2013 Volume 61 Issue 12 Pages 1282-1290
2013 Volume 61 Issue 12 Pages 1282-1290
This report describes the synthesis and in vitro anti-malarial evaluations of certain C2 or C8 and C11-disubstituted 6-methyl-5H-indolo[2,3-b]quinoline (neocryptolepine congener) derivatives. To attain higher activities, the structure–activity relationship (SAR) studies were conducted by varying the kind of alkylamino or ω-aminoalkylamino stubstituents at C11 and with Cl at the C2 position, or CO2Me at the C9 position. The anti-malarial activities of the tested compounds were significantly increased compared to the 11-non(alkylamino) derivatives. The 3-aminopropylamino group at C11 was further modified to urea and thiourea, which improved the cytotoxicity against normal cells. The best results were achieved with compounds 8 and 9d against the NF54 strain with the IC50/SI values as of 86 nM/20 and 317 nM/370, respectively. Furthermore, the compounds were tested for β-haematin inhibition. Twelve were found to have IC50 values below 100 µM and a linear correlation between the β-haematin inhibition and cell growth inhibition in the NF54 strain was found for those derivatives with basic amino side chains. A second correlation was identified between the NF54 activity and physico-chemical factors related to solvation and polarity.
Malaria is serious disease in tropical countries as a major cause of illness and death.1) According to the World Health Organization (WHO), this disease has led to about 219 million malarial infected cases in 2010, and approximately 0.66 million have died due to the non-availability of proper treatment.2) It is calculated that an African child has on average 1.6 to 5.4 episodes of malarial fever each year. The Plasmodium falciparum subspecies, which is the most virulent and deadly of the malaria parasites, is responsible in more than 90% of the cases.3) Although the intensive efforts to combat malaria have been continued, the incidence of malaria has not decreased and the endemic areas are expanding, especially in the tropical and subtropical areas.4)
For the treatment of malaria patient, chloroquine (CQ)5) and artemisinin6) are important therapeutic agents. However, P. falciparum strains resistant to CQ are spreading in recent years in the endemic areas7) and in vitro and in vivo resistances even against the most recently introduced artemisinin derivatives have also been demonstrated in therapy for uncomplicated P. falciparum infections.8,9) Therefore, the development of new chemotherapeutic treatments for this disease is urgently needed.
Alkaloids from plants are promising candidates of new lead compounds in the search for new drugs against infectious diseases and cancers.10,11) Indeed, the indoloquinoline alkaloids isolated from Cryptolepis sanguinolenta (LINDL.) SCHLTR. (Asclepiadaceae) have been used as scaffolds for drug discovery, since this plant is used as a traditional herbal medicine in West and Central Africa12,13) (Fig. 1). The structure–activity relationship (SAR) studies of the 5-Me-5H-indolo[2,3-b]quinoline core indicated that attachment of the appropriate substituents at the C2, C9, and C11 positions have significantly increased the efficacies in comparison to the natural product itself.14,15) For example, the antimalarial activity was improved 1500-fold active against the chloroquine-sensitive Plasmodium falciparum Ghana strain by introducing a ω-aminoalkylamino group at C-1116,17) and the cytotoxicity was improved 1000-fold less toxic by introduction of an ester group at C-9.18)

In contrast to these remarkable advancements in the basic research of the SAR studies with the 5-Me-5H-indolo[2,3-b]quinoline derivatives, little testing have been undertaken with the 6-Me-6H-indolo[2,3-b]quinoline, the congener and non-natural type neocryptolepines. In 2005, Opolski and colleagues reported that an evaluation of a series of derivatives of this structure, substituted at C-2, C-9 or N-6 with dialkyl(alkylamino) chains of different methylene units, showed a cyctotoxicity against KB cells (human cervix carcinoma) with IC50 values ranging from 2.1 to 9 µM.19) Additionally, these compounds showed a strong ability to inhibit the human topoisomerase II activity. In 2012, Kaczmarek and colleagues reported that indolo[2,3-b]quinoline derivatives bearing a dimethylaminoethyl group at N-6, with various substituents at C-2 or C-9, revealed an antiproliferative activity against human cancer cell lines KB (nasopharynx carcinoma), A-549 (lung carcinoma), MCF-7 (breast cancer) and Hs294T (melanoma).20) This activity was considerably higher than that of the reference 5,11-dimethyl-5H-indolo[2,3-b]quinoline (DIMIQ) and, in general, the C-2 substituted derivatives were more active than the C-9 ones.
In a previous paper, we reported that some of 6-Me-6H-indolo[2,3-b]quinoline derivatives, bearing a linear and cyclic aminoalkylamino group at C-11 and a Cl atom at C-2, had the IC50 values between 0.46–0.80 M against MV4-11 leukemia cells, which were much higher than the known anticancer drugs, cisplatin and DIMIQ.21)
However, to the best of our knowledge, the ability against the antiprotozoal activities of 6-Me-6H-indolo[2,3-b]quinoline derivatives has not yet been started. Regarding the effect of an N-Me group, Pieters et al. reported that removal of the N-methyl group from the neocryptolepine core led to a complete loss of antiplasmodial activity in the tested concentration range.22) In this paper, we discuss the antiplasmodial activity of a series of 6-Me-6H-indolo[2,3-b]quinoline derivatives to compare the activity with their respective 5-Me-5H-indolo[2,3-b]quinoline derivatives with varying substituents at the C-2, C-9, and C-11 positions. Furthermore, the compounds were tested for β-haematin inhibition for better understanding of the mechanism of action of the neocryptolepines.23)
The preparations of the 11-chloro-6-methyl-6H-indolo[2,3-b]quinolines 6 were carried out according to the method reported by Bergman et al.24) and us.25–27) Thus, as shown in Chart 1, the reaction of indole-3-carboxylate 1a and 4-chloroaniline (2) using N-chlorosuddinimide (NCS) as a coupling reagent formed the 2-anilinoindole-3-carboxylate 3a. The treatment of 3a with NaH followed by MeI afforded the corresponding 1-methyl-indole-3-carboxylate 4a as a result of exclusive deprotonation at the nitrogen of the indole ring due to low pKa of the indole NH.28) Heating of 4a at reflux in diphenyl ether induced the intramolecular acylation to form the corresponding indoloquinolinone 5a in good yield. The subsequent dehydroxylative chlorination of the quinolone 5a with POCl3 afforded the desired 6a. By analogy with this sequence, the C-9 ester-installed 6b was prepared using the indole-3,5-dicarboxylate 1b as the starting point. The diester 1b was prepared by trichloroacetylation of methyl indole-5-carboxylate in the presence of pyridine, followed by successive treatment with KOH at reflux in MeOH and MeI in N,N-dimethylformamide (DMF).29) The diester 1b was combined with the aniline 2 using NCS and the subsequent methylation of the resulting 3b by treatment with NaH-MeI, affording 4b. The thermal cyclization followed by dehydroxylative chlorination with POCl3 afforded the corresponding 11-chloro-9-methoxycarbonyl-6-methyl-6H-indolo[2,3-b]quinolone (6b) in 66% overall yield.

Reagents and conditions: (a) i. N-chlorosuccinimide, 1,4-dimethylpiperazine. ii. trichloroacetic acid; (b) NaH-MeI; (c) diphenyl ether, reflux; (d) POCl3, reflux.
In a previous paper, we reported that the SNAr aminations of 11-chloro-6-methyl-6H-indolo[2,3-b]quinolines are sluggish compared to their 5-methyl-5H-indolo[2,3-b]quinolones.26) In order to improve the amination step in short time, we employed the microwave (MW) reaction technique. Thus, the reaction of 6a and 1,3-propanediamine in DMF at 120°C under MW irradiation conditions led to completion in 2 h, forming 11-aminoindolo[2,3-b]quinolines 7 in 74% yield, while the same reaction under conventional heating took 10 h to finish the reaction, forming 7 in 61% yield.30) The uretanizations of the side-lariats of 7 with phenylisocyanate or phenylthioisocyanate smoothly produced the corresponding urethane 9 (X=O) and and 10 (X=S) (Chart 2).

Reagents and conditions: (e) appropriate amines, MW heating; (f) PhNCO or PhNCS.
The antiplasmodial efficacy was evaluated against P. falciparum (CQS: NF54),31) and the cytotoxicity toward L6 cells and all the tested compounds were compared for activity with reference to the standard drug chloroquine (Sigma C6628). Based on the literature data,25–27) we found that the halogen substitution at the C2 position of the indolo[2,3-b]quinoline core induced a favorable effect on the activity. Therefore, we employed the 6-methylindole[2,3-b]quinoline core (congener of neocryptolepine) substituted with a chlorine atom at the C2 position. The results of the studies on the antiplasmodial activity based on the synthesized derivatives 7–10 are summarized in Table 1 along with the results of the antimalarial drug, chloroquine.
| Compd | R1 | R2 | Cytotoxicity (L6 cells) | P. falciparum (NF54) | SIa) | Heamozoin inhibition | |
|---|---|---|---|---|---|---|---|
| IC50 nMb) | IC50 nMb) | (L6/NF54) | βH IC50 μM | Ratio %c) | |||
| 7a | H |  | 4834 | 424 | 11.4 | 317.1 | 51.4 |
| 7b | H |  | 1606 | 113 | 14.2 | 48.6 | 93.0 |
| 7c | H |  | 3769 | 212 | 17.8 | 113.8 | 95.7 |
| 7d | H |  | 4026 | 493 | 8.2 | 146.7 | 59.8 |
| 7e | H |  | 4531 | 1087 | 4.2 | 45.2 | 62.9 |
| 7f | H |  | 5444 | 5755 | 0.9 | 96.9 | 95.4 |
| 8 | CO2Me |  | 1732 | 86 | 20.2 | 18.4 | 73.6 |
| 9a | H |  | 10786 | 341 | 31.6 | 40.3 | 81.5 |
| 9b | H |  | 7546 | 847 | 8.9 | 26.4 | 80.8 |
| 9c | H |  | 57515 | 921 | 62.3 | 60.2 | 60.8 |
| 9d | H | 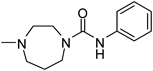 | 117141 | 317 | 369.5 | 46.0 | 78.4 |
| 10a | H |  | 16749 | 754 | 22.2 | 33.5 | 75.1 |
| 10b | H |  | 14879 | 575 | 25.9 | 17.8 | 91.4 |
| 10c | H |  | 56860 | 753 | 75.5 | 22.1 | 92.4 |
| 10d | H |  | 10738 | 1633 | 65.8 | 44.1 | 63.7 |
| 11 | >32000 | ||||||
| 6a | 68565.9 | 32739.0 | 2090 | — | — | ||
| 12 | 3482.0 | 27.7 | 125.7 | — | — | ||
| 13 | 268.6 | 11.8 | 22.8 | ||||
| Podophylotoxin | 14.5 | ||||||
| Chloroquine | 9.4 | 18.4 | |||||
| Amodiaqline | 7 | ||||||
a) Selectivity Index is the ratio of IC50 for cytotoxicity versus antiplasmodial activity (L6/P.f.).b) The IC50 values are the means of two independent assays, the individual values vary less than a factor 2.c) Haemozoin inhibition ratio is tested at 30 µM of the compound which heamozoin inhibition IC50 is below 30 µM; Haemozoin inhibition ratio is tested at 100 µM of the compound which heamozoin inhibition IC50 is between 30 to 100 µM; Haemozoin inhibition ratio is tested at 500 µM of the compound which heamozoin inhibition IC50 is between 100 to 500 µM.
As shown in Table 1, the introduction of an amino group at the C11 position of the 6-methylindole[2,3-b]quinoline core can significantly increase the antiplasmodial activities compared to the indole[2,3-b]quinoline derivatives which lacks the 6-methyl 12 and 11-alkylamino groups 11 against the chloroquine-sensitive P. falciparum Ghana strain.23) Based on these facts, we introduced various types of amino groups at C11 of the 2-chloro-6-methylindolo[2,3-b]quinoline core for the SAR study.
All the tested compounds, except for the compound 7f, have a significant cytotoxic effect against the NF54 strains of P. falciparum. Based on the data of series 7a–9c, we found that compound 7b, with a 3-aminopropylamino group at the C11 position, contributed the most to the antimalarial activity with an IC50 value of 113 nM. In general, ω-aminoalkylamino-substitutents contribute more favorably that the ω-hydroxyalkylamino-substitutent. Furthermore, ω-primary-aminoalkyl derivatives are the more active against NF54 cells compared to the ω-tert-aminoalkyl 7d or cyclic-aminoalkylamino derivatives 7e.
Moreover, the ester group of 8 at the C9 position showed a higher contribution to the antiplasmodial activity compared to the non-substituted compound 7b. Therefore, we can confirm that the substitution of the ester group at the C9 position on the neocryptolepine congener core can have a positive effect on the antimalaria activity compared to the non-substituted compound 7b.
The urea and thiourea forms significantly contribute to decreasing the cytotoxicity (L6 cells). The most significant example is the diazepane substituent (9d) in which SI (L6/NF54) was improved to 369 with ureanication from the free amine of 7e with 4.2. The thiourea forms 10b and 10c are more effective and less toxic compared to the respective urea forms 9b and 9c.
The effect of N-Me group of indolo[2,3-b]quinolone core on the antiplasmodial activity was evaluated by comparing the antiplasmodial activity of 7b, 11-(3-aminopropylamino)-2-chloro derivative 12 bearing no N-methyl group and 13 bearing 5-N-methyl group (Fig. 2). The compound 11 without both 11-alkylamino and N-methyl groups was also listed in Table 1 for a reference. Thus, the compound 11 is greatly less toxic compared with the 11-(3-aminopropylamino) derivatives 7b, 12, and 13. The antiplasmodial activity of indolo[2,3-b]quinolone derivatives with or without N-methyl group was assigned in the order of 5-methylated 13 > no-methylated 12 > 6-methylated 7b.

The β-haematin formation inhibition assay method reported by Carter et al.32,33) was modified for manual liquid delivery as previously described.34)
The formation of haemozoin by P. falciparum, the details and relevance of which has been previously described,34–36) is a suitable parasite-specific drug target for compounds able to cross the red blood cell membrane, the parasitophorous vacuole membrane, the P. falciparum plasma membrane and the acidic digestive vacuole (DV) membrane. The synthetic form of the haemozoin crystal, β-haematin, can be formed under biomimetic conditions mediated by the detergent NP-40.31) This allows for the haemozoin inhibition activity of potential antimalarial compounds to be investigated.
In order to better understand the mechanism of action of the neocryptolepine analogues, samples were tested for β-haematin inhibition (Table 1). Twelve showed IC50 values <100 µM, showing the potential of this series to inhibit haemozoin in the malaria parasite DV. If this were the case, a correlation between the β-haematin inhibition activity and the biological activity (NF54 cells) would be expected, assuming equal accumulation at the target site. Discovering these correlations is not always straightforward owing to the physico-chemical factors which influence how effectively a molecule moves through the four cell membranes to reach the site of haemozoin formation. Molecules also have different degrees of accumulation in the acidic environment of the DV (pH 4.8–5.2). One way to increase the vacuolar accumulation ratio (VAR) is through pH trapping of a weak base molecule. In this process, when equilibrium is attained the concentration of the uncharged form of the molecule, which can freely diffuse through the membrane, is equal both inside and outside the DV. Under these conditions there is a great accumulation of protonated form of the molecule in the acidic compartment. This is because the neutral unprotonated form makes up a far smaller proportion of the total species present in relation to the higher pH external environment. Therefore, for a molecule to pH trap, it must possess a moiety which is largely protonated at pH 5.
In this series, five compounds containing a basic amino group were present. Previous work on 4-aminoquinolines suggests that these five compounds should accumulate at the target site.37,38) Upon inspection, it is evident that these compounds have potent activities (<500 nM) and the three most active compounds all contain primary amines. Furthermore, a linear correlation (r2=0.84) between the logged biological and logged β-haematin inhibition activity can be seen for this subset (Fig. 3a). The correlation indicates that activity against the NF54 strain is strongly influenced by the ability of a compound to inhibit haemozoin formation, at least for those that are able to accumulate in the DV through pH trapping. Previous work on 5-Me-5H-indolo[2,3-b]quinolones34) reported no increase in activity for those derivatives with amino side chains. Rather, the compounds in this series displayed considerably greater activities against the parasite than those in the 6-Me-5H-indolo[2,3-b]quinoline series reported here. This may have masked effects arising from pH trapping. Indeed, when we re-evaluated the 5-Me-5H-indolo[2,3-b]quinoline series in the light of these findings, there was a statistically significant, albeit weak correlation between the logged biological and β-haematin inhibition activity. In the case of the 5-Me-5H-indolo[2,3-b]quinoline series however, the correlation is difficult to discern because the activities of these weak base compounds were not clearly separated from the non-base compounds.
Interestingly, if all of the compounds in the 6-Me-5H-indolo[2,3-b]quinoline series are considered together, a strong correlation between activity against the NF54 parasite strain and physico-chemical parameters excluding β-haematin inhibition is also observed. These parameters are similar to those seen for the 5-Me-5H-indolo[2,3-b]quinolines and include parameters that relate to the solvation and polarity of the molecule. More specifically, in the present series a statistically significant correlation was observed for a four parameter model involving solubility (Sol), mean water of hydration (H2O.Hyd), log P and the square of the dipole moment (μ2) (Fig. 3b). Fitted coefficients revealed that the strongest dependence was on the solubility term such that highly soluble compounds are less active, probably because they are too hydrophilic for membrane absorption. The H2O.Hyd term, which has a quarter of the weighting of the solubility term, predicts greater biological activity when the molecule is well hydrated. This can be rationalised by the favourable increase in entropy caused by the liberation of water molecules when the compound enters a lipophilic cell membrane. The activity also has a smaller dependence on the log P and μ2. Those compounds with log P>5 (10b, 9c, 10c, 10d, 7d) have some of the lowest activities, which may be a result of either poor solubility or inability of the compound to partition out of membranes. The dipole term is required to account for the inactivity of 7f. The hydroxy group in this compound results in a large dipole moment. However, if 7f is excluded, this parameter becomes superfluous.

(a) Compounds with basic amino side chains (7a, 7b, 7c, 7e, 8) were found to have haemozoin inhibition activity which correlates linearly with biological activity such that log[NF54IC50]=0.651*log[βHIC50]+1.066, r2=0.84, p=0.03. Compounds without basic groups (7d, 7f, 9, 10) showed an increasing trend but no statistically significant correlation between haemozoin and biological activity. (b) Quantitative structure–activity relationship (QSAR) analysis performed on the biological activities correlated with physico-chemical parameters. Predicted vs. experimental NF54 activity based on multiple linear regression where log(NF54IC50)=0.302(Sol)−0.188(H2O.hyd)+0.446(log P)+0.002(μ2)−4.670, (r2=0.940, p<0.0001); statistically significant for both individual parameters (t=5.33, 5.29, 8.83, 8.35, 3.30>tcrit=3.17) and overall correlation (F=39.01>Fcrit=5.99) at 99% confidence level.
The SAR studies were conducted by varying the kind of alkylamino or ω-aminoalkylamino stubstituents at C11 with a Cl at the C2 or COOMe at the C9 position of 6-methyl-6H-indolo[2,3-b]quinolone core. The anti-malarial activities of the tested compounds were significantly increased compared to the 11-nonsubstituted derivatives. The 3-aminopropylamino group at C11 was the most efficacious one compared to the other ω-aminoalkylamino of shorter or longer alkylene units. The 3-aminopropylamino group at the C11 was further modified to the urea and thiourea, which improved the cytotoxicity against normal cells, compared to the initial terminal free amine form. The best results were achieved with compounds 8 and 9d against the NF54 strain with the IC50/SI value of 86 nM/20 and 317 nM/370, respectively.
The correlations of β-haematin inhibition suggest that haemozoin inhibition probably does play a role in the activity of the neocryptolepines. This was particularly evident in the compounds that can pH trap in the 6-Me-5H-indolo[2,3-b]quinoline series, but weak correlations were found with all of the compounds in the 5-Me-5H-indolo[2,3-b]quinoline series. Even the non-base 6-Me-5H-indolo[2,3-b]quinolines showed a trend between biological activity and β-haematin inhibition, but this was not statistically significant at the 95% confidence level. Physico-chemical factors related to the solvation and polarity play a very strong role in the activities of the neocryptolepines. Therefore, it is difficult to determine whether this arises as a result of other targets, with haemozoin being a secondary target, or whether access to the DV simply masks the correlation between the activity and inhibition of the haemozoin formation. To better understand the mechanism of action of the neocryptolepines, investigation of the accumulation of free haem within P. falciparum in the presence of these compounds is required. This could be achieved using cell fractionation studies similar to those described by Combrinck et al.39)
The commercially obtained reagents were directly used without further purification. The 1H-NMR and 13C-NMR spectra were measured on the Varian INOVA-600 spectrometer (600 Hz). High resolution mass spectra (HR-MS) were obtained on a Bruker microTOF II-SKA spectrometer. Melting points were determined on a J-Science RFS-10 hot stage microscope. The scaffolds 3–6 were prepared by the method previously mentioned.25–27)
Dimethyl 2-(4-Chlorophenylamino)-1H-indole-3,5-dicarboxylate (3b)Yield: 58%, mp: 215–217°C; 1H-NMR (600 MHz, CDCl3) δ 9.04 (s, 1H), 8.53 (s, 1H), 8.46 (s, 1H), 7.78 (d, J=8.2 Hz, 1H), 7.37 (m, 2H), 7.22 (d, J=8.6 Hz, 2H), 7.18 (d, J=8.3 Hz, 1H), 3.95 (s, 3H), 3.92 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ 168.1, 167.5, 149.9, 136.6, 134.6, 1306, 130.3 (2C), 125.9, 124.0, 123.0 (2C), 123.0, 121.3, 109.6, 87.0, 51.9, 51.0. HR-MS electrospray ionization (ESI) Calcd for C18H16ClN2O4 [M+H]+ 359.0799. Found 359.0834.
Dimethyl 2-(4-Chlorophenylamino)-1-methyl-1H-indole-3,5-dicarboxylate (4b)Yield: 56%, mp: 179–181°C; 1H-NMR (600 MHz, CDCl3) δ 8.6 (d, J=1.6 Hz, 1H), 8.37 (s, 1H), 7.93 (dd, J=8.5, 1.7 Hz, 1H), 7.28 (m, 2H), 7.23 (d, J=8.5 Hz, 1H), 6.90 (m, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 3.39 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ 168.0, 167.1, 149.7, 140.6, 1377, 129.6 (2C), 128.8, 125.0, 124.1, 123.4, 122.4, 121.3 (2C), 108.6, 93.0, 52.0, 51.1, 31.7. HR-MS (ESI) Calcd for C19H18ClN2O4 [M+H]+ 373.0955. Found 373.1095.
Methyl 2-Chloro-6-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-9-Carboxylate (5b)Yield: 77%, decomposed >360°C; 1H-NMR (600 MHz, DMSO-d6) δ 8.76 (d, J=1.4 Hz, 1H), 8.14 (s, 1H), 7.88 (d, J=8.4 Hz, 1H), 7.66 (dt, J=8.8, 5.6 Hz, 2H), 7.53 (d, J=8.5 Hz, 1H), 3.88 (s, 3H), 3.85 (s, 3H); 13C-NMR (151 MHz, DMSO-d6) δ 170.6, 166.8 (2C), 146.6, 139.1, 136.9, 131.0, 126.6, 124.7, 124.3, 124.19, 122.6, 121.5, 120.0, 109.1, 101.2, 51.9, 29.5.
Methyl 2,11-Dichloro-6-methyl-6H-indolo[2,3-b]quinoline-9-carboxylate (6b)Yield: 96%, decomposed >298°C; 1H-NMR (600 MHz, CDCl3) δ 9.20 (d, J=1.4 Hz, 1H), 8.36 (d, J=2.3 Hz, 1H), 8.32 (dd, J=8.5, 1.6 Hz, 1H), 8.03 (d, J=9.0 Hz, 1H), 7.68 (dd, J=8.9, 2.3 Hz, 1H), 7.41 (d, J=8.5 Hz, 1H), 4.01 (s, 3H), 3.97 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ 167.2, 152.7, 145.6, 145.3, 135.0, 130.6, 130.5, 123.0, 129.5, 126.1, 123.1, 123.0, 122.5, 119.2, 115.8, 108.3, 52.2, 28.1. HR-MS (ESI) Calcd for C18H13Cl2N2O2 [M+H]+ 359.0354. Found 359.0355.
General Procedure for the Synthesis of 11-Aminoneocryptolepines (7–8)Method A: 11-Chloroindoloquinolines 6 (6.0 mmol) and an excess of the appropriate aminoalkylamine (3.0 mmol) in DMF were heated at 120°C for 10–15 h. TLC monitoring was used to ensure the completion of reaction. The resulting brown oil was purified by flash chromatography using AcOEt–2N ammonia in MeOH (10 : 1) as an eluent to yield pure 7–8.
Method B: 11-Chloroindoloquinolines 6 (6.0 mmol) and an excess of the appropriate aminoalkylamine (3.0 mmol) in DMF were heated at 120°C for 2–3 h. TLC monitoring was used to ensure the completion of reaction. The resulting brown oil was purified by flash chromatography using AcOEt–2N ammonia in MeOH (10 : 1) as an eluent to yield pure 7–8.
2-Chloro-11-(1,4-diazepan-1-yl)-6-methyl-6H-indolo[2,3-b]quinoline (7e)Yield: 89%, decomposed >236°C; 1H-NMR (600 MHz, CDCl3) δ 8.46 (dd, J=5.3, 2.2 Hz, 2H), 8.04 (d, J=8.9 Hz, 1H), 7.60 (m, 2H), 7.41 (d, J=8.0 Hz, 1H), 7.35 (t, J=7.4 Hz, 1H), 3.97 (s, 3H), 3.73–3.70 (m, 4H), 3.33–3.28 (m, 4H), 2.09 (d, J=4.4 Hz, 2H); 13C-NMR (151 MHz, CDCl3) δ 154.5, 151.0, 146.8, 142.4, 129.5, 129.2, 127.7 (2C), 124.6, 124.1, 123.8, 120.1, 119.4, 115.0, 108.4, 55.3, 52.7, 51.4, 49.3, 33.0, 27.8. HR-MS (ESI) Calcd for C21H22ClN4 [M+H]+ 365.1533. Found 365.1534.
Methyl 11-(3-Aminopropylamino)-2-chloro-6-methyl-6H-indolo[2,3-b]quinoline- 9-carboxylate (8)Yield: 59%, mp: 123–125°C; 1H-NMR (600 MHz, CDCl3) δ 8.92 (s, 1H), 8.20 (s, 1H), 8.14 (d, J=8.4 Hz, 1H), 7.91 (d, J=9.0 Hz, 1H), 7.55 (d, J=9.0 Hz, 1H), 7.29 (d, J=8.3 Hz, 1H), 3.96 (s, 3H), 3.95 (d, J=5.3 Hz, 2H), 3.89 (s, 3H), 3.09 (t, J=5.4 Hz, 2H), 1.89 (dd, J=11.1, 5.5 Hz, 2H).13C-NMR (151 MHz, CDCl3) δ 167.8, 154.5, 148.5, 146.7, 144.1, 129.5, 129.4, 127.4, 126.4, 124.0, 122.7, 121.2, 120.4, 118.3, 107.4, 1027, 52.0, 49.9, 41.6, 32.8, 27.8. HR-MS (ESI) Calcd for C21H22ClN4O2 [M+H]+ 397.1431. Found 397.1432.
General Procedure for the Synthesis of 11-Aminoneocryptolepines (9–10)6-Methyl-6H-indolo[2,3-b]quinoline (7, 30 mg) was completely dissolved in tetrahydrofuran (THF) (1 mL), and then a solution of isocyanate (1.2 equiv) and THF (1 mL) were added drop by drop under stirring at room temperature for 6 h. TLC monitoring was used to ensure the complete of reaction. After reaction was finished, the reaction mixture was evaporated to dryness. The crude product was purified by flash chromatography using hexane–AcOEt (1 : 1) as an eluent to yield pure products 9–10.
1-(2-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-ylamino)ethyl)-3-phenylurea (9a)Yield: 64%, mp: 137–139°C; 1H-NMR (600 MHz, DMSO-d6) δ 8.61 (d, J=2.0 Hz, 1H), 8.48 (s, 1H), 8.09 (d, J=7.8 Hz, 1H), 7.86 (d, J=8.9 Hz, 1H), 7.61 (dd, J=8.9, 2.0 Hz, 1H), 7.53 (d, J=8.0 Hz, 1H), 7.47 (t, J=7.6 Hz, 1H), 7.31 (d, J=8.4 Hz, 2H), 7.24 (t, J=7.5 Hz, 1H), 7.17 (t, J=7.9 Hz, 2H), 6.86 (td, J=7.4, 1.1 Hz, 1H), 6.71 (m, 1H), 6.28 (t, J=5.8 Hz, 1H), 3.84 (s, 3H), 3.77 (dd, J=11.8, 5.8 Hz, 2H), 3.44 (q, J=6.1 Hz, 2H); 13C-NMR (151 MHz, DMSO-d6) δ 156.0, 154.2, 148.5, 145.8, 141.2, 140.7, 129.5, 129.0 (2C), 126.3, 126.0, 123.6, 122.6, 121.5, 120.3, 120.2, 118.9, 118.6, 118.2 (2C), 109.1, 103.0, 49.3 (2C), 28.0.
1-(3-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-ylamino)propyl)-3-phenylurea (9b)Yield: 92%, mp: 189–191°C; 1H-NMR (600 MHz, DMSO-d6) δ 8.64 (s, 1H), 8.43 (s, 1H), 8.07 (d, J=7.8 Hz, 1H), 7.88 (dd, J=8.9, 1.0 Hz, 1H), 7.63 (d, J=8.9 Hz, 1H), 7.53 (d, J=8.0 Hz, 1H), 7.47 (t, J=7.6 Hz, 1H), 7.36 (d, J=8.1 Hz, 2H), 7.26 (t, J=7.4 Hz, 1H), 7.19 (t, J=7.1 Hz, 2H), 6.87 (t, J=7.3 Hz, 1H), 6.64 (s, 1H), 6.18 (t, J=5.4 Hz, 1H), 3.84 (d, J=0.9 Hz, 3H), 3.68 (d, J=6.3 Hz, 2H), 3.15 (dd, J=12.0, 5.9 Hz, 2H), 1.99–1.75 (m, 2H); 13C-NMR (151 MHz, DMSO-d6) δ 156.0, 153.9, 148.6, 141.12, 140.9, 129.6, 129.0 (2C), 128.5, 126.4, 126.0, 123.4, 122.5, 121.4, 121.3, 120.3, 120.2, 119.1, 118.1, 118.0, 109.1, 103.4, 46.0, 36.9, 32.2, 27.0. HR-MS (ESI) Calcd for C26H25ClN5O [M+H]+ 458.1748. Found 458.1748.
1-(4-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-ylamino)butyl)-3-phenylurea (9c)Yield: 98%, mp: 161–163°C; 1H-NMR (600 MHz, CDCl3) δ 7.89 (s, 1H), 7.82 (d, J=8.9 Hz, 1H), 7.75 (d, J=7.6 Hz, 1H), 7.71 (s, 1H), 7.39 (dd, J=14.1, 7.6 Hz, 2H), 7.27 (d, J=7.5 Hz, 1H), 7.16 (dd, J=17.8, 8.4 Hz, 5H), 6.92 (t, J=7.3 Hz,1H), 5.75 (s, 1H), 4.65 (s, 1H), 3.71 (s, 3H), 3.46 (d, J=5.3 Hz, 2H), 3.12 (d, J=6.0 Hz, 2H), 1.57 (m, 2H), 1.4 (m, 2H); 13C-NMR (151 MHz, CDCl3) δ 156.7, 153.1, 147.9, 145.5, 141.1, 139.2, 129.5, 129.2 (2C), 129.0, 128.7, 126.8, 126.4, 123.2, 122.1, 121.8, 120.23, 120.2, 120.0, 118.2, 108.6, 104.3, 48.8, 39.7, 29.1, 27.9, 27.7. HR-MS (ESI) Calcd for C27H27ClN5O [M+H]+ 472.1904. Found 472.1703.
4-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-yl)-1-(N-phenylcarbamoyl)-1,4-diazepane (9d)Yield: 97.5%, mp: 117–119°C; 1H-NMR (600 MHz, DMSO-d6) δ 8.49 (s, 1H), 8.26 (d, J=7.7 Hz, 1H), 8.23 (d, J=2.0 Hz, 1H), 7.88 (d, J=8.9 Hz, 1H), 7.64 (d, J=7.9 Hz, 2H), 7.50 (t, J=7.6 Hz, 2H), 7.47 (d, J=8.0 Hz, 1H), 7.26 (t, J=7.8 Hz, 2H), 7.14 (t, J=7.4 Hz, 1H), 6.96 (t, J=7.4 Hz, 1H), 3.93 (t, J=5.1 Hz, 2H), 3.82 (s, 2H), 3.75 (s, 3H), 3.66 (d, J=4.4 Hz, 2H), 3.62 (s, 2H), 1.94 (s, 2H); 13C-NMR (151 MHz, DMSO-d6) δ 155.5, 153.9, 151.1, 146.4, 142.3, 141.2, 130.0, 129.3, 128.6 (2C), 128.3, 127.2, 124.6, 124.0, 123.6, 122.1, 120.6 (2C), 120.5, 119.0, 114.9, 109.34, 53.2, 52.7, 48.0, 46.7, 32.5, 28.0. HR-MS (ESI) Calcd for C28H27ClN5O [M+H]+ 484.1904. Found 484.1906.
1-(2-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-ylamino)ethyl)-3-phenylthiourea (10a)Yield: 75%, mp: 109–111°C; 1H-NMR (600 MHz, DMSO-d6) δ 9.52 (s, 1H), 8.63 (d, J=1.9 Hz, 1H), 8.08 (d, J=7.8 Hz, 1H), 7.89 (d, J=8.9 Hz, 1H), 7.72 (s, 1H), 7.63 (dd, J=8.9, 2.2 Hz, 1H), 7.54 (d, J=8.0 Hz, 1H), 7.49 (t, J=7.6 Hz, 1H), 7.29–7.25 (m, 1H), 7.16 (t, J=7.7 Hz, 2H), 7.09 (d, J=7.5 Hz, 2H), 7.03 (t, J=7.3 Hz, 1H), 6.60 (s, 1H), 3.89 (dd, J=11.4, 5.6 Hz, 2H), 3.85 (s, 3H), 3.35 (s, 2H); 13C-NMR (151 MHz, DMSO-d6) δ 180.9, 169.4, 154.4, 148.3, 145.89, 141.3, 139.1, 129.5, 129.1 (2C), 126.4, 126.0, 124.7 (2C), 123.7, 123.5, 122.6, 120.3, 120.2 (2C), 119.1, 109.2, 103.4, 47.7, 45.2, 28.0.
1-(3-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-ylamino)propyl)-3-phenylthiourea (10b)Yield: 97%, mp: 92–94°C; 1H-NMR (600 MHz, CDCl3) δ 8.23 (s, 1H), 8.19 (s, 1H), 8.00 (d, J=7.7 Hz, 1H), 7.96 (d, J=8.8 Hz, 1H), 7.46 (t, J=7.6 Hz, 2H), 7.28 (tt, J=15.7, 7.9 Hz, 4H), 7.20 (d, J=6.6 Hz, 3H), 6.49 (s, 1H), 5.57 (m, 1H), 3.90 (dd, J=11.7, 5.7 Hz, 2H), 3.86 (s, 3H), 3.77 (d, J=4.9 Hz, 2H), 1.92 (dd, J=12.1, 6.0 Hz, 2H); 13C-NMR (151 MHz, DMSO-d6) δ 180.7, 154.5, 148.4, 146.0, 141.4, 139.4, 129.8, 129.5, 129.1, 126.5, 126.0, 124.7, 123.6, 123.5 (2C), 122.6 (2C), 120.4, 120.2, 119.3, 109.1, 103.7, 46.3, 42.0,31.0, 28.0. HR-MS (ESI) Calcd for C26H25ClN5S [M+H]+ 474.1519. Found 474.1520.
1-(4-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-ylamino)butyl)-3-phenylthiourea (10c)Yield: 92%, mp: 123–125°C; 1H-NMR (600 MHz, CDCl3) δ 8.04 (d, J=2.0 Hz, 1H), 7.99 (m, 2H), 7.93 (d, J=7.7 Hz, 1H), 7.52 (m, 2H), 7.34 (m, 3H), 7.29 (t, J=7.5 Hz, 1H), 7.24 (t, J=7.5 Hz, 1H), 7.17 (d, J=7.7 Hz, 2H), 6.21 (s, 1H), 4.85 (s, 1H), 3.88 (s, 3H), 3.74–3.63 (m, 4H), 1.82–1.67 (m, 4H); 13C-NMR (151 MHz, CDCl3) δ 180.9, 153.0, 147.8, 145.5, 141.2, 136.1, 130.1 (2C), 129.5, 128.8, 127.3, 126.9, 126.4, 125.2 (2C), 121.8, 120.2, 120.0, 119.94, 118.3, 108.5, 104.7, 48.6, 44.8, 28.9, 27.9, 26.5. HR-MS (ESI) Calcd for C27H27ClN5S [M+H]+ 488.1676. Found 488.1649.
4-(2-Chloro-6-methyl-6H-indolo[2,3-b]quinolin-11-yl)-1-(N-phenylcarbamoyl)-1,4-diazepane (10d)Yield: 91%, mp: 198–200°C; 1H-NMR (600 MHz, CDCl3) δ 8.29 (d, J=7.7 Hz, 1H), 8.16 (s, 1H), 8.03 (d, J=8.9 Hz, 1H), 7.58 (t, J=7.5 Hz, 2H), 7.37–7.31 (m, 7H), 7.16 (m, 1H), 4.31 (d, J=5.4 Hz, 2H), 4.14 (d, J=24.8 Hz, 2H), 3.90 (s, 3H), 3.76 (s, 2H), 3.66 (t, J=5.0 Hz, 2H), 2.22 (s, 2H); 13C-NMR (151 MHz, CDCl3) δ 182.6, 154.0, 150.4, 146.3, 142.4, 139.8, 129.4, 128.7(2C), 128.2, 125.8, 125.5 (2C), 124.4, 124.0, 123.1, 120.5, 120.4, 119.1, 115.3, 108.6, 54.1, 53.1 (2C), 50.6, 30.1 (2C), 27.9. HR-MS (ESI) Calcd for C28H27ClN5S [M+H]+ 500.1676. Found 500.1676.
In vitro activity against erythrocytic stages of P. falciparum was determined using a 3H-hypoxanthine incorporation assay,40,41) using the chloroquine and pyrimethamine resistant P. falciparum K1 strain that originate from Thailand (Thaitong et al.)42) and strain susceptible to known antimalarial drugs (P. falciparum NF54) (Ponnudurai et al.),31) and all the test compounds were compared for activity with the standard drug chloroquine (Sigma C6628). Compounds were dissolved in dimethyl sulfoxide (DMSO) at 10 mg/mL and added to parasite cultures incubated in RPMI 1640 medium without hypoxanthine, supplemented with N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) (5.94 g/L), NaHCO3 (2.1 g/L), neomycin (100 U/mL), Albumax® (5 g/L) and washed human red cells A+ at 2.5% haematocrit (0.3% parasitaemia). Serial drug dilutions of eleven 3-fold dilution steps covering a range from 100 to 0.002 µg/mL were prepared. The 96-well plates were incubated in a humidified atmosphere at 37°C; 4% CO2, 3% O2, 93% N2. After 48 h 50 µL of 3H-hypoxanthine (=0.5 µCi) was added to each well of the plate. The plates were incubated for a further 24 h under the same conditions. The plates were then harvested with a Betaplate™ cell harvester (Wallac, Zurich, Switzerland), and the red blood cells transferred onto a glass fibre filter then washed with distilled water. The dried filters were inserted into a plastic foil with 10 mL of scintillation fluid, and counted in a Betaplate™ liquid scintillation counter (Wallac, Zürich, Switzerland). IC50 values were calculated from sigmoidal inhibition curves by linear regression (Huber and Koella)43) using Microsoft Excel.
In Vitro Cytotoxicity against L6 CellsAssays were performed in 96-well microtiter plates, each well containing 100 L of RPMI 1640 medium supplemented with 1% L-glutamine (200 mM) and 10% fetal bovine serum, and 4000 L6 cells (a primary cell line derived from rat skeletal myoblasts) (Page et al., and Ahmed et al.).44,45) Serial drug dilutions of eleven 3-fold dilution steps covering a range from 100 to 0.002 µg/mL were prepared. After 70 h of incubation the plates were inspected under an inverted microscope to assure growth of the controls and sterile conditions. Ten liters of Alamar Blue was then added to each well and the plates incubated for another 2 h. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, U.S.A.) using an excitation wave length of 536 nm and an emission wave length of 588 nm. The IC50 values were calculated by linear regression43) from the sigmoidal dose inhibition curves using SoftmaxPro software (Molecular Devices Cooperation, Sunnyvale, CA, U.S.A.).
Detergent Mediated Assay for β-Haematin InhibitionThe β-haematin formation inhibition assay method described by Carter et al.46,47) was modified for manual liquid delivery. Three stock solutions of the samples were prepared by dissolving the pre-weighed compound in DMSO and after sonication, diluting with DMSO to give 20 mM, 2 mM and 0.4 mM solutions of each sample. These were delivered to a 96-well plate in duplicate to give concentrations ranging from 0–1000 µM (final concentration) with a total DMSO volume of 10 µL in each well after which deionised H2O (70 µL) and NP-40 (20 µL; 30.55 µM) were added. A 25 mM haematin stock solution was prepared by sonicating haemin in DMSO for one minute and then suspending 178 µL of this in a 1 M acetate buffer (pH 4.8). The homogenous suspension (100 µL) was then added to the wells to give final buffer and haematin concentrations of 0.5 M and 100 µM respectively. The plate was covered and incubated at 37°C for 5–6 h in a water bath. Analysis was carried out using the pyridine-ferrichrome method developed by Ncokazi and Egan.48) A solution of 50% (v/v) pyridine, 30% (v/v) H2O, 20% (v/v) acetone and 0.2 M HEPES buffer (pH 7.4) was prepared and 32 µL added to each well to give a final pyridine concentration of 5% (v/v). Acetone (60 µL) was then added to assist with haematin dispersion. The UV-Vis absorbance of the plate wells was read on a SpectaMax plate reader. Sigmoidal dose–response curves were fitted to the absorbance data using GraphPad Prism v3.02 to obtain a 50% inhibitory concentration (IC50) for each compound. Prediction of physical properties and multiple correlation analysis were carried out using the ChemSW Molecular Modeling Pro Plus v.6.36 software.
We are grateful to Okayama University for its support and to the Advanced Science Research Center for the NMR experiments and EA analyses. We are thankful to Prof. S. Nakashima, Okayama University, and Prof. X.-Q. Yu, Sichuan University, for the HR-MS analyses. Part of this study was supported by the Adaptable and Seamless Technology Transfer Program of JST. We thank JASSO for the scholarship supports to NW. TJE acknowledges the National Research Foundation and Medical Research Council of South Africa and University of Cape Town for the financial support.