c) MBI values were shown in % remaining at 100 µM concentration of the compounds reacted with CYP3A4 probe substrates after 30 min preincubation in human liver microsomes. d) Not tested.
Among these, we found out that the biological profile of the 1,3,4-oxadiazole 29 differed from that of the 1,2,4-oxadiazole 33. Compound 29 showed more potent activity for PPARα (EC50=2.9 nM) than for PPARγ (EC50=33 nM), which might be preferable to reduce the side effects of PPARγ activation, i.e. weight gain. Besides, we assumed that compound 33, possessing a similar structure to 29 and more potent PPARγ activity (EC50=6.9 nM), would be an interesting counterpart with regard to the comparison of in vivo pharmacological profiles. Thus, those two compounds were compared in db/db mice, an obese animal model of type 2 diabetes characterized by severe insulin resistance and marked hypertriglyceridemia. The results are displayed in Fig. 3 and Table 3. While both compounds showed a similar and significant glucose lowering effect, compound 29, a PPARα dominant variant, showed less weight gain than compound 33 (dosed orally at 10 mg/kg in db/db mice [six per group] for 14 d).

Blood glucose change (A) and body weight change (B) were conducted with 14 d of treatment.
| Exp. | Compd. | Dose (mg/kg) | Plasma glucosea) (mg/dL) | Change (%) | Plasma triglyceridea) (mg/dL) | Change (%) | BW change (%) |
|---|---|---|---|---|---|---|---|
| 1 | Vehicle | 404±28 | 227±154 | ||||
| 29 | 10 | 151±39 | −63b) | 79±15 | −65b) | +4.8 | |
| 33 | 10 | 195±32 | −52b) | 76±16 | −67b) | +7.6d) | |
| Rosiglitazone | 10 | 174±50 | −57b) | 90±38 | −60b) | +11.2c) |
a) Mean±standard deviation (n=6). b) p<0.001. c) p<0.01. d) p<0.05 versus vehicle control (t-test).
Since compound 29 showed preferable effects, we carried out further derivatization on N-(5-methyl-1,3,4-oxadiazol-2-yl)methane derivatives. The results are summarized in Table 4. All compounds showed very potent PPARα activity. Compound 40a, bearing the methyl group at the 3-position of the phenyl ring at the lipophilic tail, showed more potent PPARγ activity compared to 4-methylated compound 40b. Similarly, compounds 40h and 40i, which also had substituents at the 3-position, showed high potency for PPARγ. Moreover, 40e or 40f, which possessed bulky substituents such as the trifluoromethoxy group or ethoxy group at the 4-position, also showed potent PPARγ activity. It was revealed that changing furan to 1,3,4-oxadiazole led to a decrease of lipophilicity. Concerning the CYP3A4 inhibitory activity on 1,3,4-oxadiazole derivatives, compounds with high Log D (e.g. 2.2=40e or 1.9=40g) showed potent CYP3A4 direct inhibitory activity, while low lipophilic compounds showed the reduction of the CYP3A4 inhibitory activity, which was similar to the result of furanyl variant as described. In addition, any 1,3,4-oxadiazole derivatives did not show MBI activity, regardless of the Log D values.
 | ||||||
|---|---|---|---|---|---|---|
| Compd. | R | PPARα EC50 (nM) | PPARγ EC50 (nM) | Log Da) | CYP3A4 | |
| Direct inhibitionb) (%) | MBI remainingc) (%) | |||||
| 40a | 3-CH3 | 0.51 | 2.7 | 1.6 | 47 | 91 |
| 40b | 4-CH3 | 0.55 | 18 | 1.4 | 32 | 101 |
| 40c | 4-Cl | 1.2 | 26 | 1.6 | 32 | 99 |
| 40d | 4-OCH3 | 0.56 | 22 | 1.1 | 25 | 104 |
| 40e | 4-OCF3 | 0.47 | 0.54 | 2.2 | 69 | NTd) |
| 40f | 4-OC2H5 | 0.67 | 3.6 | 1.5 | 61 | 88 |
| 40g | 4-CF3 | 0.47 | 12 | 1.9 | 74 | 98 |
| 40h | 3,4-CH3 | 0.19 | 1.8 | 1.8 | 53 | 89 |
| 40i | 3-CH3-4-OCH3 | 0.15 | 0.69 | 1.7 | 57 | NT |
a) Log D values were determined from the partition coefficient for 1-octanol/phosphate buffer saline (PBS) at pH 7.4. b) Direct CYP3A4 inhibition values were shown in % inhibition at 10 µM concentration of the compounds for 60 min incubation. c) MBI values were shown in % remaining at 100 µM concentration of the compounds reacted with CYP3A4 probe substrates after 30 min preincubation in human liver microsomes. d) Not tested.
While having potent agonist 1,3,4-oxadiazole derivatives with a distinguished ADME profile at hand, in vivo studies were carried out; oral dosing, 3 mg/kg quaque die (QD) for 14 d in db/db mice (six per group). All PPARα dominant compounds exhibited a significant decrease in plasma glucose and plasma triglyceride without marked weight gain as depicted in Table 5.
| Exp. | Compd. | Dose (mg/kg) | Plasma glucosea) (mg/dL) | Change (%) | Plasma triglyceridea) (mg/dL) | Change (%) | BW change (%) |
|---|---|---|---|---|---|---|---|
| 1 | Vehicle | 404±28 | 258±104 | ||||
| 40b | 3 | 134±35 | −67b) | 60±14 | −77b) | −1.7 | |
| 40c | 3 | 159±41 | −61b) | 76±21 | −71b) | −1.1 | |
| Rosiglitazone | 10 | 203±42 | −50b) | 92±26 | −64b) | +10.2c) | |
| 2 | Vehicle | 564±45 | 198±217 | ||||
| 40a | 3 | 263±31 | −53b) | 61±17 | −69b) | +1.2 | |
| 40d | 3 | 220±57 | −61b) | 68±24 | −66b) | +0.6 | |
| 3 | Vehicle | 552±29 | 239±232 | ||||
| 40f | 3 | 195±48 | −65b) | 65±18 | −73b) | +2.1 | |
| 40h | 3 | 135±49 | −76b) | 68±12 | −71b) | −2.1 | |
| 4 | Vehicle | 548±51 | 209±91 | ||||
| 40g | 3 | 162±23 | −70b) | 59±17 | −72b) | −2.6 |
a) Mean±standard deviation (n=6). b) p<0.001. c) p<0.01 versus vehicle control (t-test).
We described the design, syntheses and evaluation of novel zwitterionic compounds as PPARα/γ dual agonists. The issues faced by the furan derivatives, e.g. the high lipophilicity and the potent CYP3A4 direct or mechanism-based inhibitory activities, were addressed by changing the furan ring to a low lipophilic 1,3,4-oxadiazole ring. Additionally, these oxadiazole derivatives exhibited a significant decrease in plasma glucose and plasma triglyceride levels without marked weight gain. Further pharmacological studies on these derivatives are now underway, and will be reported in due course.
Unless otherwise noted, materials were obtained from commercial suppliers and used without further purification. 1H-NMR spectra were determined on a JEOL JNM-EX400 spectrometer. Chemical shifts are reported in parts per million relative to tetramethylsilane as an internal standard. Significant 1H-NMR data are tabulated in the following order: number of protons, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad), and coupling constant(s) in hertz. Electron spray ionization condition (ESI) mass spectra were recorded on an Agilent 1100 and SCIEX API-150EX spectrometer. Fast atom bombardment ionization condition (FAB) mass spectra were recorded on a JEOL JMSHX110 spectrometer. Electron impact ionization condition (EI) mass spectra were recorded on a JEOL JMS-AX505W. Column chromatography was performed with Merck silica gel 60 (particle size 0.060–0.200 or 0.040–0.063 mm). Flash column chromatography was performed with Biotage FLASH Si packed columns. Thin layer chromatography (TLC) was performed on Merck pre-coated TLC glass sheets with silica gel 60 F254, and compound visualization was effected with a 5% solution of phosphomolybdic acid in ethanol, UV lamp, iodine, or Wako ninhydrin spray. Elemental analysis was performed using a PerkinElmer, Inc. CHNS/O 2400II or a Yokokawa Analysis IC7000RS, and analytical results were within ±0.4% of the theoretical values unless otherwise noted.
General Procedure for 2-[(Substituted)-fluorophenyl]-4,5-dimethyloxazole 3-Oxide (4a–c)To a solution of HCl (4 M EtOAc solution, 5 mL) were added corresponding aldehyde (3a−c, 5.0 mmol) and diacetyl monooxyme (0.51 g, 5.0 mmol) at 0°C and stirred at room temperature overnight. After the solvent was removed in vacuo, the residue was solidified with diethyl ether to provide 4a–c HCl salts.
2-(2-Fluorophenyl)-4,5-dimethyloxazole 3-Oxide (4a): This compound was obtained as a pale yellow solid in 81% yield: 1H-NMR (400 MHz, DMSO-d6) δ: 2.15 (3H, s), 2.41 (3H, s), 7.41–7.49 (2H, m), 7.63 (1H, dd, J=13.7, 6.3 Hz), 8.82–8.86 (1H, m). MS (ESI) m/z 208 (M+H)+.
2-(3-Fluorophenyl)-4,5-dimethyl-oxazole 3-Oxide (4b): This compound was obtained as a pale yellow solid in 85% yield: 1H-NMR (400 MHz, CDCl3) δ: 2.48 (3H, s), 2.50 (3H, s), 7.38–7.43 (1H, m), 7.58–7.64 (1H, m), 8.01–8.04 (1H, m), 8.18–8.20 (1H, m). MS (ESI) m/z: 208 (M+H)+.
2-(4-Fluorophenyl)-4,5-dimethyloxazole 3-Oxide (4c): This compound was obtained as a pale yellow solid in 47% yield: 1H-NMR (400 MHz, CDCl3) δ: 1.45 (3H, s), 1.57 (3H, s), 7.34–7.37 (4H, m). MS (ESI) m/z: 208 (M+H)+.
General Procedure for 4-(Chloromethyl)-2-[(substituted)-fluorophenyl]-5-methyl-1,3-oxazole (5a–c)To a solution of 4a–c (969 mg, 3.98 mmol) in MeCN (40 mL) was added phosphoryl chloride (730 µL, 7.95 mmol) at 0°C and stirred at room temperature, overnight. After the solvent was removed in vacuo, the resulting solid was collected by filtration and washed with water to provide 5a–c.
4-(Chloromethyl)-2-(2-fluorophenyl)-5-methyl-1,3-oxazole (5a): This compound was obtained as a colorless solid in 79% yield by using 4a (3.98 mmol), MeCN (40 mL) and phosphoryl chloride (7.95 mmol): 1H-NMR (400 MHz, CDCl3) δ: 2.45 (3H, s), 4.58 (2H, s), 7.16–7.27 (2H, m), 7.39–7.45 (1H, m), 7.98–8.03 (1H, m). MS (ESI) m/z: 226 (M+H)+.
4-(Chloromethyl)-2-(3-fluorophenyl)-5-methyl-1,3-oxazole (5b): This compound was obtained as a colorless solid in 14% yield by using 4b (3.00 mmol), MeCN (5 mL) and phosphoryl chloride (3.00 mmol): 1H-NMR (400 MHz, CDCl3) δ: 2.43 (3H, s), 4.54 (2H, s), 7.09–7.15 (1H, m), 7.38–7.43 (1H, m), 7.67–7.71 (1H, m), 7.77–7.80 (1H, m). MS (ESI) m/z: 226 (M+H)+.
4-(Chloromethyl)-2-(4-fluorophenyl)-5-methyl-1,3-oxazole (5c): This compound was obtained as a colorless solid in 42% yield by using 4c (2.34 mmol), MeCN (20 mL) and phosphoryl chloride (4.68 mmol): 1H-NMR (400 MHz, DMSO-d6) δ: 2.42 (3H, s), 4.54 (2H, s), 7.10–7.15 (2H, m), 7.98–8.02 (2H, m). MS (ESI) m/z: 226 (M+H)+.
[2-Phenyl-5-(trifluoromethyl)-1,3-oxazol-4-yl]methanol (7)To a solution of 6 (483 mg, 1.88 mmol) in tetrahydrofuran (THF) (30 mL), BH3–THF complex (1 M THF solution, 9.4 mL) was added and stirred at room temperature for 3 h. After the solvent was removed in vacuo, the residue was dissolved in CH2Cl2, washed with water, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=3/1, v/v) to provide 7 as a colorless solid (396 mg, 87%): 1H-NMR (400 MHz, CDCl3) δ: 2.17 (1H, br s), 4.76–4.77 (2H, m), 7.47–7.56 (3H, m), 8.07–8.10 (2H, m): MS (ESI) m/z: 244 (M+H)+.
4-(Bromomethyl)-2-phenyl-5-(trifluoromethyl)-1,3-oxazole (8)To a solution of 7 (185 mg, 761 µmol) in CH2Cl2 (20 mL) were added triphenylphosphine (400 mg, 1.52 mmol) and N-bromosuccinimide (356 mg, 1.52 mmol), and stirred at room temperature for 3 h. Water was added to the reaction mixture and extracted with CH2Cl2. The organic layer was dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=4/1, v/v) to provide 8 as a colorless solid (214 mg, 92%): 1H-NMR (400 MHz, CDCl3) δ: 4.48 (2H, s), 7.47–7.57 (3H, m), 8.07–8.10 (2H, m): MS (ESI) m/z: 306 (M+H)+.
Ethyl 4-(Bromomethyl)-2-phenyl-1,3-oxazole-5-carboxylate (10)To a solution of 9 (971 mg, 4.20 mmol) in CCl4 (20 mL) were added N-bromosuccinimide (748 mg, 4.20 mmol) and 2,2′-azobis(isobutyronitrile) (138 mg, 0.84 mmol), and stirred under a reflux condition overnight. After precipitates were filtered out, the filtrate was concentrated and then the crude product was purified by column chromatography on silica gel (hexane/EtOAc=93/7 then 9/1 as eluent, v/v) to provide 8 as a colorless solid (766 mg, 45%): 1H-NMR (CDCl3) δ: 1.45 (3H, t, J=7.3 Hz), 4.46 (2H, q, J=7.3 Hz), 4.73 (2H, s), 7.47–7.53 (3H, m), 8.14–8.15 (2H, m).
General Procedure for 2-(Substituted)amino-3-oxo-(alkanoic) Acid Ethyl Ester (14a–c)After 11a–c was added to acetic acid, a solution of sodium nitrite in water was added dropwise to this at 0°C. After the reaction mixture was stirred at room temperature overnight, saturated NaHCO3 aqueous solution was added to the reaction mixture, extracted with EtOAc, dried over Na2SO4 and concentrated to afford 12a–c. After 12a–c was dissolved in HCl (1 N ethanol solution), 10% palladium on carbon was added and then the reaction mixture was stirred under hydrogen atmosphere at room temperature overnight. Palladium on carbon was filtered out and then the filtrate was concentrated to provide 13a–c HCl salts. To a solution of 13a–c in CH2Cl2 were added triethylamine and a corresponding acid chloride. The mixture was stirred at room temperature for 2 h. Water was added to the reaction mixture, extracted with CH2Cl2, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel to provide 14a–c.
2-[(Tetrahydropyran-4-ylcarbonyl)amino]-3-oxo-butyric Acid Ethyl Ester (14a): This compound was obtained as a pale yellow solid in 48% yield by using 11a (20.0 mmol), acetic acid (20 mL), sodium nitrite (30.0 mmol) and 10% palladium on carbon (400 mg), followed by 13a (10.0 mmol), triethylamine acetate (TEA) (30.0 mmol), tetrahydropyran-4-carbonyl chloride (12.0 mmol) and CH2Cl2 (100 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.32 (3H, t, J=7.1 Hz), 1.77–1.84 (4H, m), 2.41 (3H, s), 2.44–2.52 (1H, m), 3.40–3.47 (2H, m), 3.99–4.04 (2H, m), 4.24–4.32 (2H, m), 5.23 (1H, d, J=6.6 Hz), 6.65 (1H, d, J=5.1 Hz). MS (ESI) m/z: 258 (M+H)+.
2-Benzoylamino-3-oxo-pentanoic Acid Ethyl Ester (14b): This compound was obtained as a colorless oil in 78% yield by using 11b (8.67 mmol), acetic acid (10 mL), sodium nitrite (13.0 mmol), 10% palladium on carbon (200 mg), TEA (17.3 mmol), benzoyl chloride (8.67 mmol) and CH2Cl2 (50 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.15 (3H, t, J=7.1 Hz), 1.32 (3H, t, J=7.1 Hz), 2.75–2.91 (2H, m), 4.30 (2H, q, J=7.1 Hz), 5.44 (1H, d, J=6.6 Hz), 7.33–7.56 (4H, m), 7.84–7.86 (2H, m). MS (ESI) m/z: 264 (M+H)+.
2-Benzoylamino-4-methyl-3-oxo-pentanoic Acid Ethyl Ester (14c): This compound was obtained as a pale yellow solid in 67% yield by using 11c (31.6 mmol), acetic acid (40 mL), sodium nitrite (47.4 mmol) and 10% palladium on carbon (500 mg), followed by 13c (10.6 mmol), TEA (21.2 mmol), benzoyl chloride (15.9 mmol) and CH2Cl2 (100 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.16 (3H, d, J=6.8 Hz), 1.26 (3H, d, J=6.8 Hz), 1.32 (3H, t, J=7.1 Hz), 3.16 (1H, hept., J=6.8 Hz), 4.26–4.34 (2H, m), 5.60 (1H, d, J=6.5 Hz), 7.31 (1H, br d, J=6.5 Hz), 7.44–7.56 (3H, m), 7.83–7.87 (2H, m). MS (ESI) m/z: 278 (M+H)+.
General Procedure for Ethyl 5-(Substituted)-2-(substituted)-1,3-oxazole-4-carboxylate (15a–c)To a solution of 14a–c in chloroform (0–5 mL) was added phosphoryl chloride and stirred under reflux conditions for 1 h. The reaction mixture was dropped into an ice-cold saturated NaHCO3 aqueous solution and then the mixture was stirred for 2 h. Organics was extracted with chloroform, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc) to provide 15a–c.
Ethyl 5-Methyl-2-(tetrahydro-2H-pyran-4yl)-1,3-oxazole-4-carboxylate (15a): This compound was obtained as a pale yellow oil in 63% yield by using 14a (2.24 mmol), CHCl3 (10 mL) and phosphoryl chloride (5 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.32 (3H, t, J=6.7 Hz), 1.97 (4H, br s), 2.61 (3H, s), 3.08 (1H, br s), 3.47–3.55 (2H, m), 4.00–4.06 (2H, m), 4.39 (2H, d, J=6.7 Hz). MS (ESI) m/z: 240 (M+H)+.
Ethyl 5-Ethyl-2-phenyl-1,3-oxazole-4-carboxylate (15b): This compound was obtained as a colorless solid in 88% yield by using 14b (6.68 mmol), and phosphoryl chloride (11 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.6 Hz), 1.42 (3H, t, J=7.1 Hz), 3.14 (2H, t, J=7.6 Hz), 4.43 (2H, t, J=7.1 Hz), 7.45–7.47 (3H, m), 8.07–8.10 (2H, m). MS (ESI) m/z: 246 (M+H)+.
Ethyl 5-(Propan-2-yl)-2-phenyl-1,3-oxazole-4-carboxylate (15c): This compound was obtained as a pale yellow solid in 90% yield by using 14c (9.30 mmol), and phosphoryl chloride (16 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.37 (6H, d, J=7.0 Hz), 1.43 (3H, t, J=7.1 Hz), 3.85 (1H, hept., J=7.0 Hz), 4.43 (2H, q, J=7.1 Hz), 7.44–7.48 (3H, m), 8.06–8.10 (2H, m). MS (ESI) m/z: 260 (M+H)+.
General Procedure for 4-Chloromethyl-5-(substituted)-2-(substituted)-1,3-oxazole (17a–c)To a solution of 15a–c in THF was added lithium borohydride and stirred under a reflux condition for 1 h. After the solvent was removed in vacuo, water was added to the residue, extracted with CH2Cl2, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (chloroform/methanol) to provide 16a–c. To a solution of 16a–c in chloroform was added thionyl chloride and stirred at room temperature for 2 h. After the solvent was removed in vacuo, the crude product was purified by column chromatography on silica gel (hexane/EtOAc) to provide 17a–c.
4-(Chloromethyl)-5-methyl-2-(tetrahydro-2H-pyran-4-yl)-1,3-oxazole (17a): This compound was obtained as a pale yellow solid in 18% yield by using 15a (3.34 mmol), lithium borohydride (10.0 mmol), THF (50 mL), thionyl chloride (10 mmol) and chloroform (15 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.86–1.99 (4H, m), 2.32 (3H, s), 2.95–3.03 (1H, m), 3.47–3.54 (2H, m), 3.99–4.04 (2H, m), 4.47 (2H, s). MS (ESI) m/z: 216 (M+H)+.
4-(Chloromethyl)-5-ethyl-2-phenyl-1,3-oxazole (17b): This compound was obtained as a yellow oil in 86% yield by using 15b (5.71 mmol), lithium borohydride (8.57 mmol), THF (70 mL), thionyl chloride (14.8 mmol) and chloroform (30 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.33 (3H, t, J=7.6 Hz), 2.79 (2H, q, J=7.6 Hz), 4.57 (2H, s), 7.42–7.45 (3H, m), 8.00–8.04 (2H, m). MS (ESI) m/z: 222 (M+H)+.
4-(Chloromethyl)-5-(propan-2-yl)-2-phenyl-1,3-oxazole (17c): This compound was obtained as a yellow oil in 89% yield by using 15c (8.25 mmol), lithium borohydride (12.4 mmol) and THF (100 mL), followed by 16c (3.00 mmol), thionyl chloride (9.00 mmol) and chloroform (20 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.37 (6H, d, J=7.1 Hz), 3.19 (1H, hept., J=7.1 Hz), 4.59 (2H, s), 7.42–7.47 (3H, m), 8.00–8.04 (2H, m).
General Procedure for 2-[4-({[2-(Substituted)-5-(substituted)oxazol-4-ylmethyl]furan-2-ylmethylamino}methyl)-2,6-dimethylphenoxy]-2-methylpropionic Acid tert-Butyl Ester (19a–c, f–i and m)To a solution of 5a–c, 8, 10 or 17a–c and 18 in DMF was added potassium carbonate and stirred at 40°C for 1–3 d. Water was added to the reaction mixture and extracted with EtOAc. The organic layer was washed with brine, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc) to provide 19.
tert-Butyl 2-(4-{[{[2-(2-Fluorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19a): This compound was obtained as a pale yellow solid in 81% yield by using 5a (1.00 mmol), 18 (1.00 mmol), potassium carbonate (1.20 mmol) and DMF (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.41 (6H, s), 1.51 (9H, s), 2.21 (6H, s), 2.29 (3H, s), 3.54 (2H, s), 3.57 (2H, s), 3.76 (2H, s), 6.26–6.28 (1H, m), 6.33–6.35 (1H, m), 6.98 (2H, s), 7.14–7.26 (2H, m), 7.37–7.40 (2H, m), 8.00–8.04 (1H, m). MS (EI) m/z: 562 M+.
tert-Butyl 2-(4-{[{[2-(3-Fluorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19b): This compound was obtained as a colorless solid in 34% yield by using 5b (0.34 mmol), 18 (0.90 mmol), potassium carbonate (0.38 mmol) and DMF (5 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.41 (6H, s), 1.51 (9H, s), 2.21 (6H, s), 2.26 (3H, s), 3.54 (4H, s), 3.77 (2H, s), 6.25–6.27 (1H, m), 6.35 (1H, br s), 6.97 (2H, s), 7.06–7.13 (1H, m), 7.37–7.44 (2H, m), 7.68–7.72 (1H, m), 7.80 (1H, d, J=7.4 Hz). MS (ESI) m/z: 563 (M+H)+.
tert-Butyl 2-(4-{[{[2-(4-Fluorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19c): This compound was obtained as a colorless solid in 86% yield by using 5c (0.91 mmol), 18 (0.91 mmol), potassium carbonate (1.00 mmol) and DMF (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.41 (6H, s), 1.51 (9H, s), 2.21 (6H, s), 2.25 (3H, s), 3.53 (4H, s), 3.77 (2H, s), 6.25–6.26 (1H, m), 6.33–6.35 (1H, m), 6.97 (2H, s), 7.08–7.15 (2H, m), 7.40–7.41 (1H, m), 7.98–8.02 (2H, m). MS (ESI) m/z: 563 (M+H)+.
tert-Butyl 2-[4-({[(2-Phenyl-5-ethyl-1,3-oxazol-4-yl)methyl](furan-2-ylmethyl)amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (19f): This compound was obtained as a colorless solid in 22% yield by using 17b (1.13 mmol), 18 (1.13 mmol), potassium carbonate (1.24 mmol) and DMF (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.23 (3H, t, J=7.6 Hz), 1.41 (6H, s), 1.51 (9H, s), 2.21 (6H, s), 2.64 (2H, q, J=7.6 Hz), 3.53 (2H, s), 3.56 (2H, s), 3.76 (2H, s), 6.26 (1H, d, J=2.9 Hz), 6.34 (1H, dd, J=2.9, 1.7 Hz), 6.98 (2H, s), 7.39–7.46 (4H, m), 8.01–8.04 (2H, m). MS (ESI) m/z: 559 (M+H)+.
tert-Butyl 2-(4-{[{[2-Phenyl-5-(propan-2-yl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19g): This compound was obtained as a colorless solid in 57% yield by using 17c (0.90 mmol), 18 (0.90 mmol), potassium carbonate (1.35 mmol) and DMF (10 mL): 1H-NMR (DMSO-d6) δ: 1.20 (6H, d, J=6.8 Hz), 1.33 (9H, s), 2.13 (6H, s), 2.97–3.03 (1H, m), 3.46 (2H, s), 3.49 (2H, s), 3.69 (2H, s), 6.37 (1H, d, J=3.2 Hz), 6.43–6.44 (1H, m), 6.97 (2H, s), 7.48–7.54 (3H, m), 7.62–7.63 (1H, m), 7.92–7.96 (2H, m).
tert-Butyl 2-(4-{[{[2-Phenyl-5-(trifluoromethyl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19h): This compound was obtained as a colorless solid in 87% yield by using 8 (0.69 mmol), 18 (0.69 mmol), potassium carbonate (0.76 mmol) and DMF (5 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.40 (6H, s), 1.51 (9H, s), 2.20 (6H, s), 3.59 (2H, s), 3.75 (2H, s), 3.82 (2H, s), 6.27 (1H, d, J=3.2 Hz), 6.33–6.35 (1H, m), 6.98 (2H, s),7.40–7.41 (1H, m), 7.47–7.52 (3H, m), 8.09–8.12 (2H, m). MS (ESI) m/z: 599 (M+H)+.
Ethyl 4-{[{4-[(1-tert-Butoxy-2-methyl-1-oxopropan-2yl)oxy]-3,5-dimethylbenzyl}(furan-2-ylmethyl)amino]methyl}-2-phenyl-1,3-oxazole-5-carboxylate (19i): This compound was obtained as a pale yellow oil in 91% yield by using 10 (1.90 mmol), 18 (1.76 mmol), potassium carbonate (1.94 mmol) and DMF (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.40 (6H, s), 1.50 (9H, s), 2.20 (6H, s), 3.65 (2H, s), 3.84 (2H, s), 4.03 (2H, s), 4.34 (2H, q, J=7.1 Hz), 6.24–6.36 (2H, m), 7.00 (2H, s), 7.40 (1H, br s), 7.48–7.53 (3H, m), 8.18–8.21 (2H, m).
tert-Butyl 2-(4-{[{[2-(Tetrahydro-2H-pyran-4-yl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19m): This compound was obtained as a colorless solid in 77% yield by using 17a (0.59 mmol), 18 (0.59 mmol), potassium carbonate (0.88 mmol) and DMF (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.42 (6H, s), 1.51 (9H, s), 1.89–1.97 (4H, m), 2.14–2.23 (9H, m), 2.94–2.99 (1H, m), 3.42–3.54 (6H, m), 3.70 (2H, s), 3.98–4.04 (2H, m), 6.20–6.23 (1H, m), 6.32 (1H, br s), 6.96 (2H, s), 7.38 (1H, br s); MS (ESI) m/z: 553 (M+H)+.
tert-Butyl 2-[4-({(Furan-2-ylmethyl)[(2-phenyl-1,3-oxazol-4-yl)carbonyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (19d)To a solution of methyl 2-phenyl-1,3-oxazole-4carboxylate (208 mg, 1.02 mmol) in THF (7 mL) and water (2 mL) was added lithium hydroxide (49 mg, 2.04 mmol) and stirred at room temperature overnight. HCl (1 N aqueous solution, 1.02 mmol) was added to the reaction mixture and then stirred at room temperature. After the solvent was removed in vacuo, DMF (10 mL), 18 (381 mg, 1.02 mmol), EDCI (391 mg, 2.04 mmol) and HOBt (69 mg, 0.51 mmol) were added to the residue and stirred at room temperature for 2 d. After the solvent was removed in vacuo, water was added to the residue and extracted with EtOAc. The organic layer was dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=3/1, v/v) to provide 19d as a colorless solid (230 mg, 41%): 1H-NMR (400 MHz, CDCl3) δ: 1.43 (6H, s), 1.51 (9H, s), 2.22 (6H, s), 4.61, 4.64 (2H, each s), 5.11, 5.14 (2H, each s), 6.27–6.32 (2H, m), 6.94 (2H, s), 7.36–7.49 (4H, m), 7.95–8.07 (2H, m), 8.26–8.33 (1H, m); MS (ESI) m/z: 545 (M+H)+.
tert-Butyl 2-[4-({[(2-Phenyl−1,3-oxazol-4-yl)methyl](furan-2-ylmethyl)amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (19e)To a solution of 19d (230 mg, 422 µmol) in THF (20 mL) was added BH3-THF complex (1 M THF solution, 4.2 mL) and stirred at 50°C overnight. After the solvent was removed in vacuo, EtOH (8 mL), water (2 mL) and TEA (2 mL) were added to the residue and stirred under a reflux condition for 2 h. After the solvent was removed in vacuo, water was added to the residue, extracted with DCM, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=2/1, v/v) to provide 19e as a colorless solid (111 mg, 50%): 1H-NMR (400 MHz, CDCl3) δ: 1.42 (6H, s), 1.51 (9H, s), 2.23 (6H, s), 3.58 (2H, s), 3.69 (2H, s), 3.75 (2H, s), 6.24 (1H, d, J=3.1 Hz), 6.34 (1H, dd, J=3.1, 2.0 Hz), 7.00 (2H, s), 7.41–7.47 (4H, m), 7.63 (1H, s), 8.04–8.06 (2H, m). MS (ESI) m/z: 531 (M+H)+.
tert-Butyl 2-(4-{[{[2-Phenyl−5-(hydroxymethyl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19k)To a mixture of 19i (845 mg, 1.40 mmol), THF (15 mL) and water (3 mL) was added NaOH (1 N aqueous solution, 3.1 mL) and stirred at 50°C for 1.5 h and then stirred at room temperature for 3 d. After HCl (1 N aqueous solution, 3.1 mL) was added to the reaction mixture, the solvent was removed in vacuo. After CH2Cl2 was added to the residue, precipitates were filtered out and the filtrate was concentrated. After the residue was dissolved in THF (20 mL), N-methylmorpholine (186 µL, 1.69 mmol) and isobutyl chloroformate (222 µL, 1.69 mmol) were added to the reaction solution at −40°C and stirred at the same temperature for 15 min. After precipitates were filtered out, sodium borohydride (79 mg, 2.11 mmol) was added to the filtrate and stirred at room temperature for 1 h. After the solvent was removed in vacuo, water was added to the residue, extracted with CH2Cl2, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=3/1, v/v) to provide 19k as a pale yellow solid (587 mg, 65%): 1H-NMR (400 MHz, CDCl3) δ: 1.42 (6H, s), 1.51 (9H, s), 2.22 (6H, s), 3.56 (2H, br s), 3.70 (4H, br s), 4.69 (2H, s), 6.28–6.36 (2H, m), 6.94 (2H, br s), 7.40–7.48 (4H, m), 7.95–8.01 (2H, m); MS (ESI) m/z: 561 (M+H)+.
tert-Butyl 2-(4-{[{[2-Phenyl-5-(dimethylcarbamoyl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (19l)To a mixture of 19i (680 mg, 1.13 mmol), THF (20 mL) and water (5 mL) was added NaOH (1 N aqueous solution, 3.38 mL) and stirred at 50°C for 4 h. After DMF (20 mL), dimethylamine HCl salts (139 mg, 1.70 mmol), EDCI (433 mg, 2.26 mmol) and HOBt (76 mg, 0.57 mmol) were added to the reaction mixture at room temperature and the mixture was stirred overnight. After the solvent was removed in vacuo, water was added to the residue, extracted with EtOAc, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=1/1, v/v) to provide 19l as a pale yellow solid (639 mg, 94%): 1H-NMR (400 MHz, CDCl3) δ: 1.40 (6H, s), 1.51 (9H, s), 2.21 (6H, s), 3.10 (3H, s), 3.16 (3H, s), 3.61 (2H, s), 3.79 (2H, s), 3.93 (2H, s), 6.25 (1H, s), 6.32 (1H, s), 7.00 (2H, s), 7.38–7.51 (4H, m), 8.09–8.10 (2H, m); MS (ESI) m/z: 602 (M+H)+.
General Procedure for 2-[4-({[2-(Substituted)-5-(substituted)oxazol-4-ylmethyl]furan-2-ylmethylamino}methyl)-2,6-dimethylphenoxy]-2-methylpropionic Acid (20a–m)To a solution of 19 in CH2Cl2 was added HCl (4 M dioxane solution) and stirred at room temperature overnight. After the solvent was removed in vacuo, the residue was purified by preparative thin-layer chromatography (dichloromethane/MeOH) to provide 20a–m.
2-(4-{[{[2-(2-Fluorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20a): This compound was obtained as a pale yellow solid in 89% yield by using 19a (0.79 mmol), CH2Cl2 (3 mL) and HCl (4 M dioxane solution, 6 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.14 (6H, s), 2.25 (3H, s), 3.48 (2H, s), 3.50 (2H, s), 3.69 (2H, s), 6.37 (1H, d, J=3.2 Hz), 6.43 (1H, d, J=3.2, 1.7 Hz), 6.97 (2H, s), 7.33–7.40 (2H, m), 7.51–7.57 (1H, m), 7.62–7.63 (1H, m), 7.96–8.00 (1H, m). MS (ESI) m/z : 507 (M+H)+. Anal. Calcd for C29H31FN2O5·0.3H2O·0.25 dioxane: C, 67.48; H, 6.34; F,3.56; N, 5.25. Found: C, 67.51; H, 6.22; F, 3.51; N, 5.42.
2-(4-{[{[2-(3-Fluorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20b): This compound was obtained as a colorless solid in 73% yield by using 19b (0.12 mmol), CH2Cl2 (3 mL) and HCl (4 M dioxane solution, 3 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.26 (6H, s), 2.06 (6H, s), 2.17 (3H, s), 3.40 (2H, s), 3.42 (2H, s), 3.62 (2H, s), 6.29 (1H, d, J=3.2 Hz), 6.35–6.37 (1H, m), 6.89 (2H, s), 7.24–7.30 (1H, m), 7.47–7.60 (3H, m), 7.70 (1H, d, J=6.8 Hz), 12.75 (1H, br s). MS (ESI) m/z: 507 (M+H)+. Anal. Calcd for C29H31FN2O5·0.3H2O: C, 68.03; H, 6.22; F, 3.71; N, 5.47. Found: C, 67.98; H, 6.14; F, 3.82; N, 5.39.
2-(4-{[{[2-(4-Fluorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20c): This compound was obtained as a colorless solid in 47% yield by using 19c (0.87 mmol), CH2Cl2 (5 mL) and HCl (4 M dioxane solution, 5 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.32 (6H, s), 2.13 (6H, s), 2.23 (3H, s), 3.45 (2H, s), 3.49 (2H, s), 3.69 (2H, s), 6.36 (1H, d, J=2.9 Hz), 6.43 (1H, dd, J=2.9, 1.8 Hz), 6.95 (2H, s), 7.33–7.37 (2H, m), 7.62 (1H, d, J=1.8 Hz), 7.95–7.99 (2H, m), 12.81 (1H, br s). MS (ESI) m/z: 507 (M+H)+. Anal. Calcd for C29H31FN2O5·0.3H2O·0.15 dioxane: C, 67.93; H, 6.28; F,3.63; N, 5.35. Found: C, 67.77; H, 6.25; F, 3.63; N, 5.29.
2-[4-({[(2-Phenyl-1,3-oxazol-4-yl)methyl](furan-2-ylmethyl)amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoic Acid (20e): This compound was obtained as a colorless solid in 67% yield by using 19e (0.21 mmol), CH2Cl2 (5 mL) and HCl (4 M dioxane solution, 10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.31 (6H, s), 2.18 (6H, s), 3.51 (2H, s), 3.56 (2H, s), 3.65 (2H, s), 6.37 (1H, d, J=2.9 Hz), 6.43 (1H, dd, J=2.9, 2.0 Hz), 6.98 (2H, s), 7.52–7.56 (3H, m), 7.63 (1H, dd, J=1.7, 0.7 Hz), 7.97–8.00 (2H, m), 8.08 (1H, s). MS (ESI) m/z: 475 (M+H)+.
2-[4-({[(2-Phenyl-5-ethyl-1,3-oxazol-4-yl)methyl](furan-2-ylmethyl)amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoic Acid (20f): This compound was obtained as a colorless solid in 50% yield by using 19f (0.25 mmol), CH2Cl2 (5 mL) and HCl (4 M dioxane solution, 5 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.16 (3H, t, J=7.5 Hz), 1.32 (6H, s), 2.14 (6H, s), 2.60 (2H, q, J=7.5 Hz), 3.46 (2H, s), 3.49 (2H, s), 3.69 (2H, s), 6.36 (1H, d, J=3.2 Hz), 6.43 (1H, dd, J=3.2, 2.0 Hz), 6.96 (2H, s), 7.46–7.54 (3H, m), 7.62–7.63 (1H, m), 7.93–7.95 (2H, m). MS (ESI) m/z: 503 (M+H)+. Anal. Calcd for C30H34N2O5·0.3H2O: C, 70.93; H, 6.86; N, 5.51. Found: C, 70.89; H, 6.86; N, 5.42.
2-(4-{[{[2-Phenyl-5-(propan-2-yl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20g): This compound was obtained as a colorless solid in 37% yield by using 19g (0.50 mmol), CH2Cl2 (5 mL) and HCl (4 M dioxane solution, 5 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.20 (6H, d, J=6.8 Hz), 1.33 (6H, s), 2.13 (6H, s), 2.97–3.03 (1H, m), 3.46 (2H, s), 3.49 (2H, s), 3.69 (2H, s), 6.36–6.38 (1H, m), 6.42–6.44 (1H, m), 6.97 (2H, s), 7.46–7.55 (3H, m), 7.62–7.63 (1H, m), 7.92–7.96 (2H, m), 12.80 (1H, br s). MS (FAB) m/z: 517 (M+H)+. Anal. Calcd for C31H36N2O5·0.3H2O: C, 71.32; H, 7.07; N, 5.37. Found: C, 71.35; H, 7.00; N, 5.17.
2-(4-{[{[2-Phenyl-5-(trifluoromethyl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20h): This compound was obtained as a pale yellow solid in 82% yield by using 19h (0.60 mmol), CH2Cl2 (5 mL) and HCl (4 M dioxane solution, 5 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.29 (6H, s), 2.09 (6H, s), 3.55 (2H, s), 3.67 (2H, s), 3.78 (2H, s), 6.37 (1H, d, J=3.2 Hz), 6.43 (1H, dd, J=3.2, 2.0 Hz), 6.94 (2H, s), 7.57–7.66 (4H, m), 8.03–8.05 (2H, m). MS (ESI) m/z: 543 (M+H)+. Anal. Calcd for C29H29N2O5·0.4H2O: C, 63.36; H, 5.46; N, 5.10; F, 10.37. Found: C, 63.28; H, 5.39; N, 5.16; F, 10.46.
2-(4-{[{[2-Phenyl-5-(hydroxymethyl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20k): This compound was obtained as a colorless solid in 77% yield by using 19k (0.31 mmol) CH2Cl2 (10 mL) and HCl (4 M dioxane solution, 10 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.14 (6H, s), 3.50 (2H, s), 3.53 (2H, s), 3.70 (2H, s), 4.43 (2H, s), 5.33 (1H, br s), 6.37 (1H, d, J=3.2 Hz), 6.43 (1H, dd, J=3.2, 1.9 Hz), 6.98 (2H, s), 7.51–7.56 (3H, m), 7.63 (1H, dd, J=1.9, 0.7 Hz), 7.96–7.99 (2H, m). MS (ESI) m/z: 505 (M+H)+. Anal. Calcd for C29H32N2O6·0.3H2O: C, 68.30; H, 6.44; N, 5.49. Found: C, 68.26; H, 6.38; N, 5.38.
2-(4-{[{[2-Phenyl-5-(dimethylcarbamoyl)-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20l): This compound was obtained as a colorless solid in 77% yield by using 19l (0.27 mmol) CH2Cl2 (3 mL) and HCl (4 M dioxane solution, 6 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.32 (6H, s), 2.13 (6H, s), 2.98 (3H, br s), 3.11 (3H, br s), 3.53 (2H, s), 3.70 (2H, s), 3.75 (2H, s), 6.33 (1H, d, J=3.1 Hz), 6.41 (1H, dd, J=3.1, 2.0 Hz), 6.95 (2H, s), 7.56–7.61 (4H, m), 8.03–8.06 (2H, m), 12.79 (1H, br s). MS (FAB) m/z: 546 (M+H)+. Anal. Calcd for C31H35N3O6·0.6H2O: C, 66.91; H, 6.56; N, 7.55. Found: C, 66.88; H, 6.48; N, 7.45.
2-(4-{[{[2-(Tetrahydro-2H-pyran-4-yl)-5-methyl-1,3-oxazol-4-yl]methyl}(furan-2-ylmethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoic Acid (20m): This compound was obtained as a colorless solid in 75% yield by using 19m (0.46 mmol) CH2Cl2 (5 mL) and HCl (4 M dioxane solution, 5 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.34 (6H, s), 1.62–1.75 (2H, m), 1.84–1.91 (2H, m), 2.12 (3H, s), 2.14 (6H, s), 2.97–3.02 (1H, m), 3.30–3.47 (5H, m), 3.56–3.64 (3H, m), 3.84–3.89 (2H, m), 6.31–6.33 (1H, m), 6.40–6.42 (1H, m), 6.94 (2H, s), 7.61 (1H, s), 12.80 (1H, br s). MS (FAB) m/z: 497 (M+H)+. Anal. Calcd for C28H36N2O6·0.5H2O: C, 66.52; H, 7.38; N, 5.54. Found: C, 66.47; H, 7.40; N, 5.26.
General Procedure for 2-[2,6-Dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl][(1,3-azol)-2-ylmethyl]amino}methyl)phenoxy]-2-methylpropanoic Acid (22a–c)To a solution of 21 in CH2Cl2 were added a corresponding aldehyde and sodium triacethoxyborohydride and stirred at room temperature overnight. The reaction mixture was diluted with EtOAc, washed with water, saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (n-hexane/EtOAc) to provide tertiary amine compounds. To a solution of the prepared compound in methanol was added NaOH (2 N aqueous solution) and stirred at room temperature overnight. The reaction mixture was quenched by HCl (1 N aqueous solution). After methanol was removed in vacuo, the organics were extracted with EtOAc and then the organic layer was washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CHCl3/methanol) to provide the free form of 22a–c. 22a and 22b were hydrochlorinated with HCl (4 M dioxane solution), concentrated and recrystallized from (hexane/EtOAc) to afford 22a and 22b HCl salts, respectively.
2-[2,6-Dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl](1,3-oxazol-2-ylmethyl)amino}methyl)phenoxy]-2-methylpropanoic Acid (22a): This compound was obtained as HCl salt form of a colorless solid in 56% yield: 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.14 (6H, s), 2.32 (3H, s), 4.11–4.35 (6H, m), 7.14 (2H, s), 7.30 (1H, m), 7.51–7.55 (3H, m), 7.93–7.95 (2H, m), 8.19 (1H, m). MS (ESI) m/z: 490 (M+H)+. Anal. Calcd for C28H31N3O5·HCl: C, 63.93; H, 6.13; N, 7.99. Found: C, 63.63; H, 6.42; N, 7.58.
2-[2,6-Dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl](1,3-thiazol-2-ylmethyl)amino}methyl)phenoxy]-2-methylpropanoic Acid (22b): This compound was obtained as HCl salt form of a colorless solid in 32% yield: 1H-NMR (CDCl3) δ: 1.41 (6H, s), 2.22 (6H, s), 2.25 (3H, s), 3.64 (2H, s), 3.65 (2H, s), 4.09 (2H, s), 7.04 (2H, s), 7.28 (1H, d, J=3.2 Hz), 7.39–7.45 (3H, m), 7.69 (1H, d, J=3.2 Hz), 7.98–8.01 (2H, m). MS (ESI) m/z: 506 (M+H)+. Anal. Calcd for C28H31N3O4S 1.25HCl·0.75H2O: C, 59.55; H, 6.02; N, 7.45; S, 5.67; Cl, 7.84. Found: C, 59.67; H, 5.92; N, 6.99; S, 5.45; Cl, 8.14.
2-[2,6-Dimethyl-4-({[(1-methyl-1H-imidazol-2yl)methyl][(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl] amino}methyl)phenoxy]-2-methylpropanoic Acid (22c): This compound was obtained as a colorless solid in 44% yield: 1H-NMR (400 MHz, DMSO-d6) δ: 1.32 (6H, s), 2.12 (6H, s), 2.22 (3H, s), 3.47 (2H, s), 3.48 (2H, s), 3.51 (3H, s), 3.68 (2H, s), 6.76 (1H, d, J=1.2 Hz), 6.91 (2H, s), 7.03 (1H, d, J=1.2 Hz), 7.48–7.54 (3H, m), 7.92–7.94 (2H, m). MS (ESI) m/z: 503 (M+H)+. Anal. Calcd for C29H34N4O4·0.25H2O: C, 68.69; H, 6.86; N, 11.05. Found: C, 68.49; H, 6.93; N, 10.76.
Ethyl 2-(4-{[(2-tert-Butoxy-2-oxoethyl)amino]methyl}-2,6-dimethylphenoxy)-2-methylpropanoate (24)To a solution of 23 (15.8 g, 59.9 mmol) in THF (300 mL) were added tert-butylglycinate (9 mL, 95.9 mmol) and MgSO4 (50 g), and then the mixture was stirred under a reflux condition for 4 h. After the insoluble matter was filtered through Celite, the filtrate was concentrated. After the residue was dissolved with methanol (100 mL), sodium borohydride (2.30 g, 59.9 mmol) was added at 0°C and then the reaction mixture was stirred at room temperature for 4 h. The solvent was removed in vacuo and the residue was diluted with EtOAc. The organic layer was washed with saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CHCl3/MeOH=99/1 then 19/1 as eluent) to give compound 24 (21.6 g, 95%) as a pale yellow oil. 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.46 (6H, s), 1.48 (9H, s), 2.18 (6H, s), 3.30 (2H, s), 3.65 (2H, s), 4.29 (2H, q, J=7.1 Hz), 6.93 (2H, s). MS (ESI) m/z: 380 (M+H)+.
Ethyl 2-[4-({(2-tert-Butoxy-2-oxoethyl)[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (25)To a solution of 4-(chloromethyl)-5-methyl-2-phenyl-1,3-oxazole (1.87 g, 9.00 mmol) and 24 (3.90 g, 10.3 mmol) in MeCN (30 mL) was added K2CO3 (2.50 g, 18.0 mmol). The reaction mixture was stirred under a reflux condition for 24 h. After cooling the mixture to an ambient temperature, the solvent was removed in vacuo. The residue was diluted with EtOAc. The organic layer was washed with water and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (n-hexane/EtOAc=9/1 then 2/1 as eluent) to provide compound 21 (4.0 g, 81%) as a pale yellow oil. 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.45 (6H, s), 1.48 (9H, s), 2.17 (6H, s), 2.30 (3H, s), 3.30 (2H, s), 3.71 (2H, s), 3.75 (2H, s), 4.28 (2H, q, J=7.1 Hz), 6.99 (2H, s), 7.38–7.46 (3H, m), 8.00–8.02 (2H, m). MS (ESI) m/z: 551 (M+H)+.
N-{4-[(1-Ethoxy-2-methyl-1-oxopropan-2-yl)oxy]-3,5-dimethylbenzyl}-N-[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]glycine (26)To a solution of 25 (4.0 g, 7.26 mmol) in CH2Cl2 was added HCl (4 M dioxane solution, 30 mL) at 0°C and stirred at room temperature for 14 h. After the solvent was removed in vacuo, the residue was solidified with n-hexane to provide compound 26 HCl salt (3.17 g, 82%) as a pale yellow solid. MS m/z: 495 (M+H)+.
Ethyl 2-[4-({[2-(2-Acetylhydrazinyl)-2-oxoethyl][(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (28)To a solution of 26 (0.40 g, 0.86 mmol) in DMF (3 mL) were added acetohydrazide (77 mg, 1.0 mmol), EDCI (250 mg, 1.3 mmol) and HOBt (200 mg, 1.3 mmol). The reaction mixture was stirred at room temperature for 20 h. The mixture was diluted with EtOAc, washed with water, 10% citric acid aqueous solution, saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated to give 28 (500 mg) as a pale yellow oil. This compound was used for the next reaction without further purification. 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.45 (6H, s), 2.02 (3H, s), 2.18 (6H, s), 2.23 (3H, s), 3.40 (2H, s), 3.60 (2H, s), 3.65 (2H, s), 4.28 (2H, q, J=7.1 Hz), 6.98 (2H, s), 7.42–7.45 (3H, m), 8.00–8.02 (2H, m), 8.62 (1H, br s), 10.12 (1H, br s). MS (ESI) m/z: 551 (M +H)+.
2-[2,6-Dimethyl-4-({[(5-methyl-1,3,4-oxadiazol-2-yl)methyl][(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoic Acid (29)To a solution of triphenylphosphine (680 mg, 2.6 mmol) in CH2Cl2 (15 mL) were added hexachloroethane (510 mg, 2.2 mmol) and triethylamine (720 µL, 5.1 mmol). After the reaction mixture was stirred at room temperature for 10 min, a solution of 28 (500 mg, 1.0 mmol) in CH2Cl2 (5.0 mL) was added and stirred at the same temperature overnight. Saturated NaHCO3 aqueous solution was added to the reaction mixture and stirred at an ambient temperature for 1 h. The organics was extracted with CH2Cl2 and washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (n-hexane/EtOAc=4/1 then 1/2 as eluent) to provide ethyl 2-[2,6-dimethyl-4-({[(5-methyl-1,3,4-oxadiazol-2-yl)methyl][(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoate as a yellow oil. 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.45 (6H, s), 2.18 (6H, s), 2.27 (3H, s), 2.51 (3H, s), 3.67 (4H, br s), 3.99 (2H, s), 4.28 (2H, q, J=7.1 Hz), 6.98 (2H, s), 7.40–7.45 (3H, m), 8.00–8.02 (2H, m). MS m/z: 533 (M+H)+. To a solution of the prepared ester compound in MeOH (10 mL) was added NaOH (1 N aqueous solution, 3.0 mL) at room temperature and stirred under a reflux condition for 1 h. After cooling the mixture to an ambient temperature, the reaction mixture was quenched by the addition of HCl (1 N aqueous solution, 3.0 mL). The solvent was removed in vacuo and the residue was diluted with EtOAc. The organic layer was washed with water, brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CHCl3/MeOH=19/1 then 9/1 as eluent). HCl (4 M dioxane solution) was added to the obtained the free form and then the solvent was removed in vacuo to give 29 HCl salt as a colorless solid (315 mg, 70%): 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.13 (6H, s), 2.29 (3H, s), 2.46 (3H, s), 3.66–4.26 (6H, m), 7.05 (2H, s), 7.50–7.52 (3H, m), 7.91–7.93 (2H, m). MS (ESI) m/z: 505 (M+H)+; Anal. Calcd for C28H32N4O5·HCl·0.75H2O: C, 60.64; H, 6.27; N, 10.10; Cl, 6.39. Found: C, 61.04; H, 6.47; N, 9.69; Cl, 6.22.
Ethyl 2-[4-({(2-Amino-2-oxoethyl)[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (32)To a solution of 26 (0.40 g, 0.86 mmol) in DMF (3 mL) were added ammonium chloride (55 mg, 1.03 mmol), EDCI (250 mg, 1.3 mmol) and HOBt (200 mg, 1.3 mmol), and then the reaction mixture was stirred at room temperature for 14 h. The mixture was diluted with EtOAc, washed with water, 10% citric acid aqueous solution, saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated to give compound 32 (528 mg) as a pale yellow oil. This compound was used for the next reaction without further purification: 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.45 (6H, s), 2.17 (6H, s), 2.25 (3H, s), 3.24 (2H, s), 3.54 (2H, s), 3.60 (2H, s), 4.28 (2H, q, J=7.1 Hz), 6.91 (2H, s), 7.44–7.45 (3H, m), 7.98–8.02 (2H, m). MS m/z: 494 (M +H)+.
2-[2,6-Dimethyl-4-({[(3-methyl-1,2,4-oxadiazol-5-yl)methyl][(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoic Acid (33)To a solution of 32 (0.808 mmol) was added (1,1-dimethoxyethyl)-dimethylamine (5 mL) and stirred at 120°C for 2 h. After the solvent was removed in vacuo, hydroxylamine (50% aqueous solution, 70 µL, 0.515 mmol) and acetic acid (70% aqueous solution, 6 mL) were added to the residue and then stirred at room temperature for 5 d. The mixture was diluted with EtOAc, washed with water, saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (n-hexane/EtOAc=9/1 then 3/1 as eluent) to provide ethyl 2-[2,6-dimethyl-4-({[(3-methyl-1,2,4-oxadiazol-5-yl)methyl][(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoate as a colorless oil (379 mg, 88%): 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.45 (6H, s), 2.18 (6H, s), 2.27 (3H, s), 2.41 (3H, s), 3.67 (2H, s), 3.70 (2H, s), 4.04 (2H, s), 4.28 (2H, q, J=7.1 Hz), 7.00 (2H, s), 7.40–7.46 (3H, m), 7.99–8.01 (2H, m). MS (ESI) m/z: 533 (M+H)+. To a solution of the prepared ester (375 mg, 0.704 mmol) in methanol (10 mL) was added NaOH (1 N aqueous solution, 3.0 mL) at room temperature and stirred under a reflux condition overnight. After cooling the mixture to ambient temperature, the reaction mixture was quenched by the addition of HCl (1 N aqueous solution, 3.0 mL). The solvent was removed in vacuo and the residue was diluted with EtOAc. The organic layer was washed with water, brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CHCl3/MeOH=19/1 then 9/1 as eluent). HCl (4 M dioxane solution) was added to the obtained free form and the solvent was removed in vacuo to give 33 HCl salt as a colorless solid (273 mg, 71%): 1H-NMR (400 MHz, DMSO-d6) δ: 1.32 (6H, s), 2.13 (6H, s), 2.27 (3H, s), 2.31 (3H, s), 3.81 (4H, br s), 4.15 (2H, br s), 7.01 (2H, s), 7.49–7.53 (3H, m), 7.90–7.92 (2H, m). MS (ESI) m/z: 505 (M+H)+. Anal. Calcd for C28H32N4O5·HCl·0.25H2O: C, 61.65; H, 6.19; N, 10.27. Found: C, 62.02; H, 6.53; N, 9.87.
Ethyl 2-[4-({(2-Hydrazinyl-2-oxoethyl)[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (27)To a solution of 26 (1 g, 2.02 mmol) in DMF (3 mL) were added hydrazine carboxylic acid tert-butyl ester (400 mg, 3.03 mmol), EDCI (776 mg, 4.04 mmol) and HOBt (622 mg, 4.04 mmol) and then stirred at room temperature for overnight. The mixture was diluted with EtOAc, washed with water, 10% citric acid aqueous solution, saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated. To a solution of the residue in CH2Cl2 (5 mL) was added TFA (5 mL) and stirred at room temperature for 14 h. The mixture was diluted with EtOAc, washed with saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated to give 27 (1.14 g) as a pale yellow oil. This compound was used for the next reaction without further purification: MS (ESI) m/z: 509 (M+H)+.
2-[2,6-Dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl][5-oxo-4,5-dihydro-1,3,4-oxadiazol-2-ylmethyl]amino}methyl)phenoxy]-2-methylpropionic Acid (30)To a solution of 27 (200 mg, 0.394 mmol) in dioxane (5 mL) was added triphosgene (468 mg, 1.58 mmol) and stirred at 60°C for 4 h. After the solvent was removed in vacuo, water was added to the residue, extracted with EtOAc, washed with saturated NaHCO3 aqueous solution and brine, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=3/1 then 1/2 as eluent) to provide ethyl 2-[2,6-dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl][5-oxo-4,5-dihydro-1,3,4-oxadiazol-2-ylmethyl]amino}methyl)phenoxy]-2-methylpropanoate as a colorless oil (176 mg, 84%): 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.57 (6H, s), 2.18 (6H, s), 2.28 (3H, s), 3.65 (2H, s), 3.67 (2H, s), 3.73 (2H, s), 4.28 (2H, q, J=7.1 Hz), 6.97 (2H, s), 7.40–7.46 (3H, m), 7.99–8.01 (2H, m), 8.54 (1H, s). MS (ESI) m/z: 535 (M+H)+. The prepared ethyl ester form was hydrolyzed in a similar manner with 22 to afford 30 as a colorless solid (100 mg, 62%): 1H-NMR (400 MHz, DMSO-d6) δ: 1.32 (6H, s), 2.12 (6H, s), 2.26 (3H, s), 3.57 (2H, s), 3.59 (2H, s), 3.62 (2H, s), 6.95 (2H, s), 7.46–7.53 (3H, m), 7.90–7.92 (2H, m). MS (ESI) m/z: 505 (M−H)−. Anal. Calcd for C27H30N4O6·0.25H2O: C, 63.46; H, 6.02; N, 10.96. Found: C, 63.44; H, 6.11; N, 10.73.
2-[2,6-Dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl][(5-methyl-1,3,4-thiadiazol-2-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoic Acid (31)To a solution of 28 (498 mg, 0.90 mmol) in THF (50 mL) was added Lawesson’s reagent (732 mg, 1.81 mmol) and stirred under a reflux condition for 30 min. After the solvent was removed in vacuo, the residue was purified by column chromatography on silica gel (CH2Cl2/methanol=19/1, v/v) to provide ethyl 2-[2,6-dimethyl-4-({[(5-methyl-2-phenyl-1,3-oxazol-4-yl)methyl][(5-methyl-1,3,4-thiadiazol-2-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoate as a pale green solid (437 mg, 88%): 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.2 Hz), 1.46 (6H, s), 2.20 (6H, s), 2.24 (3H, s), 2.74 (3H, s), 3.61 (2H, s), 3.63 (2H, s), 4.13 (2H, s), 4.29 (2H, q, J=7.2 Hz), 6.99 (2H, s), 7.40–7.47 (3H, m), 7.98–8.01 (2H, m). The prepared ethyl ester form was hydrolyzed in a similar manner with 22 to afford 31 as a colorless solid (243 mg, 56%): 1H-NMR (400 MHz, DMSO-d6) δ: 1.34 (6H, s), 2.16 (6H, s), 2.24 (3H, s), 2.68 (3H, s), 3.58 (2H, s), 3.59 (2H, s), 4.08 (2H, s), 7.01 (2H, s), 7.50–7.55 (3H, m), 7.92–7.96 (2H, m). MS (FAB) m/z: 521 (M+H)+. Anal. Calcd for C28H32N4O4S·0.1dioxane·0.8H2O: C, 62.72; H, 6.38, N, 10.30; S, 5.90. Found: C, 62.84; H, 6.24, N, 10.02; S, 5.87.
Ethyl 2-[4-({(2-tert-Butoxy-2-oxoethyl)[(9H-fluoren-9-ylmethoxy)carbonyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (34)To a solution of 24 (3.5 g, 9.23 mmol) in MeCN (30 mL) was added 9-fluorenylmethylsuccinimidyl carbonate (3.74 g, 11.1 mmol) at 0°C and stirred at room temperature for 4 h. After the solvent was removed in vacuo, water was added to the residue, extracted with EtOAc, washed with saturated NaHCO3 aqueous solution, 10% citric acid aqueous solution and brine, dried over Na2SO4 and concentrated. The crude product was purified by column chromatography on silica gel (hexane/EtOAc=9/1 then 3/1 as eluent) to provide 34 as a pale yellow solid (5.24 g, 94%): 1H-NMR (400 MHz, CDCl3) δ: 1.34–1.38 (3H, m), 1.44 (9H, d, J=5.9 Hz), 1.47 (6H, d, J=2.5 Hz), 2.18 (6H, d, J=2.7 Hz), 3.81 (2H, d, J=23.0 Hz), 4.26–4.30 (3H, m), 4.44–4.49 (4H, m), 6.77 (1H, s), 6.85 (1H, s), 7.25–7.29 (2H, m), 7.36–7.41 (2H, m), 7.53 (1H, d, J=7.4 Hz), 7.60 (1H, d, J=7.4 Hz), 7.74 (1H, d, J=11.8 Hz), 7.76 (1H, d, J=11.8 Hz). MS (ESI) m/z: 602 (M+H)+.
N-{4-[(1-Ethoxy-2-methyl-1-oxopropan-2-yl)oxy]-3,5-dimethylbenzyl}-N-[(9H-fluoren-9-ylmethoxy)carbonyl]glycine (35)To a solution of 34 (5.24 g, 8.71 mmol) in CH2Cl2 (60 mL) was added TFA (20 mL) at 0°C and stirred at room temperature overnight. The mixture was diluted with EtOAc, washed with water and brine, dried over Na2SO4, filtered and concentrated. The crude product was purified by column chromatography on silica gel (CHCl3/methanol=19/1 then 9/1 as eluent) to provide 35 as a pale yellow oil (5.70 g): 1H-NMR (400 MHz, CDCl3) δ: 1.33–1.38 (3H, m), 1.46 (6H, d, J=5.9 Hz), 2.17 (6H, s), 3.88 (2H, d, J=81.3 Hz), 4.25–4.32 (3H, m), 4.44 (2H, s), 4.52–4.55 (2H, m), 6.75 (1H, s), 6.82 (1H, s), 7.23–7.31 (2H, m), 7.38 (2H, t, J=7.4 Hz), 7.53–7.56 (2H, m), 7.74 (2H, d, J=7.4 Hz); MS (ESI) m/z: 568 (M+Na)+.
Ethyl 2-[4-({[2-(2-Acetylhydrazinyl)-2-oxoethyl][(9H-fluoren-9-ylmethoxy)carbonyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (36)35 was amidated in a similar manner with 28 to afford 36 as colorless solid (1.1 g, 67%): MS (ESI) m/z: 602 (M+H)+.
Ethyl 2-[4-({[(9H-Fluoren-9-ylmethoxy)carbonyl][(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino}methyl)-2,6-dimethylphenoxy]-2-methylpropanoate (37)To a solution of triphenylphosphine (1.53 g, 5.85 mmol) in CH2Cl2 (30 mL) were added hexachloroethane (1.17 g, 4.94 mmol), triethylamine (1.63 mL, 11.7 mmol) and 36 (1.1 g, 1.83 mmol). After the mixture was stirred at room temperature overnight, the reaction mixture was diluted with EtOAc. The organic layer was washed with water, 10% citric acid aqueous solution, saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (n-hexane/EtOAc=3/1 then 1/3 as eluent) to provide 37 as a pale yellow oil (1.0 g, 94%): 1H-NMR (400 MHz, CDCl3) δ: 1.36 (3H, t, J=7.1 Hz), 1.46 (6H, s), 2.16 (6H, s), 2.47 (3H, d, J=10.5 Hz), 4.26–4.31 (3H, m), 4.39–4.44 (3H, m), 4.55–4.59 (3H, m), 6.79 (2H, d, J=47.6 Hz), 7.36–7.40 (2H, m), 7.46–7.49 (1H, m), 7.53–7.57 (3H, m), 7.65–7.70 (1H, m), 7.73–7.75 (2H, m). MS (ESI) m/z: 584 (M+H)+.
Ethyl 2-[2,6-Dimethyl-4-({[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino}methyl)phenoxy]-2-methylpropanoate (38)To a solution of 37 (1.0 g, 1.83 mmol) in THF (50 mL) was added DBU (2% THF solution, 42 mL, 5.48 mmol) at 0°C and stirred at the same temperature for 2 h. The reaction mixture was diluted with EtOAc, washed with saturated NaHCO3 aqueous solution and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CHCl3/methanol=99/1 then 9/1 as eluent) to provide 38 as a yellow oil (0.54 g, 82%): 1H-NMR (400 MHz, CDCl3) δ: 1.35 (3H, t, J=7.1 Hz), 1.46 (7H, s), 2.19 (6H, s), 2.53 (3H, s), 3.73 (2H, s), 3.98 (2H, s), 4.29 (2H, q, J=7.1 Hz), 6.92 (2H, s). MS (ESI) m/z: 362 (M+H)+.
General Procedure for the Preparation of Compounds (40a–i)To a solution of 38 in MeCN were added 39, Cs2CO3 and KI and then the mixture was stirred at 75°C overnight. The reaction mixture was diluted with EtOAc, washed with water and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (hexane/EtOAc). To a solution of the prepared tertiary amine compounds in methanol was added NaOH (1 N aqueous solution) and stirred at room temperature under a reflux condition. After the reaction mixture was quenched by HCl (1 N aqueous solution), the solvent was removed in vacuo. The residue was diluted with EtOAc, washed with water and brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CHCl3/methanol) to provide 40a–i. 40a, 40d and 40e was hydrochlorinated with HCl (4 M dioxane solution), concentrated and recrystallized from (hexane/EtOAc) to afford 40a HCl salt, 40d HCl salt and 40e HCl salt.
2-{2,6-Dimethyl-4-[({[5-methyl-2-(3-methylphenyl)-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]phenoxy}-2-methylpropanoic Acid (40a): This compound was obtained as the HCl salt form of a colorless solid in 25% yield by using 38 (0.42 mmol), 39a (0.42 mmol), Cs2CO3 (0.62 mmol), KI (0.12 mmol) and MeCN (5 mL), followed by NaOH (2.0 mmol), MeOH (5 mL) and HCl (4 M dioxane solution, 2 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.14 (6H, s), 2.25 (3H, s), 2.36 (3H, s), 2.45 (3H, s), 3.57 (2H, s), 3.61 (2H, s), 3.91 (2H, s), 6.96 (2H, s), 7.32 (2H, d, J=8.1 Hz), 7.81 (2H, d, J=8.1 Hz). MS (ESI) m/z: 519 (M+H)+. Anal. Calcd for C29H34N4O5·HCl·0.5H2O: C, 61.75; H, 6.43; N, 9.93. Found: C, 61.47; H, 6.43; N, 9.65.
2-{2,6-Dimethyl-4-[({[5-methyl-2-(4-methylphenyl)-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]phenoxy}-2-methylpropanoic Acid (40b): This compound was obtained as a colorless solid in 39% yield by using 38 (0.41 mmol), 39b (0.41 mmol), Cs2CO3 (0.61 mmol), KI (0.12 mmol) and MeCN (5 mL), followed by NaOH (2.0 mmol) and MeOH (10 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.14 (6H, s), 2.25 (3H, s), 2.36 (3H, s), 2.45 (3H, s), 3.57 (2H, s), 3.61 (2H, s), 3.91 (2H, s), 6.96 (2H, s), 7.32 (2H, d, J=8.1 Hz), 7.81 (2H, d, J=8.1 Hz). MS (ESI) m/z: 519 (M+H)+. Anal. Calcd for C29H34N4O5·0.6H2O: C, 65.79; H, 6.70; N, 10.58. Found: C, 66.10; H, 6.75; N, 10.30.
2-{4-[({[2-(4-Chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]-2,6-dimethylphenoxy}-2-methylpropanoic Acid (40c): This compound was obtained as a colorless solid in 43% yield by using 38 (0.41 mmol), 39c (0.41 mmol), Cs2CO3 (0.61 mmol), KI (0.12 mmol) and MeCN (5 mL), followed by NaOH (2.0 mmol) and MeOH (10 mL): 1H-NMR (400 MHz, DMSO-d6) δ: 1.33 (6H, s), 2.13 (6H, s), 2.26 (3H, s), 2.46 (3H, s), 3.59 (2H, s), 3.61 (2H, s), 3.91 (2H, s), 6.96 (2H, s), 7.58 (2H, d, J=8.6 Hz), 7.92 (2H, d, J=8.6 Hz). MS (ESI) m/z: 540 (M+H)+. Anal. Calcd for C28H31ClN4O5·0.75H2O: C, 60.87; H, 5.93; N, 10.14. Found: C, 61.36; H, 5.92; N, 9.64.
2-{4-[({[2-(4-Methoxyphenyl)-5-methyl-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]-2,6-dimethylphenoxy}-2-methylpropanoic Acid (40d): This compound was obtained as the HCl salt form of a colorless solid in 24% yield by using 38 (0.42 mmol), 39d (0.42 mmol), Cs2CO3 (0.62 mmol), KI (0.12 mmol) and MeCN (5 mL), followed by NaOH (2.0 mmol), MeOH (10 mL) and HCl (4 M dioxane solution, 2 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.52 (6H, s), 2.25 (6H, s), 2.46 (3H, s), 2.58 (3H, s), 3.87 (3H, s), 4.31 (2H, s), 4.46 (2H, s), 4.56 (2H, s), 6.96 (2H, d, J=8.8 Hz), 7.45 (2H, s), 7.88 (2H, d, J=8.8 Hz). MS (ESI) m/z: 535 (M+H)+. Anal. Calcd for C29H34N4O6·HCl·0.5H2O: C, 60.05; H, 6.26; N, 9.66. Found: C, 60.31; H, 6.30; N, 9.46.
2-[2,6-Dimethyl-4-({[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]({5-methyl-2-[4-(trifluoromethoxy)phenyl]-1,3-oxazol-4-yl}methyl)amino}methyl)phenoxy]-2-methylpropanoic Acid (40e): This compound was obtained as HCl salt form of a colorless solid in 58% yield by using 38 (0.28 mmol), 39e (0.28 mmol), Cs2CO3 (0.41 mmol), KI (0.08 mmol) and MeCN (10 mL), followed by NaOH (2.0 mmol), MeOH (10 mL) and HCl (4 M dioxane solution, 2 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.52 (6H, s), 2.25 (6H, s), 2.46 (3H, s), 2.59 (3H, s), 4.19 (2H, s), 4.32–4.35 (4H, m), 7.29 (2H, d, J=8.8 Hz), 7.36 (2H, s), 7.98 (2H, d, J=8.8 Hz). MS (ESI) m/z: 589 (M+H)+. Anal. Calcd for C29H31F3N4O6·HCl·0.25H2O: C, 55.33; H, 5.20; N, 8.90. Found: C, 55.31; H, 5.22; N, 8.65.
2-{4-[({[2-(4-Ethoxyphenyl)-5-methyl-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]-2,6-dimethylphenoxy}-2-methylpropanoic Acid (40f): This compound was obtained as a colorless solid in 46% yield by using 38 (0.28 mmol), 39f (0.28 mmol), Cs2CO3 (0.41 mmol), KI (0.08 mmol) and MeCN (10 mL), followed by NaOH (2.0 mmol) and MeOH (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.44 (3H, t, J=7.0 Hz), 1.50 (6H, s), 2.22 (6H, s), 2.28 (3H, s), 2.52 (3H, s), 3.67 (2H, s), 3.69 (2H, s), 3.98 (2H, s), 4.08 (2H, q, J=6.9 Hz), 6.93 (2H, d, J=8.8 Hz), 7.04 (2H, s), 7.92 (2H, d, J=8.8 Hz). MS (ESI) m/z: 549 (M+H)+. Anal. Calcd for C30H36N4O6·0.75H2O: C, 64.10; H, 6.72; N, 9.97. Found: C, 64.33; H, 6.64; N, 9.81.
2-[2,6-Dimethyl-4-({[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]({5-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-oxazol-4-yl}methyl)amino}methyl)phenoxy]-2-methylpropanoic Acid (40g): This compound was obtained as a colorless solid in 51% yield by using 38 (0.28 mmol), 39g (0.28 mmol), Cs2CO3 (0.41 mmol), KI (0.08 mmol) and MeCN (10 mL), followed by NaOH (2.0 mmol) and MeOH (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.51 (6H, s), 2.23 (6H, s), 2.33 (3H, s), 2.53 (3H, s), 3.70–3.71 (4H, m), 3.98 (2H, s), 7.04 (2H, s), 7.70 (2H, d, J=8.3 Hz), 8.12 (2H, d, J=8.1 Hz). MS (ESI) m/z: 573 (M+H)+. Anal. Calcd for C29H31F3N4O5·0.5H2O: C, 59.89; H, 5.55; N, 9.63. Found: C, 59.95; H, 5.48; N, 9.49.
2-{4-[({[2-(3,4-Dimethylphenyl)-5-methyl-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]-2,6-dimethylphenoxy}-2-methylpropanoic Acid (40h): This compound was obtained as a colorless solid in 61% yield by using 38 (0.28 mmol), 39h (0.28 mmol), Cs2CO3 (0.41 mmol), KI (0.08 mmol) and MeCN (10 mL), followed by NaOH (2.0 mmol) and MeOH (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.50 (6H, s), 2.22 (6H, s), 2.29 (3H, s), 2.30 (3H, s), 2.32 (3H, s), 2.52 (3H, s), 3.68–3.69 (4H, m), 3.98 (2H, s), 7.03 (2H, s), 7.19 (1H, d, J=7.8 Hz), 7.72 (1H, d, J=7.8 Hz), 7.80 (1H, s). MS (ESI) m/z: 533 (M+H)+. Anal. Calcd for C30H36N4O5·0.5H2O: C, 66.52; H, 6.89; N, 10.34. Found: C, 66.91; H, 6.92; N, 10.13.
2-{4-[({[2-(4-Methoxy-3-methylphenyl)-5-methyl-1,3-oxazol-4-yl]methyl}[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]amino)methyl]-2,6-dimethylphenoxy}-2-methylpropanoic Acid (40i): This compound was obtained as a colorless solid in 50% yield by using 38 (0.28 mmol), 39i (0.28 mmol), Cs2CO3 (0.41 mmol), KI (0.08 mmol) and MeCN (10 mL), followed by NaOH (2.0 mmol) and MeOH (10 mL): 1H-NMR (400 MHz, CDCl3) δ: 1.50 (6H, s), 2.22 (6H, s), 2.26 (3H, s), 2.28 (3H, s), 2.52 (3H, s), 3.67 (2H, s), 3.68 (2H, s), 3.88 (3H, s), 3.98 (2H, s), 6.86 (1H, d, J=9.1 Hz), 7.04 (2H, s), 7.80–7.81 (2H, m). MS (ESI) m/z: 549 (M+H)+. Anal. Calcd for C30H36N4O6·0.5H2O: C, 64.62; H, 6.69; N, 10.05. Found: C, 64.87; H, 6.75; N, 9.81.
PPAR Transactivation AssayThe fusion protein (PPAR ligand binding domain-GAL4 DNA binding domain) expression plasmid (pFA-PPARγ/GAL4 or pFA-PPARα/GAL4) and the reporter plasmid (pFA-SEAP, Stratagene) were used. HEK 293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in 5% CO2. After 24 h of culture, cells were co-transfected with pFA-PPAR/GAL and pFA-SEAP using Lipofectamine (Invitrogen) and Plus Reagents (Invitrogen) according to the manufacturer’s protocol. After 5 h of transfection, cells were treated with DMEM-FBS containing a test compound at 37°C in 5% CO2. After 48 h of incubation, the conditioned medium was collected and the SEAP activity in it was measured by the use of the Reporter assay kit—SEAP (TOYOBO) according to the manufacturer’s protocol. Chemiluminescence was read by ARVOsx, PerkinElmer, Inc. The fold increase in chemiluminescence in the presence of a test compound compared to that in the absence of it was calculated, and then the EC50 value was obtained.
AnimalAnimal facilities, animal care, and study programs were in accordance with the in-house guideline of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd. Female db/db (C57BLKS/J-m+/+Leprdb) mice were purchased at 10 weeks olds from CREA Japan, Inc. (Tokyo, Japan) and used as a type 2 diabetic animal model. The db/db mice were housed six per cage and maintained on an 8 a.m. light/8 p.m. dark schedule. Rodent chow and water were given ad libitum.
In Vivo db/db Mouse StudiesMice (six per group) received a once daily oral dosing of a test compound or vehicle (0.5% methylcellulose) by oral gavage for 10 d. Blood was collected from the tail vein immediately prior to the next dosing on Days 0, 5 and 10 for measurement of the plasma glucose and triglyceride levels.
Distribution CoefficientThe distribution coefficients (Log D) between 1-octanol and phosphate buffered saline (PBS) were assayed by a shaking flask method.19) Equal amounts of PBS and 1-octanol were shaken and left for over 12 h. The upper layer (1-octanol) and lower layer (PBS) were collected individually. Each compound was dissolved in 1-octanol or PBS (200 µM). The same amount of either PBS or 1-octanol was added and the mixture was shaken vigorously for 30 min at room temperature. Then, both phases were separated and assayed using LC-MS methodologies (LC-Mass spectrometer: 1100 Series LC/MSD, Agilent; Analytical Column: X Terra® MSC18 3.5 µm, 3.0×30 mm, Waters; Mobile Phase: 10 mM ammonium acetate buffer (pH 4.5)/0.05% acetic acid in acetonitrile=95/5 to 10/90 v/v). The values of Log D were analyzed using Analyst software program (version 1.4, Applied Bio Systems).
CYP3A4 Direct Inhibition AssayP450 3A4 inhibition activities were measured with a high throughput inhibitor screening kit (Baculovirus-insect cell-expression system, Supersomes™, and a fluorescent substrate (7-benzyloxy-trifluoromethylcoumarin)) available from BD GENTEST.14) % Inhibition was estimated by fluorescence at 10 µM of the test compounds.
MBI AssayMechanism based inactivation against CYP3A4 is estimated as the percentage of the enzymatic activity (1′-hydroxylation of midazolam) remaining, after the 30-min preincubation of the test compounds in pooled human liver microsomes.16)
We are grateful to the members in Biological Research Laboratory for performing the biological assays. Also, we would like to acknowledge Dr. Kiyoshi Nakayama for his helpful scientific guidance and support in the preparation of this manuscript.